Abstract
To determine how intracellular Ca2+ and membrane voltage regulate the gating of large conductance Ca2+-activated K+ (BK) channels, we examined the steady-state and kinetic properties of mSlo1 ionic and gating currents in the presence and absence of Ca2+ over a wide range of voltage. The activation of unliganded mSlo1 channels can be accounted for by allosteric coupling between voltage sensor activation and the closed (C) to open (O) conformational change (Horrigan, F.T., and R.W. Aldrich. 1999. J. Gen. Physiol. 114:305–336; Horrigan, F.T., J. Cui, and R.W. Aldrich. 1999. J. Gen. Physiol. 114:277–304). In 0 Ca2+, the steady-state gating charge-voltage (QSS-V) relationship is shallower and shifted to more negative voltages than the conductance-voltage (GK-V) relationship. Calcium alters the relationship between Q-V and G-V, shifting both to more negative voltages such that they almost superimpose in 70 μM Ca2+. This change reflects a differential effect of Ca2+ on voltage sensor activation and channel opening. Ca2+ has only a small effect on the fast component of ON gating current, indicating that Ca2+ binding has little effect on voltage sensor activation when channels are closed. In contrast, open probability measured at very negative voltages (less than −80 mV) increases more than 1,000-fold in 70 μM Ca2+, demonstrating that Ca2+ increases the C-O equilibrium constant under conditions where voltage sensors are not activated. Thus, Ca2+ binding and voltage sensor activation act almost independently, to enhance channel opening. This dual-allosteric mechanism can reproduce the steady-state behavior of mSlo1 over a wide range of conditions, with the assumption that activation of individual Ca2+ sensors or voltage sensors additively affect the energy of the C-O transition and that a weak interaction between Ca2+ sensors and voltage sensors occurs independent of channel opening. By contrast, macroscopic IK kinetics indicate that Ca2+ and voltage dependencies of C-O transition rates are complex, leading us to propose that the C-O conformational change may be described by a complex energy landscape.
Keywords: calcium, potassium channel, BK channel, gating current, ion channel gating
INTRODUCTION
Ion channels activate by sensing stimuli such as membrane voltage or ligand binding and transducing this information into conformational changes that open the channel pore. Thus, a key question to understanding ion channel function is how the protein domains involved in sensing stimuli (sensors) and opening the pore (gates) communicate. We addressed this question in mSlo1 large conductance Ca2+-activated K+ (BK) channels by measuring ionic and gating currents over a wide range of voltage and [Ca2+] (for abbreviations and parameters used in this paper, see Table I) .
TABLE I.
Commonly Used Abbreviations and Parameters
| BK | Large conductance Ca2+-activated K+ channel. |
| MWC | Monod-Wyman-Changeux. |
| LFER | Linear-free energy relationship. |
| C,O | Closed and open conformations of the channel. |
| R,A | Resting and activated conformations of voltage sensor. |
| X,Xca | Unliganded and Ca2+-bound state of Ca2+-sensor. |
| L | C-O equilibrium constant (unliganded channel, resting voltage sensors). |
| L0,zL | The zero voltage value of L and its partial charge, respectively. |
| J | R-A equilibrium constant (closed, unliganded channel). |
| J0,zJ | The zero voltage value of J and its partial charge, respectively. |
| Vh(J) | The voltage where J = 1, corresponding to the half-activation voltage of QC. |
| KD | Ca2+ dissociation constant (closed channel, resting voltage sensors). |
| K | Equilibrium constant for Ca2+-binding (K = [Ca2+]/KD). |
| C | Allosteric factor describing interaction between channel opening and Ca2+-binding. The ratio of KDs for closed and open channels. |
| D | Allosteric factor describing interaction between channel opening and voltage sensor activation. The ratio of R-A equilibrium constants for open and closed channels. |
| E | Allosteric factor describing interaction between voltage-sensor activation and Ca2+-binding. The ratio of KDs for activated and resting voltage sensor. |
| Vh(P0) | Half activation voltage of the PO-V relationship. |
| R0 | Ratio of NPO in the presence and absence of Ca2+ at extreme negative voltages where voltage-sensors are not activated. |
| QP | Gating charge moved during a voltage pulse. |
| IgFast | The fast component of ON or OFF gating current. |
| Qfast | Fast component of gating charge movement. |
| τgFast | Time constant of fast gating charge movement. |
| τgSLOW | Time constant of slow gating charge movement (limited by C-O transition). |
| QSS | Steady-state gating charge. |
| QC,QO | Steady-state gating charge distribution for closed or open channels. |
| QT | Total gating charge associated with opening and voltage-sensor activation. |
| <qa> | Mean activation charge displacement/slope of log(PO)-V (Eq. 7). |
| Cg | Gating capacitance determined by admittance analysis. |
| α,β | Forward and backward rate constant, respectively, for R-A transition. |
| δ,γ | Forward and backward rate constant, respectively, for C-O transition. |
| i,j | The number of voltage sensors activated and Ca2+ ions bound, respectively. |
| τ(IK) | The time constant of IK relaxation after voltage-sensor activation. |
| τN,τP | τ(IK) at extreme negative or positive voltages, respectively. |
| zN,zP | Partial charges of τN,τP, respectively. |
| VdN | The voltage where τ(IK) deviates from an exponential voltage-dependence at extreme negative voltages. |
To characterize sensor–gate interaction ideally, one would like to monitor both sensor and gate activity and determine what effect the state of one has on the functional properties of the other. However, this approach is problematic for many channels because the kinetics and equilibrium properties of sensor activation and channel opening are too similar to allow one process to be studied while the other remains in a fixed state. In addition, for cases in which certain combinations of sensor and gate conformation are prohibited, the conditions under which interactions can be probed will be restricted. For instance, channel opening in many voltage-gated channels cannot be studied when voltage sensors are in a nonactivated state (Islas and Sigworth, 1999).
