Abstract
Biochemical experiments by others have indicated that protein kinase C activity is present in the rod outer segment, with potential or demonstrated targets including rhodopsin, transducin, cGMP-phosphodiesterase (PDE), guanylate cyclase, and arrestin, all of which are components of the phototransduction cascade. In particular, PKC phosphorylations of rhodopsin and the inhibitory subunit of PDE (PDE γ) have been studied in some detail, and suggested to have roles in downregulating the sensitivity of rod photoreceptors to light during illumination. We have examined this question under physiological conditions by recording from a single, dissociated salamander rod with a suction pipette while exposing its outer segment to the PKC activators phorbol-12-myristate,13-acetate (PMA) or phorbol-12,13-dibutyrate (PDBu), or to the PKC-inhibitor GF109203X. No significant effect of any of these agents on rod sensitivity was detected, whether in the absence or presence of a background light, or after a low bleach. These results suggest that PKC probably does not produce any acute downregulation of rod sensitivity as a mechanism of light adaptation, at least for isolated amphibian rods.
Keywords: retina, photoreceptor, phorbol ester, GF109203X
INTRODUCTION
Phototransduction in retinal rods involves a cGMP signaling cascade (for recent reviews, see Lagnado and Baylor, 1992; Kawamura, 1993; Pugh and Lamb, 1993; Yarfitz and Hurley, 1994; Yau, 1994; Fain et al., 1996; Koutalos and Yau, 1996; Palczewski and Saari, 1997). In this process, light isomerizes the visual pigment rhodopsin into an active form, which, via the G protein transducin, stimulates a cGMP-phosphodiesterase to lead to an increase in cGMP hydrolysis. In darkness, cytoplasmic cGMP binds to and opens cGMP-activated cation channels on the plasma membrane of the rod's outer segment (see Yau and Baylor, 1989; Finn et al., 1996). These open channels sustain an inward dark current to keep the cell partially depolarized and maintain a steady release of glutamate from the rod's synaptic terminal. In the light, the hydrolysis of cGMP leads to the closure of these channels, producing a membrane hyperpolarization as the light response and reducing the glutamate release from the cell. The turn-off of phototransduction involves several mechanisms, an important one being the phosphorylation of the photoactivated rhodopsin by a rhodopsin kinase, which reduces its ability to activate transducin; this is followed by the binding of a protein called arrestin to the phosphorylated rhodopsin to cap its activity.
For many years, there have also been reports of protein kinase C activity in the rod outer segment (Kapoor and Chader, 1984; Kelleher and Johnson, 1985; Hayashi et al., 1987; Kapoor et al., 1987; Binder et al., 1989; Wolbring and Cook, 1991), with potential or demonstrated substrates including rhodopsin (Kelleher and Johnson, 1986; Newton and Williams, 1991, 1993a ; Greene et al., 1995, 1997; Udovichenko et al., 1997), the α-subunit of transducin (Zick et al., 1986), the cGMP-phosphodiesterase (PDE)1 (Hayashi et al., 1991; Udovichenko et al., 1993, 1994, 1996), guanylate cyclase (Wolbring and Schnetkamp, 1995) and arrestin (Weyand and Kühn, 1990). In particular, the PKC phosphorylations of rhodopsin and the inhibitory subunit of PDE (PDEγ) have been studied in some detail. The phosphorylation of rhodopsin by PKC is reported to be triggered or enhanced by light, and involves both photoactivated rhodopsin and rhodopsin that has not absorbed any light (Newton and Williams, 1991, 1993a , 1993b ; Kelleher and Johnson, 1986). Because PKC phosphorylation of rhodopsin also weakens the latter's ability to activate transducin (Kelleher and Johnson, 1986), the action of PKC on both photoactivated and nonphotoactivated rhodopsin has been proposed to be a highly effective mechanism for reducing the light sensitivity of rods, much analogous to the heterologous desensitization of the α-adrenergic receptor through phosphorylation by protein kinase A and possibly protein kinase C (Newton and Williams, 1993b ). As for the PDEγ, PKC phosphorylation is reported to have several consequences, including an increased ability of PDEγ to inhibit the catalytic subunits PDEαβ, a decreased ability of transducin to activate the PDEαβγ holoenzyme, and an accelerated GTPase activity of the transducin bound to PDEγ, all of which would also reduce the sensitivity of rods to light (Udovichenko et al., 1994, 1996; see also Tsuboi et al., 1994a , 1994b ).
In view of the above biochemical findings, we have undertaken experiments to look for a physiological role of PKC in phototransduction, by recording from single, mechanically dissociated salamander rods and testing the effects of agents that either activate or inhibit PKC. However, we have not found any significant effect of these chemicals on the sensitivity of rods to light under various illumination conditions.
MATERIALS AND METHODS
Preparation
Single dissociated rods from the retina of the dark-adapted, larval tiger salamander (Ambystoma tigrinum) were used. The animals were purchased from Kons Scientific Co., Inc. (Germantown, WI). The procedure for isolating the cells was as described elsewhere (Nakatani and Yau, 1988a ). Briefly, the eyes were removed from the pithed animal and coronally hemisected. The retina was peeled from the back half of the eye, divided into several pieces, and stored under normal Ringer at room temperature for up to several hours until use. When needed, a piece of retina was finely chopped with a razor blade under Ringer solution and on a bed of cured Sylgard elastomer (Dow Corning Corp., Midland, MI), yielding many dissociated rod photoreceptors with intact outer and inner segments. These intact cells were selected for recording. The enucleation of the eyes was done in deep red light, and all other procedures were carried out in infrared light with the help of an infrared viewing scope or TV system.
Recordings and Optics
The suction-pipette recording technique (Baylor et al., 1979a ) was used to record membrane current from a rod. In all experiments, carried out at room temperature (19–24°C), the inner segment of the cell was drawn into the pipette for recording membrane current, and the outer segment was subjected to bath perfusion (see, for example, Nakatani and Yau, 1989). The position of the cell was adjusted so that the cilium between the outer and inner segments was situated near the point of narrowest constriction at the tip lumen. With the electrode resistance typically around 1–2 Mohm when empty and 5–10 Mohm with a rod in place, the fraction of membrane current recorded should be >80% (assuming that the resistance of the empty electrode was equally distributed between its very tip and the shank). No corrections were made for this imperfect current collection. However, the time course of the light response, as well as the light sensitivity when expressed as a fractional suppression of the dark current, should not be affected. All records were stored on an FM tape recorder and simultaneously digitized on-line on a PC computer. Data acquisition and analysis were carried out using the pCLAMP software. Low-pass filtering was set at 100 Hz. In figures where flash-response families are shown, the traces were generally derived from averages of multiple flash trials.
The optical bench design was as previously described (Baylor et al., 1979a ). Diffuse, unpolarized light at 520 nm (peak absorption wavelength for the “red” rods studied here; see Harosi, 1975) was used in all experiments, incident approximately perpendicular to the longitudinal axis of the recorded cell. Illumination consisted of 8-ms test flashes, 1- or 5-s bleaching light, or long steps of background light. For sensitivity calculations, we obtained the number of photoisomerized rhodopsin molecules from light calibrations and an individual cell's effective collecting area derived from video images. The effective collecting area, A (μm2), of an outer segment for incident light normal to the longitudinal axis of the outer segment is given by A = πr2l × Q × 2.303αf (Baylor et al., 1979b ), where r and l are the radius and length of the rod outer segment (in microns), respectively, Q is the quantum efficiency, α is the transverse specific optical density of the outer segment, and f is a correction factor depending on the polarization of the illuminating light. This expression for A is a linear approximation of an exponential function for absorption, and holds when 2.303αf × 2r << 1, roughly true for tiger-salamander rods (r ≅ 4 μm). For unpolarized light used in our experiments, f is 0.5 (Baylor et al., 1979b ). We have also adopted an α of 0.012 μm−1 at the peak absorption wavelength and a Q of 0.5 (see Harosi, 1975; Baylor et al., 1979b ; Nakatani and Yau, 1989).
In the bleaching experiments, the fractional bleach can be calculated as follows. For a bleaching light of intensity I (photons μm−2 s−1 at 520 nm) and duration T (s), the number of photoisomerizations (Rh*) is then I × T × A, or I × T × πr2lQ × 2.303αf = I × T × πr2l × 6.9 × 10−3, from above. With a rhodopsin concentration in the outer segment of 3.5 mM (Harosi, 1975), the total number of rhodopsin molecules is πr2l × 2.1 × 106. Thus the fractional bleach is simply I × T × 3.3 × 10−9. Again, this expression only holds when the percentage of pigment bleach is low, which applies to our experiments.
Perfusion and Solutions
The perfusion system and the recording chamber were as described previously (Nakatani and Yau, 1988a , 1989). The tip of the pipette was bent at right angles to the main shank so that during the experiment the long axis of the recorded cell was oriented parallel to the solution flow (Nakatani and Yau, 1988a , 1989).
Normal Ringer solution was at pH 7.6 and contained (mM): 110 NaCl, 2.5 KCl, 1.6 MgCl2, 1.0 CaCl2, 5.0 NaHEPES, 5.0 dextrose. The phorbol esters PMA and PDBu (phorbol-12,13-dibutyrate) were purchased from Research Biochemicals International (Natick, MA). The PKC inhibitor GF109203X {2-[1-(3-dimethyl-aminopropyl)indol-3-yl]-3-(indol-3-yl)maleimide} was purchased from Tocris Cookson (St. Louis, MO). Stock solutions of the chemicals were prepared by dissolving in DMSO at 16.2 mM for PMA, 19.8 mM for PDBu, and 24.2 mM for GF109203X, and stored at −20°C. Immediately before the experiments, the stock solutions were diluted to the final concentrations (1 μM for PMA, 20 μM for PDBu, and 1 or 5 μM for GF109203X) with Ringer solution. The 0.1% or less DMSO by itself had no effects in control experiments. The outer segment of a recorded rod was usually perfused with a chemical-containing solution for >10 min before any effect on light sensitivity was examined. In the literature, this interval is generally more than sufficient for an effect to take place when these chemicals are applied extracellularly at comparable or much lower concentrations to other cell types (see, for example, Stea et al., 1995; Gillette and Dacheux, 1996; Toullec et al., 1991; Li and Cathcart, 1994; Qiu and Leslie, 1994; Ikeuchi et al., 1996; Poulin et al., 1996). During the entire period of sensitivity testing, which could take many minutes, perfusion with the chemical-containing solution was continued. In experiments where sensitivity was retested after washout of the chemical, again at least 10 min was allowed after chemical removal before testing of sensitivity began.
RESULTS
PKC Activators
The phorbol esters PMA and PDBu were chosen as PKC activators because of their potency and general effectiveness in other cell types (see, for example, Stratton et al., 1989; Stea et al., 1995; Gillette and Dacheux, 1996). PMA was also reported to be effective in promoting PKC phosphorylation of rhodopsin in biochemical experiments with intact retinae (Newton and Williams, 1991,1993a).
Flash responses in the absence of background light.
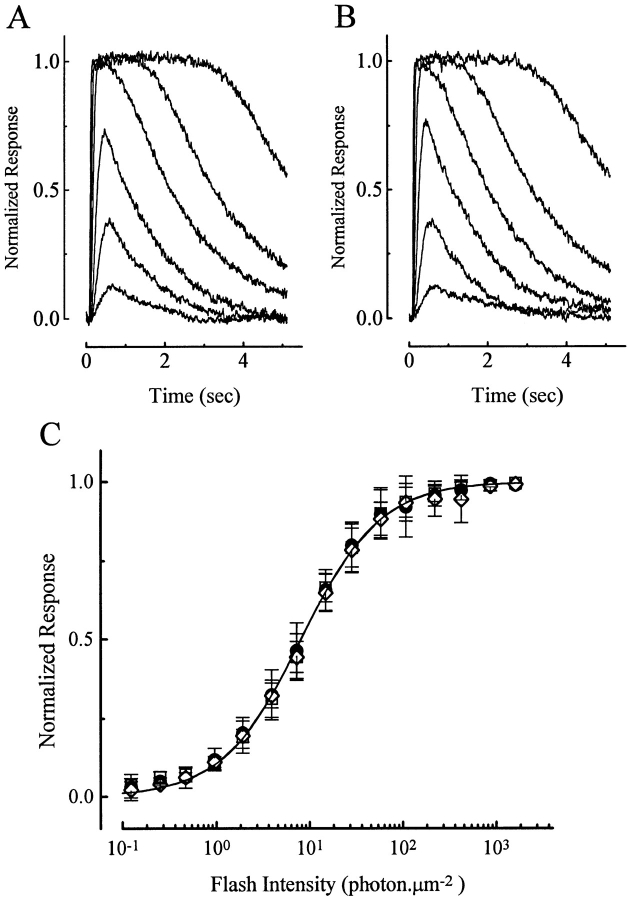
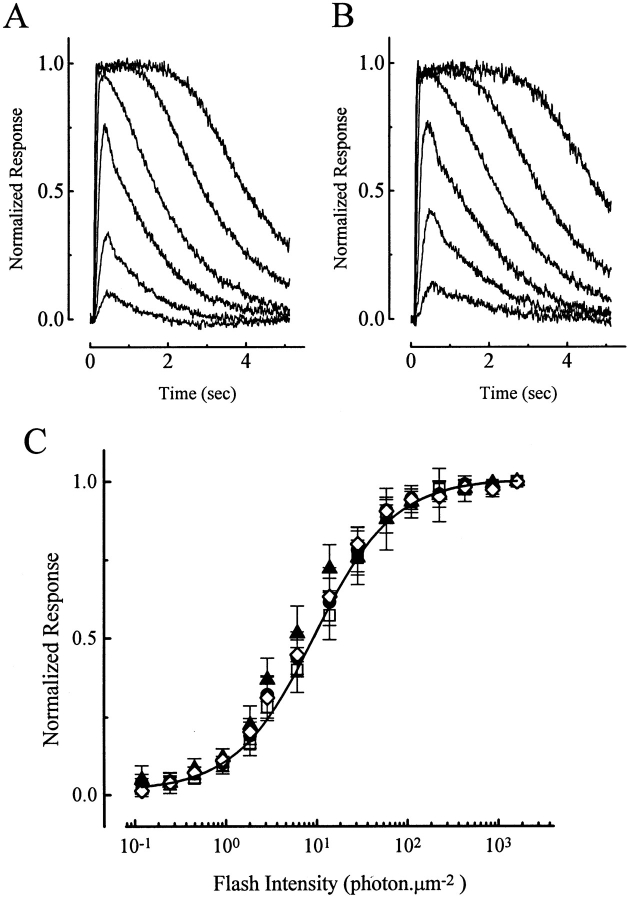
We first examined any effect of the two phorbol esters on rod sensitivity in the absence of background light. Fig. 1 A shows a family of responses of a salamander rod to flashes of increasing intensity in normal Ringer, and Fig. 1 B shows the responses of the same cell to identical flashes after being exposed to 1 μM PMA in the perfusing Ringer. The two families of responses are extremely similar, both in amplitudes (absolute and relative) and kinetics. Results from six cells are averaged and plotted in the form of peak response–intensity relations in Fig. 1 C, in the presence (filled symbols) and absence (open symbols) of 1 μM PMA. It is clear that 1 μM PMA had practically no effect on rod sensitivity. The smooth curve is the Michaelis equation with a half-saturating flash intensity at ∼8 Rh*/μm2, which is broadly similar to previous measurements (Nakatani and Yau, 1989).
Figure 1.
Responses of salamander rods to light flashes in the absence and presence of 1 μM PMA. No background light. (A) Response family from one cell in control condition. (B) Response family from the same cell in the presence of PMA. (C) Averaged data from six cells, with response peak plotted against flash intensity. Error bars indicate standard deviations. □, before PMA application; •, during PMA application; and ⋄, after PMA removal. Saturated response amplitude was 22.0 ± 2.5 pA (mean ± SD). Smooth curve is the Michaelis equation, with a half-saturating intensity of 8 photons μm−2.
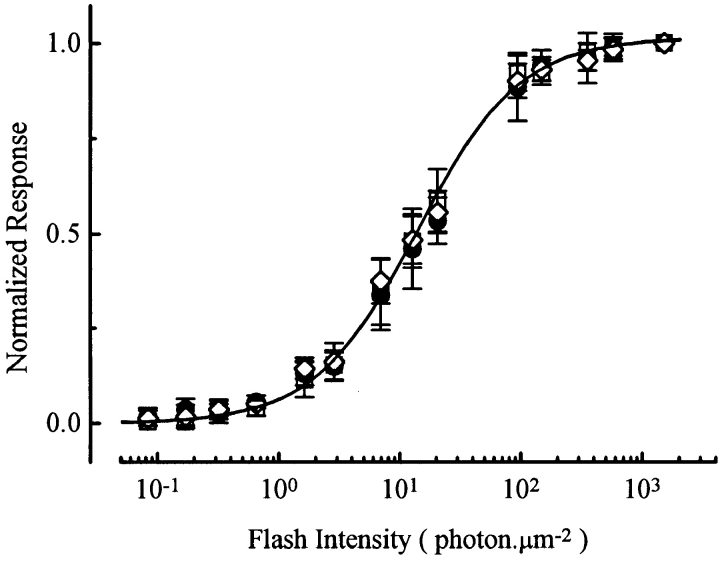
Identical experiments were carried out with 20 μM PDBu. During PDBu application, the dark current generally decreased by ∼10%. The reason for this current decrease is unknown, but the change is opposite to what would be expected for an increase in desensitization due to activation of PKC. Despite this small reduction in dark current, there was no change in flash sensitivity, based on averaged results from six experiments (Fig. 2). The dark current reduction was reversible upon PDBu removal. Previously, a similar inhibitory effect on the dark current by oleoylacetylglycerol, another PKC activator, was reported (Binder et al., 1989). Occasional effects of PKC activators unconnected with PKC activity have also been described for other retinal neurons (Gillette and Dacheux, 1996).