Large conductance BK channels possess many advantages as a system for studying interactions between sensors and gates. First, voltage- and ligand-dependent gating mechanisms can be studied in the same channel. Second, the mechanisms of voltage sensor activation, Ca2+-binding, and channel opening appear simple and reasonably represented as two-state processes. The homotetrameric nature of the channel and the absence of inactivation also help to limit the potential complexity of interactions between sensors and gates. Third, closed to open state transition kinetics in Slo1 channels are slow relative to voltage sensor activation and Ca2+-binding (Cox et al., 1997a; Horrigan and Aldrich, 1999; Horrigan et al., 1999). This simplifies the analysis of macroscopic IK kinetics and allows sensor function to be examined while channels are maintained in either an open or closed conformation. Fourth, voltage sensors and Ca2+ sensors appear to interact with channel opening through allosteric mechanisms such that no combination of sensor and gate conformation is prohibited. Finally, the sensitivity of BK channels to both Ca2+ and voltage allows the manipulation of channel function in ways that facilitate analysis of sensor–gate interaction.
In the present study, we address several features of BK channel gating that are readily explained by allosteric models. Our ability to test this mechanism and to characterize sensor–gate interaction in BK channels depends on isolating subsets of transitions under extreme conditions and measuring both ionic and gating currents. For example, we show that the effect of Ca2+-binding on channel opening is best characterized when the C-O equilibrium constant is reduced by forcing voltage sensors into a resting state at extreme negative voltages. Conversely, some effects of channel opening on voltage sensor activation are best detected when the open conformation is stabilized by high [Ca2+]. The observed properties of Ca2+- and voltage-dependent transitions and their relationship to each other define how Ca2+ and voltage interact to determine mSlo1 channel activity.
The BK Channel Gating Mechanism
Because the response of BK channels to Ca2+ and voltage is complex, it is useful to present our results in the framework of a plausible general model. BK channel gating involves voltage sensor activation, Ca2+ binding, channel opening, and some interaction among these three processes. Previous analysis of ionic and gating currents from mSlo1 in 0 Ca2+ ([Ca2+] < 1 nM) showed that voltage sensor activation promotes channel opening through an allosteric mechanism, illustrated in Fig. 1 A, Scheme I (Horrigan and Aldrich, 1999; Horrigan et al., 1999). In Scheme I, voltage sensors in each of the four identical subunits can undergo transitions between resting (R) and activated (A) conformations. In addition, the channel can undergo a transition between closed (C) and open (O) conformations. Voltage sensor activation and channel opening interact through an allosteric mechanism, represented by a factor D, such that the C-O equilibrium constant increases D-fold for each voltage sensor activated, and the R-A equilibrium constant increases D-fold when the channel opens.
Figure 1.

Slo1 gating mechanisms. (A) The unliganded gating mechanism (Scheme I) involves an allosteric interaction between channel opening (C-O) and voltage sensor activation (R-A). L is the C-O equilibrium constant when all voltage sensors are in the resting (R) state. J is the R-A equilibrium constant when channels are closed. D is the allosteric interaction factor such that the C-O equilibrium constant increases D-fold for each voltage sensor activated, and the R-A equilibrium constant increases D-fold when the channel opens. (B) Scheme I specifies a 10-state gating scheme (Scheme I*) with the indicated equilibrium constants. Subscripts for closed and open states denote 0–4 activated voltage sensors. (C) A general allosteric gating mechanism (Scheme II) includes a Ca2+ binding transition (X-XCa) for each subunit with an equilibrium constant K = [Ca2+]/KD when channels are closed and voltage sensors are not activated. Allosteric interactions of Ca2+ binding with channel opening and voltage sensor activation are determined by allosteric factors C and E, respectively, as described in the text.
A key feature of an allosteric interaction is that the two processes involved influence each other but are not coupled obligatorily. That is, channels can open even if voltage sensors are not activated and voltage sensors can activate whether channels are closed or open. Therefore, Scheme I specifies a 10-state gating scheme (Fig. 1 B, Scheme I*) with 5 closed and 5 open states representing the C and O conformations with 0–4 activated voltage sensors. Similar schemes have been proposed to describe the activation of other voltage-dependent channels (Marks and Jones, 1992; Rios et al., 1993; McCormack et al., 1994), and the basic finding that channels undergo both independent and concerted (or cooperative) transitions is well established (Sigworth, 1994; Zagotta et al., 1994). The kinetic and equilibrium properties of mSlo1 channels allow us to study these transitions in isolation (Horrigan and Aldrich, 1999; Horrigan et al., 1999), an advantage that will be exploited here to determine the mechanism of Ca2+ action.