Figure 2.
Flash response–intensity relations in the absence and presence of 20 μM PDBu. Averaged data from six cells. Same experimental protocol as in Fig. 1. □, before PDBu application; •, during PDBu application; ⋄, after PDBu removal. Saturated response amplitude was 27.3 ± 7.0 pA. Smooth curve is the Michaelis equation, with a half-saturating intensity of 12 photons μm−2.
Flash responses in the presence of background light.
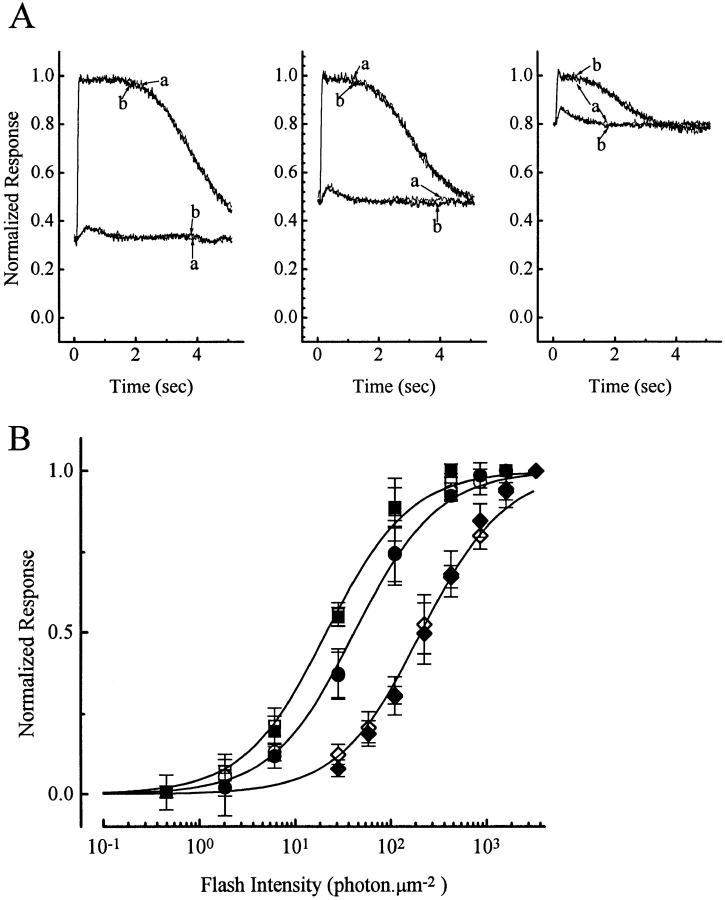
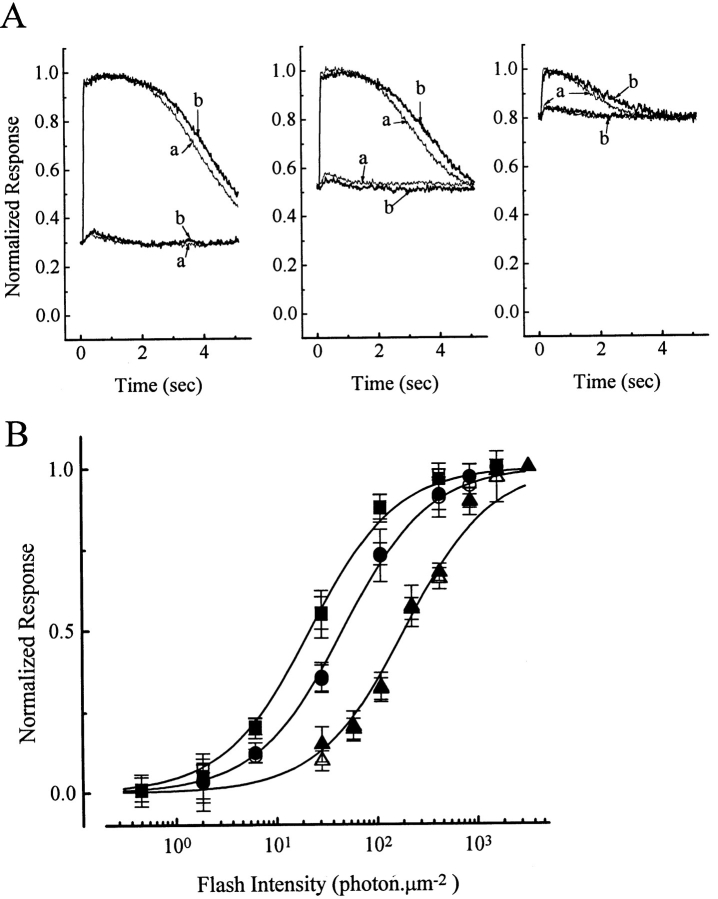
Next we examined rod sensitivity in the presence of background light. Three background intensities were chosen, producing steady responses of ∼30, 50, and 80% to saturation, respectively, in order to cover a fairly broad range. Superposed on these backgrounds were incremental flashes to test for sensitivity. Fig. 3 A shows selected records from one such experiment in which the effect of PMA was examined. Each panel in Fig. 3 A corresponds to a different background light, with the traces showing the averaged responses to an incremental dim and bright flash, respectively; the DC level of the traces corresponds to the steady response of the cell to a particular background light, and the labels a and b indicate control condition and the presence of 1 μM PMA, respectively. Again, no effect of the chemical is evident for either the steady response to background light or the incremental flash response. Fig. 3 B shows averaged data from five complete experiments, plotted in the form of incremental flash response–intensity relations under the three different background lights, as indicated by square, circle, and diamond symbols, respectively. As expected, the response–intensity relation shifted progressively to the right with increasing background light. However, at each background intensity there was no apparent difference in rod behavior whether PMA was absent (Fig. 3 B, open symbols) or present (Fig. 3 B, filled symbols).
Figure 3.
Flash responses of salamander rods in the presence of a background light, with and without 1 μM PMA. (A) Sample results from one cell, obtained with three different background intensities. In each panel, the responses to an incremental dim and bright flash, respectively, are shown, with the DC level of the traces corresponding to the steady response to the background light. Traces labeled a indicate no PMA, those labeled b indicate PMA being present. (B) Collected results from five cells, showing averaged incremental flash response–intensity relations in the presence of the three different background lights. Background light intensities are 8 (squares), 29 (circles), and 520 (diamonds) photons μm−2 s−1. Open symbols, no PMA; filled symbols, 1 μM PMA. Smooth curves are the Michaelis equation. Saturated response amplitude was 24.4 ± 3.0 pA.
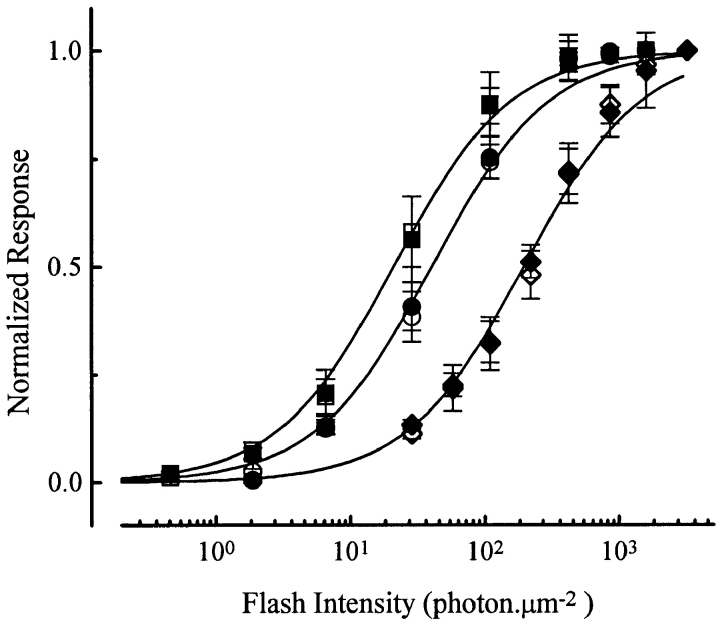
Four experiments with 20 μM PDBu led to the same conclusion (Fig. 4).
Figure 4.
Incremental-flash-on-background experiments identical to those in Fig. 3, but with 20 μM PDBu. Averaged data from four cells. Same background intensities as in Fig. 3. Symbols have similar meanings as in Fig. 3. Saturated response amplitude was 28.0 ± 5.7 pA.
After a bleach
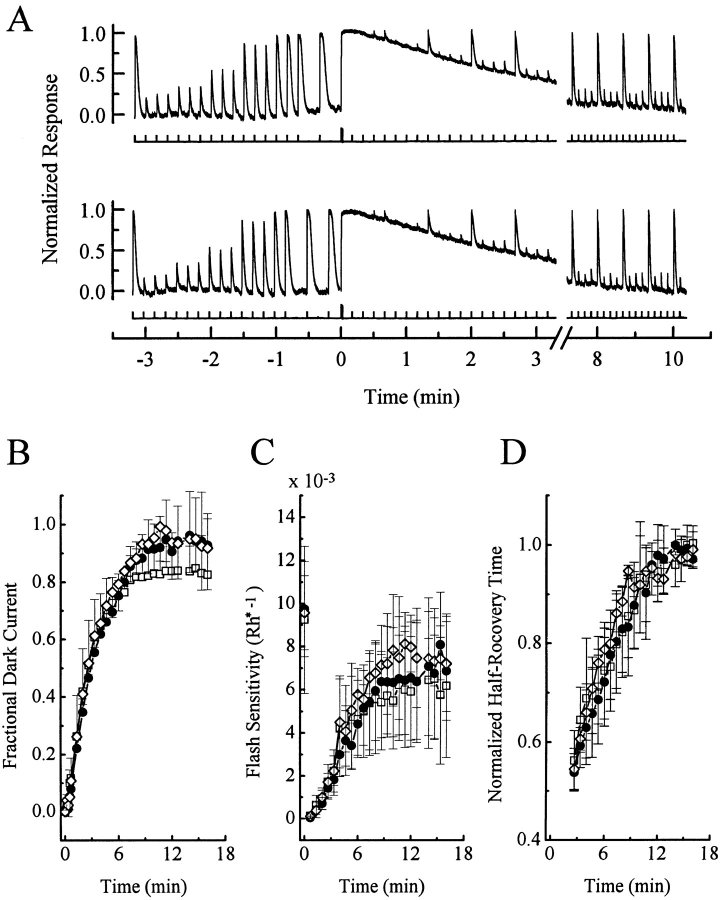
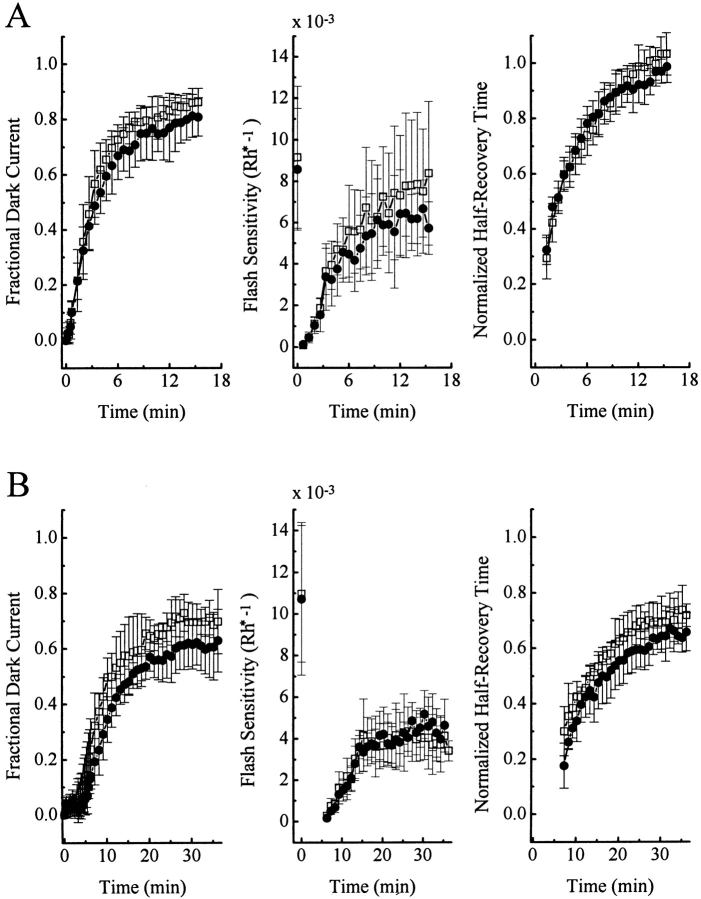
Finally we examined rod sensitivity after a low bleach because Newton and Williams (1991, 1993a ) have reported that the phosphorylation of rhodopsin in the presence of phorbol ester is strongest in intact rat retina after a ≤10% bleach. Fig. 5 A shows such an experiment, carried out initially in control condition (upper trace), and then in the presence of 1 μM PMA (lower trace). In each condition, a series of flashes at different intensities were delivered to a rod before bleaching, after which an ∼2% bleach was affected by a 1-s intense flash at time zero. As a result, the dark current stayed completely suppressed for seconds before slowly recovering. During this recovery, dim and bright flashes were again delivered to produce small and saturating responses for comparison with those elicited before the bleach. The time course of recovery of the light sensitivity paralleled that of the dark current. After the run in the presence of PMA, the chemical was removed from the bath solution and a second control run was repeated (not shown in Fig. 5 A, but see data analysis in B–D). Fig. 5, B–D show analyzed results averaged from six cells. Three parameters were measured as a function of time after the bleach: (a) the amplitude of the dark current, (b) the sensitivity to a dim flash, expressed as a fractional suppression of the dark current per Rh*, and (c) the half-recovery time of a (saturated) response to a bright flash of fixed intensity. For the dark current, there was a slight indication that the final phase of recovery was more complete when PMA was present (Fig. 5, •) than absent (at least the first run; Fig. 5, □), which would be consistent with a desensitization effect induced by hyperactivated PKC. On the other hand, this was not corroborated by the recoveries of the dim-flash sensitivity and of the half-recovery time of the responses to bright flashes, which appeared similar with and without PMA.
Figure 5.
Recovery of dark current and flash sensitivity of salamander rods after an ∼2% bleach, in the absence and presence of 1 μM PMA. (A) Recordings from one cell. Top, Ringer solution; bottom, Ringer solution plus 1 μM PMA. The flash intensities were the same in both panels, as follows. Before the bleach: flash #1, 219; #2–4, 3; #5–7, 6; #8–10, 14; #11–13, 57; #14, 219; #15, 856; #16, 1,594 photons/μm−2; after the bleach: flash #1–4, 1,594; #5–7, 57; #8, 856; #9–11, 14; #12, 856; #13–15, 14; #16, 421; #17–19, 6; #20, 219; #21–23, 3 photons μm−2; after which #20–23 were reiterated. (B–D) Averaged, analyzed data from six cells. Error bars are standard deviations. The 1-s bleaching light, delivering altogether 6 × 106 photons μm−2, occurred at time 0 and produced a 1.98% bleach (see materials and methods for calculations). In C, the flash sensitivity was calculated by dividing the fractional response to a dim flash (response amplitude at peak divided by the dark current just before a given bleach) by the calculated number of photoisomerizations (Rh*) produced by the flash; see materials and methods. Note that, after a bleach, the dark current recovered mostly, but not completely; this new dark current was used for normalizations in the subsequent bleach. In D, the normalized half-recovery time was derived from saturated responses to bright flashes, calculated by dividing the half-recovery time of a given bright flash response after the bleach by that of the response elicited by an identical flash before the bleach. □, before PMA exposure; •, during PMA exposure; ⋄, after PMA removal. Saturated response amplitude was 23.5 ± 2.2 pA.
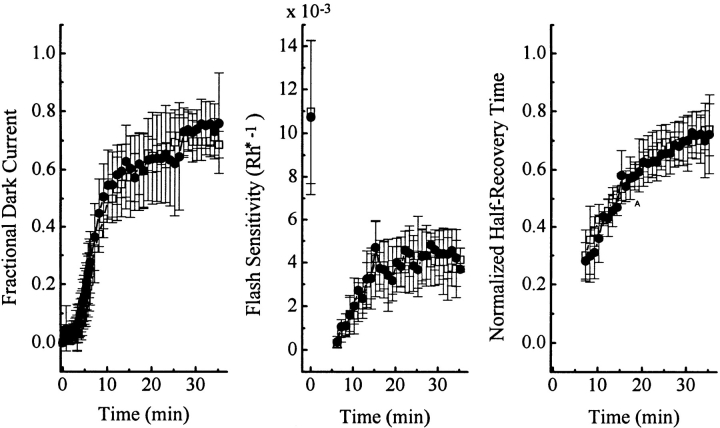
Four experiments were also carried out with a more intense bleach, namely ∼10%. After such a bleach, the cells took much longer to recover, but again PMA had no obvious effect (Fig. 6). We did not carry out still more intense bleaches because the extremely slow recoveries in these cases would preclude any comparison between absence and presence of the chemical from the same cell.
Figure 6.
Same bleaching experiment as in Fig. 5, except the bleach was ∼10% instead. Averaged data from four experiments. The 5-s bleaching light, delivering altogether 3 × 107 photons μm−2, occurred at time 0. □, before PMA exposure; and •, during PMA treatment. No data were collected after PMA washout. Saturated response amplitude was 25.3 ± 2.5 pA.
Finally, we performed identical bleach experiments with 20 μM PDBu, and obtained the same results (Fig. 7).
Figure 7.