Several lines of evidence indicate that Ca2+-binding also influences channel opening through an allosteric mechanism. The voltage-dependent activation of unliganded channels shows that Ca2+-binding is not obligatory for channel opening or for voltage sensor activation (Cui et al., 1997; Nimigean and Magleby, 2000; Talukder and Aldrich, 2000). Similarly, macroscopic IK relaxation kinetics are altered by Ca2+ in a saturable manner, indicating that Ca2+ binding is not a rate-limiting step in channel activation. The kinetic and steady-state properties of mSlo1 activation in the presence and absence of Ca2+ exhibit similar voltage dependencies (Cox et al., 1997a; Cui et al., 1997; Rothberg and Magleby, 2000), suggesting that a similar gating scheme with altered rate constants might account for the activation of unliganded and Ca2+-bound channels. In line with this prediction, single channel data from native BK channels in high Ca2+ are consistent with a two-tiered gating scheme for Ca2+-bound channels similar to Scheme I* (Rothberg and Magleby, 1999). Moreover, an allosteric model of Ca2+ action reproduces many features of macroscopic mSlo1 IK over a wide range of Ca2+ despite the use of a simplified voltage-gating mechanism (Cox et al., 1997a; Cui et al., 1997).
A General Allosteric Model of BK Channel Gating
Any BK channel gating scheme that incorporates four identical subunits and accounts for the effects of both Ca2+ and voltage must necessarily contain a large number of states (Cox et al., 1997a). In addition to the four voltage sensors, each channel presumably contains at least four high affinity Ca2+ binding sites, since dose-response relationships describing the effect of micro molar Ca2+ on steady-state open probability require Hill coefficients greater than three (Cox et al., 1997a; Rothberg and Magleby, 2000). To describe a channel that can be open or closed with any number (0–4) of Ca2+-bound and voltage sensors activated requires a minimum of 50 states, divided into two interconnected tiers of open and closed states (Horrigan et al., 1999; Rothberg and Magleby, 1999; Cox and Aldrich, 2000; Cui and Aldrich, 2000; Rothberg and Magleby, 2000).
Despite the apparent complexity of a large gating scheme, the mechanism underlying such a model and the parameters required to describe it may be relatively simple as illustrated by Fig. 1 C, Scheme II. The homotetrameric nature of the mSlo1 channel implies that high affinity binding sites are identical. Thus, in Scheme II, Ca2+ binds to these subunits with identical equilibrium constants K. The independence of the binding sites is an initial simplifying assumption. As in Scheme I, the gating of unliganded channels is specified by an allosteric interaction between voltage sensor activation (R-A) and channel opening (C-O). Similarly, Ca2+ is coupled to channel gating through allosteric interactions represented by the factors C and E that connect the Ca2+-binding transitions to the C-O and R-A voltage sensor transitions respectively. The steady-state properties of this model are fully described by three allosteric factors (C, D, E) and three equilibrium constants (J, K, L):
 |
(1) |
where
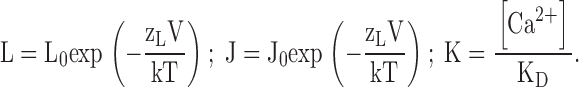 |
zL and zJ are the partial charges associated with channel opening and voltage sensor activation respectively, and KD is the elementary Ca2+ dissociation constant when the channel is closed and voltage sensors are not activated.
Scheme II highlights an important strategy used in our analysis: to define the simplest mechanism—rather than the gating scheme with the fewest kinetic states—that can account for the data. The simplest or most physically plausible mechanism often does not produce the fewest states. The number of states specified by Scheme II is determined by the nature of interactions between C-O, R-A, and X-XCa2+ transitions. Interactions of channel opening with voltage sensors and Ca2+ binding sites are the most easily defined. If the C-O transition is concerted, channel opening should affect individual subunits equally. Therefore, by energy conservation, each bound Ca2+ or activated voltage sensor must have an additive effect on the energy of the C-O transition, increasing the C-O equilibrium constant C- or D-fold, respectively. By contrast, interactions between the X-XCa2+ and R-A transitions in Scheme II do not involve a concerted transition and several mechanisms can be postulated, each resulting in a different number of functionally distinct states.