Same kinds of experiments as in Figs. 5 and 6, except 20 μM PDBu was used instead. (A) ∼2% bleach; averaged data from five cells. Saturated response amplitude was 23.8 ± 2.0 pA. (B) ∼10% bleach; averaged data from four cells. Saturated response amplitude was 23.0 ± 2.6 pA.
PKC Inhibitor
We have also tested for possible constitutive PKC activity with an inhibitor of the enzyme. The recently developed PKC inhibitor GF109203X was chosen because of its high specificity (Toullec et al., 1991).
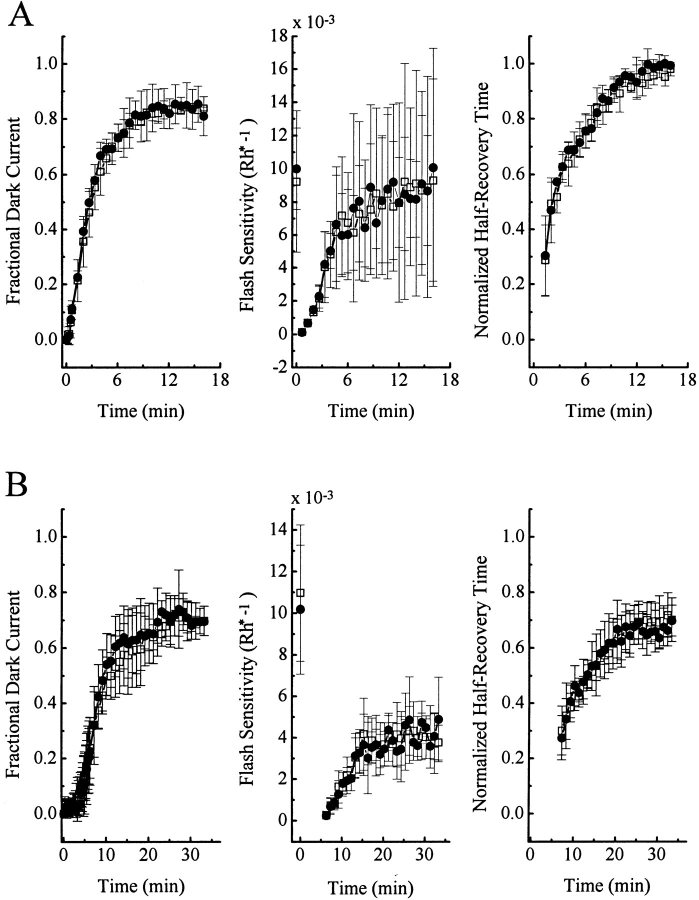
Fig. 8, A and B shows an experiment with GF109203X on a dark-adapted rod. Again, the flash response–intensity families in the absence and presence of the drug were very similar, a conclusion borne out by averaged results from six cells (Fig. 8 C). Experiments with different background light intensities (Fig. 9) likewise gave negative results. With 2 and 10% bleaches, the rod dark current and flash responses recovered perhaps a little more slowly in the presence of GF109203X (Fig. 10), which would be consistent with a role of PKC in desensitization under these conditions. However, the difference between absence and presence of the PKC inhibitor was very minor and probably not statistically significant.
Figure 8.
Flash responses of salamander rods in the absence and presence of GF109203X. No background light. (A) Response family from one cell in control condition. (B) Response family from the same cell in the presence of 1 μM GF109203X. (C) Averaged data from six cells, with response peak plotted against flash intensity. Error bars indicate standard deviations. □, before GF109203X application; •, during 1 μM GF109203X application; ▴, during 5 μM GF109203X application; ⋄, after GF109203X removal. Saturated response amplitude was 21.8 ± 2.9 pA. Smooth curve is the Michaelis equation, with a half-saturating intensity of nine photons μm−2.
Figure 9.
Incremental-flash-on-background experiments identical to those in Fig. 3, but with 1 μM GF109203X instead. Same background intensities as in Fig. 3. (A) Sample results from one cell with the three background intensities. Traces labeled a indicate no GF109203X, those labeled b indicate GF109203X being present. (B) Collected results from six cells, showing averaged incremental flash response–intensity relations in the presence of the three different background lights, indicated by squares, circles, and diamonds, respectively. Open symbols, no GF109203X; filled symbols, 1 μM GF109203X. Smooth curves are the Michaelis equation. Saturated response amplitude was 23.7 ± 2.9 pA.
Figure 10.
Same bleaching experiments as in Figs. 5 and 6, but with 1 μM GF109203X instead. (A) ∼2% bleach; averaged data from six cells. Saturated response amplitude was 21.8 ± 2.1 pA. (B) ∼10% bleach; averaged data from five cells. Saturated response amplitude was 22.0 ± 2.0 pA. □, before GF109203X application; •, during GF109203X application.
DISCUSSION
The existence of a light-activated, phosphoinositide signaling pathway in the rod outer segment has been a topic of interest for many years (Ghalayini and Anderson, 1984; Hayashi and Amakawa, 1985; Brown et al., 1987; Millar et al., 1988). The hydrolysis of phosphatidylinositol-4,5-bisphosphate (PIP2) by PLC produces two second messengers: inositol-1,4,5-trisphosphate (IP3) and diacylglycerol, with the former leading to intracellular Ca2+ release and the latter activating PKC. The role of IP3 in the rod outer segment remains elusive because so far there is no evidence of an IP3 receptor in this location (Peng et al., 1991; Day et al., 1993). The evidence of a diacylglycerol–PKC pathway in the rod outer segment, on the other hand, is more substantial (introduction). The concentration of PKC in the rod outer segment is reported to be at least as high as that of rhodopsin kinase, ∼1:1,000 in mole ratio with respect to rhodopsin (Kelleher and Johnson, 1986; Wolbring and Cook, 1991). The PKC isozymes identified so far are grouped into three classes: conventional (α, βI, βII, and γ), novel (δ, ε, η, and θ), and atypical (ζ, ι, λ, and μ) isoforms (see, for example, Asaoka et al., 1992; Nishizuka, 1992). Biochemical studies suggest that the PKC in the rod outer segment is of the conventional class (i.e., Ca2+ dependent and phorbol ester activatable [see, for example, Kelleher and Johnson, 1986; Newton and Williams, 1991, 1993a ; Greene et al., 1995; but see Wolbring and Schnetkamp, 1995]), and is perhaps the α-isoform (Wolbring and Cook, 1991; Udovichenko et al., 1993). This isoform identification in the rod outer segment, nonetheless, is still tentative and has not been confirmed immunocytochemically, despite numerous studies employing a variety of isoform-specific antibodies (Wood et al., 1988; Negishi et al., 1988; Suzuki and Kaneko, 1990; Ghalayini et al., 1991, 1994; Usuda et al., 1991; Zhang et al., 1992; Kolb et al., 1993; Osborne et al., 1992, 1994; Koistinaho and Sagar, 1994; Ohki et al., 1994). Some of these studies could have produced negative results because of the failure of a given antibody to recognize the same protein isoform across different vertebrate species.
As pointed out in introduction, several rod proteins involved in phototransduction have been found to be substrates of PKC. Many of these studies were carried out in vitro, so it is difficult to be certain that the findings are really relevant to the intact tissue. On the other hand, the light-triggered phosphorylation of bleached and unbleached rhodopsin by PKC has been reported in intact retinae of both mammalian and amphibian species (Newton and Williams, 1991, 1993a , 1993b ; Green et al., 1995, 1997; Udovichenko et al., 1997). Furthermore, this phosphorylation by PKC has been found to reduce the ability of rhodopsin to activate transducin (Kelleher and Johnson, 1986). Because the action of PKC is less specific than rhodopsin kinase, which phosphorylates only photoactivated rhodopsin (but see below), it has been proposed to provide an effective mechanism for light adaptation, by analogy to the heterologous desensitization of the β-adrenergic receptor through PKA phosphorylation (Newton and Williams, 1991, 1993a , 1993b ). To test this idea, we have examined the effect of hyperactivation of PKC by phorbol esters on the sensitivity of single, intact salamander rods under three conditions: in the absence and presence of background light, and after a low bleach. The choice of one of the phorbol esters (PMA) and its concentration (1 μM) was based on the biochemical experiments in intact rat retina (Newton and Williams, 1991, 1993a ). For the bleaching experiments, the degrees of bleach (∼2 and 10%) we used also broadly matched those (≤10%) reported in the same biochemical experiments to be effective for enhancing the phosphorylation of rhodopsin with PMA. However, in none of these conditions were we able to detect any significant effect of PMA on rod sensitivity. The second phorbol ester we used, PDBu, is an equally potent PKC activator, and the concentration adopted (20 μM) is up to 100-fold higher than that found effective in other cell types when applied extracellularly (Stratton et al., 1989; Stea et al., 1995; Gillette and Dacheux, 1996). However, we also did not find any effect of PDBu on rod sensitivity. Because our experiments generally lasted 30 min or less (longer for some of the bleaching experiments), we could have missed any effects of PKC that developed beyond these time durations upon illumination. However, this possibility seems unlikely because, most recently, Udovichenko et al. (1997) have reported that, in intact frog retina, the phosphorylation of rhodopsin due to hyperactivation of PKC by phorbol ester is quite fast and transient, peaking at 10–15 min after light onset, and declining shortly thereafter. This time window should be well within detection in our experiments.