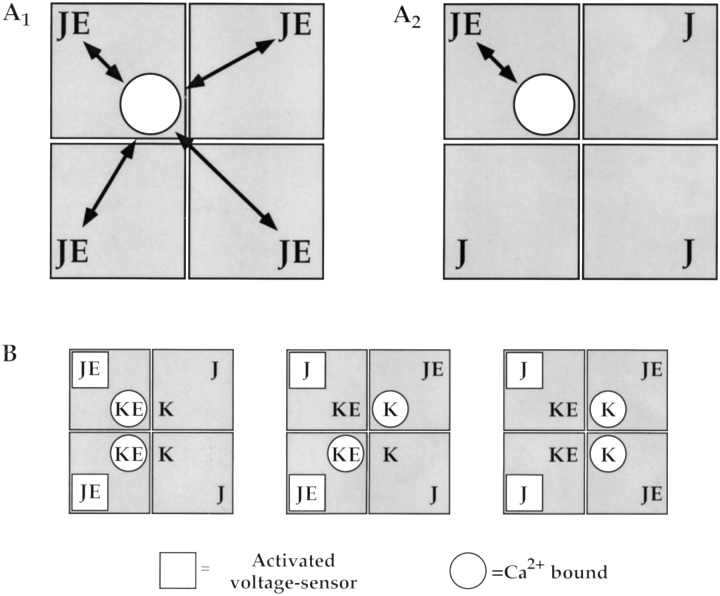
In cases where no interaction between Ca2+ binding sites and voltage sensors exist, Scheme II will define a gating scheme with the minimum 50 states and PO can be described by Eq. 1 with E = 1 (Cox and Aldrich, 2000; Shi and Cui, 2001; Zhang et al., 2001). A 50-state scheme is also generated if Ca2+ binding to one subunit affects voltage sensors in all subunits equally and the activation of a voltage sensor affects all Ca2+-binding sites equally (Fig. 2 A1). However, this would require a complex mechanism for coordinated communication among subunits independent of their relative positions in the tetramer. The equation describing such a mechanism is more complex than Eq. 1 (Cui and Aldrich, 2000). We will instead make the simplifying assumption that Ca2+ binding sites and voltage sensors can only interact within the same subunit (Fig. 2 A2). This assumption, although mechanistically and mathematically simpler, increases the number of states to 70 because interactions between voltage sensors and Ca2+ binding sites now depend on their relative location within the tetramer, and some combinations of i-activated voltage sensors and j-occupied binding sites are no longer energetically equivalent. For example, a channel with two activated voltage sensors and two Ca2+ bound can exist in three distinct states depending on whether Ca2+ and activated voltage sensors are in the same or different subunits (Fig. 2 B). More general gating schemes that distinguish the relative Ca2+ occupancy and/or voltage sensor conformation in adjacent and diagonally opposed subunits result in even more than 70 states (Cox et al., 1997a) but, like the 50-state model, require additional mechanisms to account for interactions between or among voltage sensors and Ca2+ binding sites in different subunits.
Figure 2.
Mechanisms of interaction between voltage sensors and Ca2+-binding sites. (A1) If the binding of Ca2+ to a single subunit affects voltage sensors equally then voltage sensor equilibrium constants in all four subunits will increase E-fold to JE as indicated. (A2) In Scheme II, we assume Ca2+ binding only affects the voltage sensor in the same subunit. Consequently, the A2 mechanism predicts more states than the A1 mechanism. (B) For example, when a channel has two Ca2+-bound (open circles) and two voltage sensors activated (open squares), the A2 mechanism specifies three states depending on the relative location of Ca2+ and activated voltage sensors. That the states are functionally distinct can be seen by comparing the different Ca2+-binding equilibria for the unoccupied binding sites (K,K), (K,KE), and (KE,KE). Equilibrium constants for voltage sensor activation are also different. By contrast, the A1 mechanism specifies a single state with equilibrium constants KE2 for Ca2+ binding and JE2 for voltage sensor activation (not depicted).
The general allosteric model (Scheme II) illustrates some questions and strategies that motivated this study. Does the allosteric effect of Ca2+ on BK channel activation occur via effects on voltage sensor activation, channel opening or both? In terms of Scheme II, the answer to this fundamental question reduces to the evaluation and comparison of the interaction factors C and E. To accomplish this and to characterize Ca2+-binding, voltage sensor activation, and channel opening transitions, we studied mSlo1 gating under a range of conditions that allow us to isolate subsets of the interactions in Scheme II. Fig. 3 shows the sub-schemes that describe mSlo1 for various conditions that are approximated in our results. The unliganded (sub-Scheme IIa) or Ca2+-saturated (sub-Scheme IIb) conditions were approximated in sub-nanomolar or 70–100 μM Ca2+ respectively. Voltage sensors were forced into resting (sub-Scheme IIc) or activated (sub-Scheme IId) states at extreme negative or positive voltages. Finally, by taking advantage of the relatively slow C-O transition kinetics, we studied gating while channels remain briefly in a closed (sub-Scheme IIe) or open (sub-Scheme IIf) conformation.
Figure 3.
Sub-Schemes derived from Scheme II. Under extreme conditions that limit Ca2+-binding, voltage sensor activation, or channel opening, the number of interactions that govern mSlo1 gating is reduced and Scheme II is reduced to the following subschemes. (A) unliganded, (B) Ca2+-saturated, (C) very negative voltages (voltage sensors resting), (D) very positive voltages (voltage sensors activated), (E) closed, and (F) open.