We have also tested for any possible constitutive PKC activity on rod sensitivity using a PKC inhibitor. Ideally, the inhibitor of choice for these experiments would be a chemical, such as calphostin C, that acts on the regulatory domain of PKC and is highly potent and specific (Kobayashi et al., 1989). However, the action of calphostin C is light dependent (Bruns et al., 1991), making it incompatible with the nature of our experiments. Most other PKC inhibitors act on the catalytic domain of the enzyme, which shares sequence homology with other protein kinases, and are thus less specific. Indeed, we have tried several PKC inhibitors of this class before GF109203X, including H-7 (250 μM), staurosporine (1–10 μM), and chelerythrine (10 μM), and found these to decrease the dark current substantially (∼30–50%), as well as slow down the kinetics of the light response (data not shown). Because these inhibitors may also inhibit rhodopsin kinase, we found it difficult to interpret the results. The recently developed chemical GF109203X is one of the most selective among PKC inhibitors that act on the kinase's catalytic site (Toullec et al., 1991). At the concentration of 1 (and occasionally 5) μM used here, this chemical is found to be effective in inhibiting PKC activity in other cell types when applied extracellularly (Toullec et al., 1991; Li and Cathcart, 1994; Qiu and Leslie, 1994; Ikeuchi et al., 1996; Poulin et al., 1996). However, it did not affect rod sensitivity under the different light conditions described above. Thus, there is no obvious indication of any constitutive PKC activity, in either darkness or light, that affects phototransduction, at least for isolated rod photoreceptors.
While our experiments strongly suggest that PKC is unlikely to have a significant role in light adaptation, they cannot be viewed as absolutely conclusive because of the negative findings, and because of an inevitable element of uncertainty associated with pharmacological experiments. However, judging from the degree of rhodopsin phosphorylation by PKC reported so far, it would be very surprising if the conclusion should eventually turn out to be any different. This point can be appreciated from the following order-of-magnitude calculations. Using calphostin C as a specific PKC inhibitor, Udovichenko et al. (1997) most recently estimated that, in intact frog retina, PKC accounted for ∼50% of the overall phosphorylation of rhodopsin induced by illumination. Furthermore, they found that PKC-phosphorylated rhodopsin was dephosphorylated more rapidly than rhodopsin kinase-phosphorylated rhodopsin. For argument sake, let us assume that rhodopsin kinase can phosphorylate photoactivated rhodopsin at multiple residues (though, in reality, probably just one residue is phosphorylated; see Ohguro et al., 1995), whereas PKC phosphorylates rhodopsin only at one residue (Newton and Williams, 1993a , 1993b ). Thus, even most optimistically, the maximum stoichiometry of unbleached to bleached pigment that becomes phosphorylated in the light is still less than a factor of 10 (but see below). Furthermore, let us assume that the unbleached, but phosphorylated, rhodopsin has negligible transducin-activating ability upon photoisomerization (though, in reality, this ability is only reduced by <50%; see Kelleher and Johnson, 1986). Thus, even in the extreme case, there cannot be more than 10 “inactivated,” unbleached rhodopsin molecules associated with each Rh*. A salamander rod has ∼3 × 109 rhodopsin molecules, but its response already saturates when a steady light effectively produces 105 Rh* at a given instant (see Fig. 2 a in Nakatani and Yau, 1988b ; and by adopting a typical effective collecting area of 10 μm2 for salamander rods illuminated by unpolarized light, together with an integration time of 1 s for their single-photon response). With this level of Rh*, at most 106 unbleached pigment molecules would be rendered “inactive” by PKC phosphorylation, leading to a reduction in the photon-capturing ability (or sensitivity) of the cell by no more than a factor of 10−3, or 0.1%; more realistically, this percentage could be as low as 0.01%. In either case, the change is well below detectability. With higher steady light intensities, the fraction of unbleached pigment that is “inactivated” will increase, but this is immaterial because rods cannot signal after response saturation. Similar arguments apply to mammalian rods. A complexity not mentioned so far in this paper and not included in the above calculations is the possible phenomenon of so-called “high gain” phosphorylation, in which rhodopsin kinase supposedly phosphorylates both bleached and unbleached rhodopsin in the light, much like the reported action of PKC being evaluated in this paper, and thought to lead to light adaptation as well (see, for example, Aton, 1986; Binder et al., 1990, 1996; Chen et al., 1995; Dean and Akhtar, 1993, 1996; Palczweski, 1997). The existence of this phenomenon has been controversial and, most recently, not confirmed (Rim et al., 1997); even if present, however, its contribution to light adaptation also appears insignificant, based on the most recent experiments (Binder et al., 1996). If included in our calculations above, this high gain phosphorylation will only make the phosphorylation of rhodopsin by PKC even less significant compared with that by rhodopsin kinase. The situation could be a little different for cones, if the same PKC biochemistry exists, because these cells continue to function at very high steady light intensities (see, for example, Nakatani and Yau, 1988b ).
Finally, it might be added that, while several groups have reported light-activated phosphoinositide hydrolysis in the rod outer segment (see above), this observation is by no means universal (see Gehm and McConnell, 1990; Panfoli et al., 1990 for negative results). The phosphorylation of rhodopsin by PKC is likewise not an unequivocal finding (compare Binder et al., 1989; Ohguro et al., 1996). Thus, it seems that even the basic questions of which in vivo stimuli trigger PKC and which substrates this enzyme acts on in the rod outer segment are still unsettled. Because the activation of PKC can also be enhanced or sustained by phospholipid signaling pathways involving phospholipase A2 or phospholipase D (Asaoka et al., 1992; Nishizuka, 1992), these alternative pathways perhaps need to be considered and examined. On the other hand, based on our recent immunocytochemical and immunoblotting studies, it appears that Gα11 and PLCβ4 (or isoforms immunologically identical to them) are present in the rod outer segment (Peng et al., 1997; but see Ferreira and Pak, 1994). These proteins are specific for the phosphatidylinositol-4,5-bisphosphate pathway, suggesting that this pathway is truly present in this location. The PKC in the rod outer segment may have nothing to do with the modulation of phototransduction but affects other cellular functions instead. In this respect, rod photoreceptors differ from invertebrate rhabdomeric photoreceptors, such as those in the fly, where PKC has indeed been demonstrated to be involved in light adaptation (Hardie et al., 1993). This difference may lie in the fact that a phosphoinosi-tide pathway is central for phototransduction in fly photoreceptors (see, for example, Hardie and Minke, 1995; Ranganathan et al., 1995), whereas a cGMP phototransduction pathway is used in rods.
Acknowledgments
We thank Drs. James B. Hurley, George I. King, David J. Linden, Alexandra C. Newton, Krzysztof Palczewski, and David S. Williams for discussions, and Drs. James B. Hurley, Alexandra C. Newton, and Krzysztof Palczewski for sending us preprints.
This work was supported by National Institutes of Health grant EY-06837.
Footnotes
Abbreviations used in this paper: PDBu, phorbol-12,13-dibutyrate; PDE, phosphodiesterase; Rh*, photoisomerization.