MATERIALS AND METHODS
Channel Expression
Experiments were performed with the mbr5 clone of the mouse homologue of the Slo1 gene (mSlo1) (Butler et al., 1993) expressed in Xenopus oocytes or HEK 293 cells. The clone was modified to facilitate mutagenesis and was propagated and cRNA transcribed as described previously (Cox et al., 1997b). Xenopus oocytes were injected 3–7 d before recording with ∼0.5–5 ng or ∼50 ng of cRNA for ionic current or gating current experiments respectively (50 nl, 0.01–1 ng/nl). mSlo1 was also subcloned into a mammalian expression vector (SRα, provided by Dr. A.P. Braun, University of Calgary, Calgary, Canada) containing the SV-40 promoter. HEK 293 cells expressing the large T-antigen of the SV-40 virus were cotransfected with mSlo1 and green fluorescent protein (GFP, as a marker) using LipofectAMINE (GIBCO BRL/Life Technologies, Inc.) 3 d before recording.
Electrophysiology
Currents were recorded using the patch clamp technique in the inside out configuration (Hamill et al., 1981). Upon excision, patches were transferred to a separate chamber and washed with at least 20× volumes of internal solution. K+ currents were recorded with internal solutions containing (in mM) 110 KMeSO3, 20 HEPES, and an external (pipette) solution containing 104 KMeSO3, 6 KCl, 2 MgCl2, 20 HEPES. Gating currents were recorded with internal solutions containing 135 N-methyl-d-glucamine(NMDG)-MeSO3, 20 HEPES, and an external solution containing 125 tetraethylammonium(TEA)-MeSO3, 6 TEA-Cl, 2 MgCl2, 20 HEPES. Internal solutions contained 40 μM (+)-18-crown-6-tetracarboxylic acid (18C6TA) to chelate contaminant Ba2+ (Diaz et al., 1996; Neyton, 1996; Cox et al., 1997b). “0 Ca2+” solutions contained 2 mM EGTA reducing free Ca2+ to an estimated 0.8 nM based on the presence of ∼10 μM contaminant Ca2+ (Cox et al., 1997b). Ca2+ solutions were buffered with 2 or 5 mM HEDTA and CaCl2, and free Ca2+ was measured with a Ca2+-electrode (Orion Research, Inc.). Total Cl− was adjusted to 10 mM with HCl. The pH of all solutions was adjusted to 7.2. Solutions were prepared and experiments performed at 20°C (approximately ±1°C).
Electrodes were pulled from thick walled 1010 glass (World Precision Instruments), coated with wax (KERR sticky wax) to minimize electrode capacitance (∼1 pF), and fire-polished before use. Pipette access resistance measured in the bath solution (0.5–1.5 MΩ) was used as an estimate of series resistance (Rs) to correct the pipette voltage (Vp) at which IK was recorded. The corrected pipette voltage, Vm, was used in determining membrane conductance (GK) from tail current measurements and in plotting the voltage dependence of GK or the time constant of IK relaxation (τ[IK]). Series resistance error was <15 mV for all data presented and <10 mV for τ(IK) measurements.
Data were acquired with an Axopatch 200B amplifier (Axon Instruments, Inc.) set in patch mode with the Axopatch's internal 4-pole Bessel filter set at 100 kHz. Currents were subsequently filtered by an 8-pole Bessel filter (Frequency Devices, Inc.) at 20 kHz (Ig) or 50 kHz (IK) and sampled at 100 kHz with a 16 bit A/D converter (Instrutech ITC-16). Macroscopic currents were recorded at a relatively low gain (1–2 mV/pA) to avoid saturation of capacitive transients in response to voltage steps that often exceeded 300 mV. In addition, for gating currents, the voltage command was filtered at 20 kHz to limit the speed of fast capacitive transients so that they could be sampled accurately and subtracted. Gating current records were typically signal averaged in response to at least eight voltage pulses. A P/4 protocol was used for leak subtraction (Armstrong and Bezanilla, 1974) except at voltages less than the holding potential where a P/8 protocol was used to avoid channel activation during the leak pulses. The holding potential was adjusted from −80 mV in 0 Ca2+ to −120 mV in 1,000 μM Ca2+.
A Macintosh-based computer system was used in combination with Pulse Control acquisition software (Herrington and Bookman, 1995) and Igor Pro for graphing and data analysis (WaveMetrics, Inc.). A Levenberg-Marquardt algorithm was used to perform nonlinear least-squared fits. Error estimates for fit parameters are given as ± SD.
Single Channel Analysis
Under conditions where the open probability (PO) is small (<10−3), single channel opening events were observed in patches containing hundreds of channels and NPO was determined from steady-state recordings of 5–45 s duration. Currents were filtered at 20 kHz, yielding a dead-time of ∼10 μs, and were sampled at 100 kHz. NPO was then determined from all-points amplitude histograms by measuring the fraction of time spent (Pk) at each open level (k) using a half-amplitude criteria and summing their contributions 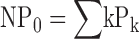 . NPO was also determined by fitting Pk with a Poisson distribution
. NPO was also determined by fitting Pk with a Poisson distribution  . The values of NPO obtained by these two methods differ by <5%, consistent with the assumption that the observed currents represent the activity of a large uniform population of channels opening with very low probability (Horrigan et al., 1999).
. The values of NPO obtained by these two methods differ by <5%, consistent with the assumption that the observed currents represent the activity of a large uniform population of channels opening with very low probability (Horrigan et al., 1999).
Normalized open probability (PO/POMAX = NPO/NPOMAX) was determined by combining NPO measurements with an estimate of NPOMAX obtained from the macroscopic GK-V relationship in the same patch (NPOMAX = GKMAX/gK, where gK is the single channel conductance). Patches that were used to measure single channel activity at negative voltages often produced currents that were too large to measure (>20 nA) at voltages that activate mSlo1 channels maximally. In these cases, Gmax was estimated by fitting the macroscopic GK-V with a Boltzmann function {1 + exp[−z(V − Vh)/kT]} raised to a power n (e.g., Fig. 4 C1), where z and n were determined at each [Ca2+] from other patches where the entire GK-V relationship was measured.
Figure 4.
Effects of Ca2+ on IK. (A) Families of IK evoked by 20-ms depolarizations to different voltages (20 mV steps over the indicted range) are compared in 0 and 70 μM Ca2+ (different patches). (B) IK evoked by 10-ms pulses to 160 mV in 0 and 100 μM Ca2+ (same patch) are normalized to steady-state current during the pulse and superimposed together with exponential fits (dashed lines) to both activation and deactivation time courses. (C1) Normalized GK-V relationships (mean ± SEM) in 0 Ca2+(n = 51) and 70 μM Ca2+(n = 3) obtained from isochronal tail currents following 20-ms pulses. G-Vs were normalized by fitting with Boltzmann functions raised to a power n (solid lines)(0 Ca: z = 0.73 e, Vh = 144 mV, n = 2.93; 70 μM Ca2+: z = 1.22 e, Vh = 12.9 mV, n = 1.39). (D1) Mean time constants of IK relaxation (τ[IK]) are plotted on a log scale versus voltage for the same experiments as in C1. Dashed lines indicate similar exponential voltage dependencies of τ(IK) at negative voltages in the presence or absence of Ca2+. The shapes of the G-V and τ(IK)-V relationships from C1 and D1 are compared in C2 and D2, respectively, by shifting the 0 Ca2+ plot along the voltage-axis by 135 mV, which is sufficient to align the τ(IK)-V relationships at voltages less than the peak voltage. (E) The 10-state gating scheme (sub-Scheme IIb*) specified by Scheme II in saturating Ca2+.
For voltages >60 mV from the reversal potential (0 mV), single channel amplitudes were large enough that false opening events due to noise were not detected using 20 kHz filtering. The prevalence of false events was assessed by evaluating the number of current transients from the closed level that exceed the 1/2 amplitude criterion in a direction opposite to that of channel opening. When determining PO-V relationships over a large voltage range, currents were digitally filtered at 5 kHz before determining NPO such that false events were not observed at 20 or −20 mV. For V > 60 mV no difference in NPO was observed with 5 or 20 kHz filtering. However, for V < −60 mV a decrease in NPO was observed at 5 kHz, reflecting the brevity of open times at these voltages. The largest decreases (∼30%) were observed at the most negative voltages in 0 Ca2+ and little change was observed for [Ca2+] > 1 μM. Thus, PO in low Ca2+ may be underestimated. To minimize this effect, dose-response relationships used to quantify the effect of Ca2+ on channel activation (Fig. 9) were determined from NPO at V < −80 mV using 20 kHz filtering.
Figure 9.