REFERENCES
- Asaoka Y, Nakamura S-I, Yoshida K, Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992;17:414–417. doi: 10.1016/0968-0004(92)90011-w. [DOI] [PubMed] [Google Scholar]
- Aton BR. Illumination of bovine photoreceptor membranes causes phosphorylation of both bleached and unbleached rhodopsin molecules. Biochemistry. 1986;25:677–680. doi: 10.1021/bi00351a025. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau K-W. The membrane current of single rod outer segments. J Physiol. 1979a;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau K-W. Responses of retinal rods to single photons. J Physiol. 1979b;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Binder RM, Biernbaum MS, Bownds MD. Light activation of one rhodopsin molecule causes the phosphorylation of hundreds of others. J Biol Chem. 1990;265:15333–15340. [PubMed] [Google Scholar]
- Binder BM, Brewer E, Bownds MD. Stimulation of protein phosphorylations in frog rod outer segments by protein kinase activators. J Biol Chem. 1989;264:8857–8864. [PubMed] [Google Scholar]
- Binder BM, O'Connor TM, Bownds MD, Arshavsky VY. Phosphorylation of non-bleached rhodopsin in intact retinas and living frogs. J Biol Chem. 1996;271:19826–19830. doi: 10.1074/jbc.271.33.19826. [DOI] [PubMed] [Google Scholar]
- Brown JE, Blazynski C, Cohen AI. Light induces a rapid and transient increase in inositol-trisphosphate in toad rod outer segments. Biochem Biophys Res Commun. 1987;146:1392–1396. doi: 10.1016/0006-291x(87)90804-7. [DOI] [PubMed] [Google Scholar]
- Bruns RF, Miller FD, Merriman RL, Howbert JJ, Heath WF, Kobayashi E, Takahashi I, Tamaoki T, Nakano H. Inhibition of protein kinase C by calphostin C is light-dependent. Biochem Biophys Res Commun. 1991;176:288–293. doi: 10.1016/0006-291x(91)90922-t. [DOI] [PubMed] [Google Scholar]
- Chen C-K, Inglese J, Lefkowitz RJ, Hurley JB. Ca2+-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem. 1995;270:18060–18066. doi: 10.1074/jbc.270.30.18060. [DOI] [PubMed] [Google Scholar]
- Day NS, Koutz CA, Anderson RE. Inositol-1,4,5-trisphosphate receptors in the vertebrate retina. Curr Eye Res. 1993;12:981–991. doi: 10.3109/02713689309029224. [DOI] [PubMed] [Google Scholar]
- Dean KR, Akhtar M. Phosphorylation of solubilized dark-adapted rhodopsin: insights into the activation of rhodopsin kinase. Eur J Biochem. 1993;213:881–890. doi: 10.1111/j.1432-1033.1993.tb17832.x. [DOI] [PubMed] [Google Scholar]
- Dean KR, Akhtar M. Novel mechanism for the activation of rhodopsin kinase: implications for other G protein-coupled receptor kinases (GRKs) Biochemistry. 1996;35:6164–6170. doi: 10.1021/bi952480q. [DOI] [PubMed] [Google Scholar]
- Fain GL, Matthews HR, Cornwall MC. Dark adaptation in vertebrate photoreceptors. Trends Neurosci. 1996;19:502–507. doi: 10.1016/S0166-2236(96)10056-4. [DOI] [PubMed] [Google Scholar]
- Ferreira PA, Pak WL. Bovine phospholipase C highly homologous to the NorpA protein of Drosophilais expressed specifically in cones. J Biol Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- Finn JT, Grunwald ME, Yau K-W. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol. 1996;58:395–426. doi: 10.1146/annurev.ph.58.030196.002143. [DOI] [PubMed] [Google Scholar]
- Gehm BD, McConnell DG. Phosphatidylinositol-4,5-bisphosphate phospholipase C in bovine rod outer segments. Biochemistry. 1990;29:5447–5452. doi: 10.1021/bi00475a006. [DOI] [PubMed] [Google Scholar]
- Ghalayini AJ, Anderson RE. Phosphatidylinositol 4,5-bisphosphate: light-mediated breakdown in the vertebrate retina. Biochem Biophys Res Commun. 1984;124:503–506. doi: 10.1016/0006-291x(84)91582-1. [DOI] [PubMed] [Google Scholar]
- Ghalayini AJ, Koutz CA, Wetsel WC, Hannun YA, Anderson RE. Immunolocalization of PKCζ in rat photoreceptor inner segments. Curr Eye Res. 1994;13:145–150. doi: 10.3109/02713689409042409. [DOI] [PubMed] [Google Scholar]
- Ghalayini AJ, Tarver AP, Mackin WM, Koutz CA, Anderson RE. Identification and immunolocalization of phospholipase C in bovine rod outer segments. J Neurochem. 1991;57:1405–1412. doi: 10.1111/j.1471-4159.1991.tb08307.x. [DOI] [PubMed] [Google Scholar]
- Gillette MA, Dacheux RF. Protein kinase modulation of GABAAcurrents in rabbit retinal rod bipolar cells. J Neurophysiol. 1996;76:3070–3086. doi: 10.1152/jn.1996.76.5.3070. [DOI] [PubMed] [Google Scholar]
- Greene NM, Williams DS, Newton AC. Kinetics and localization of the phosphorylation of rhodopsin by protein kinase C. J Biol Chem. 1995;270:6710–6717. doi: 10.1074/jbc.270.12.6710. [DOI] [PubMed] [Google Scholar]
- Greene NM, Williams DS, Newton AC. Identification of protein kinase C phosphorylation sites on bovine rhodopsin. J Biol Chem. 1997;272:10341–10344. doi: 10.1074/jbc.272.16.10341. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+and trp. Cell Calcium. 1995;18:256–274. doi: 10.1016/0143-4160(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Peretz A, Suss-Toby E, Rom-Glas A, Bishop SA, Selinger Z, Minke B. Protein kinase C is required for light adaptation in Drosophila photoreceptors. Nature (Lond) 1993;363:634–637. doi: 10.1038/363634a0. [DOI] [PubMed] [Google Scholar]
- Harosi FI. Absorption spectra and linear dichroism of cone amphibian photoreceptors. J Gen Physiol. 1975;66:357–382. doi: 10.1085/jgp.66.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Amakawa T. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem Biophys Res Commun. 1985;128:954–959. doi: 10.1016/0006-291x(85)90139-1. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Lin GY, Matsumoto H, Yamazaki A. Phosphatidylinositol-stimulated phosphorylation of an inhibitory subunit of cGMP phosphodiesterase in vertebrate rod photoreceptors. Proc Natl Acad Sci USA. 1991;88:4333–4337. doi: 10.1073/pnas.88.10.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Sumi M, Amakawa T. Phosphatidylinositol stimulates phosphorylation of protein components I and II in rod outer segments of frog photoreceptors. Biochem Biophys Res Commun. 1987;148:54–60. doi: 10.1016/0006-291x(87)91075-8. [DOI] [PubMed] [Google Scholar]
- Ikeuchi Y, Nishizaki T, Mori M, Okada Y. Adenosine activates K+ channel and enhances cytosolic Ca2+ release via a P2Ypurinoceptor in hippocampal neurons. Eur J Pharmacol. 1996;304:191–199. doi: 10.1016/0014-2999(96)00113-6. [DOI] [PubMed] [Google Scholar]
- Kapoor CL, Chader GJ. Endogenous phosphorylation of retinal photoreceptor outer segment proteins by calcium phospholipid-dependent protein kinase. Biochem Biophys Res Commun. 1984;122:1397–1403. doi: 10.1016/0006-291x(84)91246-4. [DOI] [PubMed] [Google Scholar]
- Kapoor CL, O'Brien PJ, Chader GJ. Phorbol ester– and light-induced endogenous phosphorylation of rat rod outer segment proteins. Exp Eye Res. 1987;45:545–556. doi: 10.1016/s0014-4835(87)80065-9. [DOI] [PubMed] [Google Scholar]
- Kawamura S. Molecular aspects of photoreceptor adaptation in vertebrate retina. Int Rev Neurobiol. 1993;35:43–86. doi: 10.1016/s0074-7742(08)60568-1. [DOI] [PubMed] [Google Scholar]
- Kelleher DJ, Johnson GL. Purification of protein kinase C from bovine rod outer segments. J Cyclic Nucleotide Protein Phosphorylation Res. 1985;10:579–591. [PubMed] [Google Scholar]
- Kelleher DJ, Johnson GL. Phosphorylation of rhodopsin by protein kinase C in vitro. . J Biol Chem. 1986;261:4749–4757. [PubMed] [Google Scholar]
- Kobayashi E, Nakano H, Morimoto M, Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Koistinaho J, Sagar SM. Localization of protein kinase C subspecies in the rabbit retina. Neurosci Lett. 1994;177:15–18. doi: 10.1016/0304-3940(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Kolb H, Zhang L, Dekorver L. Differential staining of neurons in the human retina with antibodies to protein kinase C isozymes. Vis Neurosci. 1993;10:341–351. doi: 10.1017/s0952523800003734. [DOI] [PubMed] [Google Scholar]
- Koutalos Y, Yau K-W. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci. 1996;19:73–81. doi: 10.1016/0166-2236(96)89624-x. [DOI] [PubMed] [Google Scholar]
- Lagnado L, Baylor DA. Signal flow in visual transduction. Neuron. 1992;8:995–1002. doi: 10.1016/0896-6273(92)90122-t. [DOI] [PubMed] [Google Scholar]
- Li Q, Cathcart MK. Protein kinase C activity is required for lipid oxidation of low density lipoprotein by activated human monocytes. J Biol Chem. 1994;269:17508–17515. [PubMed] [Google Scholar]
- Millar FA, Fisher SC, Muir CA, Edwards E, Hawthorne JN. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. Biochim Biophys Acta. 1988;970:205–211. doi: 10.1016/0167-4889(88)90180-2. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yau KW. Calcium and magnesium fluxes across the plasma membrane of the toad rod outer segment. J Physiol. 1988a;395:695–729. doi: 10.1113/jphysiol.1988.sp016942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani K, Yau K-W. Calcium and light adaptation in retinal rods and cones. Nature (Lond) 1988b;334:69–71. doi: 10.1038/334069a0. [DOI] [PubMed] [Google Scholar]