The Ca2+ dependence of PO. (A) NPO determined from the patch in Fig. 8 C is plotted on a semi-log scale vs. voltage for different [Ca2+] (in μM: 0 (•), 0.13 (○), 0.27 (▪), 0.58 (▵), 0.79 (▴), 3.8 (▾), 19 (♦), 68 ( ), 102 (
), 102 ( ), 313 (
), 313 ( ), 1030 (
), 1030 ( )). Dashed lines are exponential fits with z = 0.3 e. (B) PO-V relationships determined from normalized G-Vs at different [Ca2+](in μM: 0 (•), 0.27 (▪), 0.58 (▵), 0.81 (▴), 1.8 (▿), 3.8 (▾), 8.2 (⋄), 19 (♦), 68 (
)). Dashed lines are exponential fits with z = 0.3 e. (B) PO-V relationships determined from normalized G-Vs at different [Ca2+](in μM: 0 (•), 0.27 (▪), 0.58 (▵), 0.81 (▴), 1.8 (▿), 3.8 (▾), 8.2 (⋄), 19 (♦), 68 ( ), 99 (
), 99 ( )) are plotted on a semilog scale versus voltage (mean ± SEM). Data for PO < 10−2 were obtained from amplitude histograms as in A but were filtered at 5 kHz such that openings near the K+ reversal potential could be distinguished from noise based on a half-amplitude criterion (see materials
and
methods). The data were fit (solid lines) by Scheme I* (Fig 1 B) allowing all parameters (L0, zL, J0, zJ, D) to vary freely at each [Ca2+]. (C) A dose-response relationship for the effect of Ca2+on PO at negative voltages is obtained by plotting the log-ratio of NPO in the presence and absence of Ca2+ (log[Ro]) versus [Ca2+] for several experiments (symbols). NPO(V) was determined from exponential fits to the data with z = 0.3 e as in A. Log(RO) from A (•) spans the entire [Ca2+] range and is fit (dashed line) by Eq. 4 (C = 7.8, KD = 8.2 μM). (D) The mean log(RO)-[Ca2+] relationship is fit (solid line) by Eq. 4 (C = 7.4, KD = 9.3 μM) and compared with the half activation voltage (Vh) of PO (open symbols). Solid lines in C and D represent predictions of Scheme II for log(RO) and VH(PO), respectively, using the Fit B parameters in Table II (C = 8, KD = 11 μM; Table II, Fit B). The change in VH(PO) from 100–1,000 μM Ca2+ can be reproduced (dotted line) if Scheme II is modified to include an additional low affinity binding site in each subunit that interacts with the C-O transition through an allosteric mechanism analogous to that embodied by the C-factor in Scheme II. The parameters for the low affinity site (KDLow = 2.33 mM, CLow = 3.53) were taken from Zhang et al. (2001). However, this model predicts a marked increase of RO in 100–1,000 μM Ca2+ (dashed line) that is inconsistent with the data.
)) are plotted on a semilog scale versus voltage (mean ± SEM). Data for PO < 10−2 were obtained from amplitude histograms as in A but were filtered at 5 kHz such that openings near the K+ reversal potential could be distinguished from noise based on a half-amplitude criterion (see materials
and
methods). The data were fit (solid lines) by Scheme I* (Fig 1 B) allowing all parameters (L0, zL, J0, zJ, D) to vary freely at each [Ca2+]. (C) A dose-response relationship for the effect of Ca2+on PO at negative voltages is obtained by plotting the log-ratio of NPO in the presence and absence of Ca2+ (log[Ro]) versus [Ca2+] for several experiments (symbols). NPO(V) was determined from exponential fits to the data with z = 0.3 e as in A. Log(RO) from A (•) spans the entire [Ca2+] range and is fit (dashed line) by Eq. 4 (C = 7.8, KD = 8.2 μM). (D) The mean log(RO)-[Ca2+] relationship is fit (solid line) by Eq. 4 (C = 7.4, KD = 9.3 μM) and compared with the half activation voltage (Vh) of PO (open symbols). Solid lines in C and D represent predictions of Scheme II for log(RO) and VH(PO), respectively, using the Fit B parameters in Table II (C = 8, KD = 11 μM; Table II, Fit B). The change in VH(PO) from 100–1,000 μM Ca2+ can be reproduced (dotted line) if Scheme II is modified to include an additional low affinity binding site in each subunit that interacts with the C-O transition through an allosteric mechanism analogous to that embodied by the C-factor in Scheme II. The parameters for the low affinity site (KDLow = 2.33 mM, CLow = 3.53) were taken from Zhang et al. (2001). However, this model predicts a marked increase of RO in 100–1,000 μM Ca2+ (dashed line) that is inconsistent with the data.
Shifts in Voltage-dependent Parameters
Patch to patch variations in the half-activation voltage (Vh) of GK-V and Q-V relationships are observed for mSlo1 (Horrigan and Aldrich, 1999; Horrigan et al., 1999) and hSlo1 (Stefani et al., 1997), possibly due to differences in the redox state of channels (DiChiara and Reinhart, 1997; Tang et al., 2001). Such shifts do not appreciably alter the shape of voltage-dependent relationships but make comparison of data between different experiments difficult and cause broadening in averaged voltage-dependent relationships. To compensate for this effect, Vh was determined for each patch and compared with the mean for all experiments (<Vh>) at the same [Ca2+]. Data from individual experiments were then shifted along the voltage-axis by ΔVh = (<Vh> − Vh) before averaging. This procedure yields average relationships that accurately represent the shape of individual GK-V and Q-V relationships.
Admittance Analysis
Admittance analysis was performed as described previously (Horrigan and Aldrich, 1999). Briefly, in gating current solutions, the membrane was clamped with a sinusoidal voltage command (868 Hz, 60 mV peak to peak) superimposed on a 1 s voltage-ramp. The voltage command and current signal were both filtered at 20 kHz and current was sampled at 18-μs intervals (64 samples/period). Admittance was determined for each cycle of the sinusoid. Gating capacitance (Cg[V]) was determined as the voltage-dependent component of patch admittance appearing at a phase angle of 90° relative to the command voltage (after correction for instrumentation phase delays).