- Nakatani K, Yau KW. Sodium-dependent calcium extrusion and sensitivity regulation in retinal cones of the salamander. J Physiol. 1989;409:525–548. doi: 10.1113/jphysiol.1989.sp017511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C–like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Newton AC, Williams DS. Involvement of protein kinase C in the phosphorylation of rhodopsin. J Biol Chem. 1991;266:17725–17728. [PubMed] [Google Scholar]
- Newton AC, Williams DS. Rhodopsin is the major in situsubstrate of protein kinase C in rod outer segments of photoreceptors. J Biol Chem. 1993a;268:18181–18186. [PubMed] [Google Scholar]
- Newton AC, Williams DS. Does protein kinase C play a role in rhodopsin desensitization? . Trends Biochem Sci. 1993b;18:275–277. doi: 10.1016/0968-0004(93)90032-i. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science (Wash DC) 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ohguro H, Rudnicka-Nawrot M, Buczylko J, Zhao X, Taylor JA, Walsh KA, Palczewski K. Structural and enzymatic aspects of rhodopsin phosphorylation. J Biol Chem. 1996;271:5215–5224. doi: 10.1074/jbc.271.9.5215. [DOI] [PubMed] [Google Scholar]
- Ohguro H, Van Hooser JP, Milam AH, Palczewski K. Rhodopsin phosphorylation and dephosphorylation in vivo. . J Biol Chem. 1995;270:14259–14262. doi: 10.1074/jbc.270.24.14259. [DOI] [PubMed] [Google Scholar]
- Ohki K, Yoshida K, Imaki J, Harada T, Matsuda H. The existence of protein kinase C in cone photoreceptors in the rat retina. Curr Eye Res. 1994;13:547–550. doi: 10.3109/02713689408999887. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Barnett NL, Morris NJ, Huang FL. The occurrence of three isoenzymes of protein kinase C (α, β, and γ) in retinas of different species. Brain Res. 1992;570:161–166. doi: 10.1016/0006-8993(92)90577-v. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Wood J, Groome N. The occurrence of three calcium-independent protein kinase C subspecies (δ, ε, and ζ) in retina of different species. Brain Res. 1994;637:156–162. doi: 10.1016/0006-8993(94)91228-9. [DOI] [PubMed] [Google Scholar]
- Palczewski K. GTP-binding-protein-coupled receptor kinases: two mechanistic models. Eur J Biochem. 1997;248:261–269. doi: 10.1111/j.1432-1033.1997.00261.x. [DOI] [PubMed] [Google Scholar]
- Palczewski K, Saari JC. Activation and inactivation steps in the visual transduction pathway. Curr Opin Neurobiol. 1997;7:500–504. doi: 10.1016/s0959-4388(97)80029-3. [DOI] [PubMed] [Google Scholar]
- Panfoli I, Morelli A, Pepe I. Calcium ion-regulated phospholipase C activity in bovine rod outer segments. Biochem Biophys Res Commun. 1990;173:283–288. doi: 10.1016/s0006-291x(05)81054-x. [DOI] [PubMed] [Google Scholar]
- Peng Y-W, Rhee SG, Yu W-P, Ho YK, Schoen T, Chader GJ, Yau K-W. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc Natl Acad Sci USA. 1997;94:1995–2000. doi: 10.1073/pnas.94.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y-W, Sharp AH, Snyder SH, Yau K-W. Localization of the inositol 1,4,5-trisphosphate receptor in synaptic terminals in the vertebrate retina. Neuron. 1991;6:525–531. doi: 10.1016/0896-6273(91)90055-5. [DOI] [PubMed] [Google Scholar]
- Poulin B, Rich N, Mitev Y, Gautron J-P, Kordon C, Enjalbert A, Drouva SV. Differential involvement of calcium channels and protein kinase-C activity in GnRH-induced phospholipase-C, -A2, and -D activation in a gonadotrope cell line (αT3-1) Mol Cell Endocrinol. 1996;122:33–50. doi: 10.1016/0303-7207(96)03868-3. [DOI] [PubMed] [Google Scholar]
- Pugh EN, Jr, Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochim Biophys Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- Qiu Z-H, Leslie CC. Protein kinase C–dependent and –independent pathways of mitogen-activated protein kinase activation in macrophages by stimuli that activate phospholipase A2 . J Biol Chem. 1994;269:19480–19487. [PubMed] [Google Scholar]
- Ranganathan R, Malicki DM, Zuker CS. Signal transduction in Drosophila photoreceptors. Annu Rev Neurosci. 1995;18:283–317. doi: 10.1146/annurev.ne.18.030195.001435. [DOI] [PubMed] [Google Scholar]
- Rim J, Faurobert E, Hurley JB, Oprian DD. In vitroassay for trans-phosphorylation of rhodopsin by rhodopsin kinase. Biochemistry. 1997;36:7064–7070. doi: 10.1021/bi970470e. [DOI] [PubMed] [Google Scholar]
- Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Stratton KR, Worley PF, Huganir RL, Baraban JM. Muscarinic agonists and phorbol esters increase tyrosine phosphorylation of a 40-kilodalton protein in hippocampal slices. Proc Natl Acad Sci USA. 1989;86:2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Kaneko A. Identification of bipolar cell subtypes by protein kinase C–like immunoreactivity in the goldfish retina. Vis Neurosci. 1990;5:223–230. doi: 10.1017/s0952523800000298. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolymaleimide GF109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Tsuboi S, Matsumoto H, Jackson KW, Tsujimoto K, Williams T, Yamazaki A. Phosphorylation of an inhibitory subunit of cGMP phosphorylation in Rana catesbianarod photoreceptors. I. Characterization of the phosphorylation. J Biol Chem. 1994a;269:15016–15023. [PubMed] [Google Scholar]
- Tsuboi S, Matsumoto H, Yamazaki A. Phosphorylation of an inhibitory subunit of cGMP phosphorylation in Rana catesbianarod photoreceptors. II. A possible mechanism for the turnoff of cGMP phosphorylation without GTP hydrolysis. J Biol Chem. 1994b;269:15024–15029. [PubMed] [Google Scholar]
- Udovichenko IP, Cunnick J, Gonzalez K, Takemoto DJ. Phosphorylation of bovine rod photoreceptor cyclic GMP phosphodiesterase. Biochem J. 1993;295:49–55. doi: 10.1042/bj2950049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udovichenko IP, Cunnick J, Gonzalez K, Takemoto DJ. Functional effect of phosphorylation of the photoreceptor phosphodiesterase inhibitory subunit by protein kinase C. J Biol Chem. 1994;269:9850–9856. [PubMed] [Google Scholar]
- Udovichenko IP, Cunnick J, Gonzalez K, Yakhnin A, Takemoto DJ. Protein kinase C in rod outer segments: effects of phosphorylation of the phosphodiesterase inhibitory subunit. Biochem J. 1996;317:291–295. doi: 10.1042/bj3170291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udovichenko IP, Newton AC, Williams DS. Contribution of protein kinase C to the phosphorylation of rhodopsin in intact retinae. J Biol Chem. 1997;272:7952–7959. doi: 10.1074/jbc.272.12.7952. [DOI] [PubMed] [Google Scholar]
- Usuda N, Kong Y, Hagiwara M, Uchida C, Terasawa M, Nagata T, Hidaka H. Differential localization of protein kinase C isozymes in retinal neurons. J Cell Biol. 1991;112:1241–1247. doi: 10.1083/jcb.112.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand I, Kühn H. Subspecies of arrestin from bovine retina. FEBS Lett. 1990;193:459–467. doi: 10.1111/j.1432-1033.1990.tb19360.x. [DOI] [PubMed] [Google Scholar]
- Wolbring G, Cook NJ. Rapid purification and characterization of protein kinase C from bovine retinal rod outer segments. FEBS Lett. 1991;201:601–606. doi: 10.1111/j.1432-1033.1991.tb16320.x. [DOI] [PubMed] [Google Scholar]
- Wolbring G, Schnetkamp PPM. Activation by PKC of the Ca2+-sensitive guanylyl cyclase in bovine retinal rod outer segments measured with an optical assay. Biochemistry. 1995;34:4689–4695. doi: 10.1021/bi00014a024. [DOI] [PubMed] [Google Scholar]
- Wood JG, Hart CE, Mazzei GJ, Girard PR, Kuo JF. Distribution of protein kinase C immunoreactivity in rat retina. Histochem J. 1988;20:63–68. doi: 10.1007/BF01746605. [DOI] [PubMed] [Google Scholar]
- Yarfitz S, Hurley JB. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994;269:14329–14332. [PubMed] [Google Scholar]
- Yau K-W. Phototransduction mechanism in retinal rods and cones. The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35:9–32. [PubMed] [Google Scholar]
- Yau K-W, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dekorver L, Kolb H. Immunocytochemical staining with antibodies against protein kinase C and its isozymes in the turtle retina. J Neurocytol. 1992;21:833–847. doi: 10.1007/BF01191681. [DOI] [PubMed] [Google Scholar]
- Zick Y, Sagi-Eisenberg R, Pines M, Gierschik P, Spiegel AM. Multisite phosphorylation of the α subunit of transducin by the insulin receptor kinase and protein kinase C. Proc Natl Acad Sci USA. 1986;83:9294–9297. doi: 10.1073/pnas.83.24.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]