RESULTS
Effects of Ca2+ on mSlo1 Ionic Currents
The basic effects of micromolar Ca2+ on BK channel gating are shown in Fig. 4, which compares macroscopic IK recorded from mSlo1 channels in the presence of internal solutions containing 0 Ca2+ or 70–100 μM Ca2+, which correspond approximately to unliganded (sub-Scheme IIa) or Ca2+-saturated (sub-Scheme IIb) conditions, respectively. 70 μM Ca2+ may be insufficient to completely saturate high affinity Ca2+ binding sites, which we estimate later to have KDs on the order of 1 and 10 μM for open and closed channels, respectively (Cox et al., 1997a). However, concentrations of Ca2+ or other divalent cations in the 100 μM to 100 mM range produce distinct effects on channel gating that have been attributed to nonselective, low-affinity (millimolar) binding sites (Shi and Cui, 2001; Zhang et al., 2001). As a compromise, 70–100 μM Ca2+ was used throughout this study to approximate the Ca2+-saturated condition for high affinity binding sites while minimizing possible contributions from low affinity sites.
Fig. 4 A compares IK evoked at different voltages in 0 or 70 μM Ca2+. Ca2+ increases the steady-state open probability (PO) such that conductance-voltage (GK-V) relationships shift to more negative voltages (Fig. 4 C1). The records in Fig. 4 A indicate that Ca2+ speeds IK activation during the pulse and slows deactivation at −80 mV after the pulse. This effect is illustrated in Fig. 4 B, where currents evoked by pulses to 160 mV in 0 and 100 μM Ca2+ from the same patch have been superimposed and normalized. Activation and deactivation kinetics are well fit by exponential functions following a brief delay (dashed lines) (Horrigan et al., 1999). The time constants (τ[IK]) of such fits over a wide range of voltages in 0 and 70 μM Ca2+ are plotted in Fig. 4 D1.
Figs. 4, C1 and D1, show that Ca2+ shifts the GK-V and τ(IK)-V relationships along the voltage axis with little change in shape, suggesting that unliganded and ligand-bound channels gate by similar mechanisms. Indeed, Scheme II reduces, in saturating Ca2+ (Fig. 3, sub-Scheme IIb), to a 10-state gating scheme (Fig. 4 E, sub-Scheme IIb*) analogous to the unliganded scheme (Fig. 1, Scheme I*)(Horrigan and Aldrich, 1999; Horrigan et al., 1999). Despite this general similarity, significant changes in the shape of GK-V and τ(IK)-V relationships are observed when the plots obtained in 70 μM Ca2+ are shifted by 135 mV to align them with the 0 Ca2+ data (Fig. 4, C2 and D2). Such differences are expected if mSlo1 gating is governed by multiple voltage-dependent processes (e.g., C-O and R-A transitions).
In general, Ca2+ will shift the GK-V and τ(IK)-V relationships without changing their shape only if the rate constants in the unliganded scheme (Fig. 1, Scheme I*) at any voltage V are identical to those for the Ca2+-saturated scheme (Fig. 4 E, sub-Scheme IIb*) at V-ΔV. Since the horizontal (R-A) transitions are more voltage dependent than the vertical (C-O) transitions, this condition can only be satisfied if voltage sensor activation is more Ca2+ dependent than channel opening. In fact we will show that the opposite is true. Therefore, the relationship between the horizontal and vertical transitions in Schemes I* and IIb* is altered by Ca2+ in a manner that cannot be compensated for by voltage, producing a change in the shape of the GK-V and τ(IK)-V relations.
Effects of Ca2+ on Gating Current
The data in Fig. 4 are insufficient to determine if Ca2+ effects channel opening (C-O) or voltage sensor activation (R-A) because IK kinetics and steady-state activation are generally dependent on both processes (Horrigan et al., 1999). To help address this question mSlo1 gating currents (Ig) were compared in 0 Ca2+ and 70 μM Ca2+. As the following analysis indicates (Figs. 5–7) , the Ca2+ sensitivity of Ig can be attributed mainly to an effect on channel opening and the interaction between Ca2+ binding and voltage sensor activation is weak.
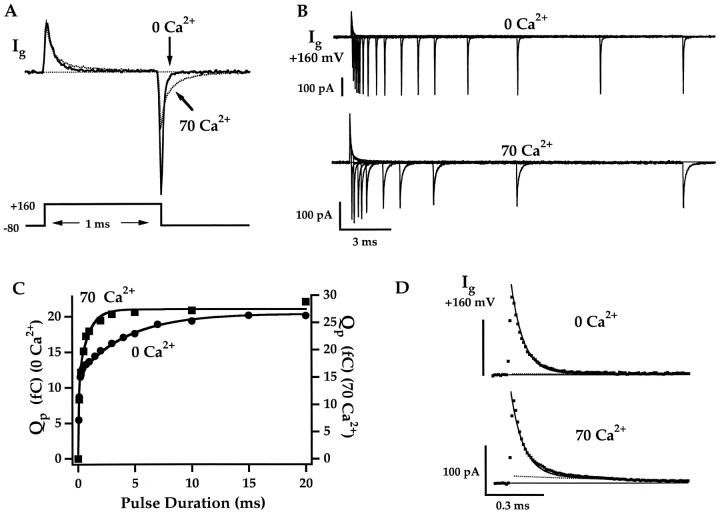
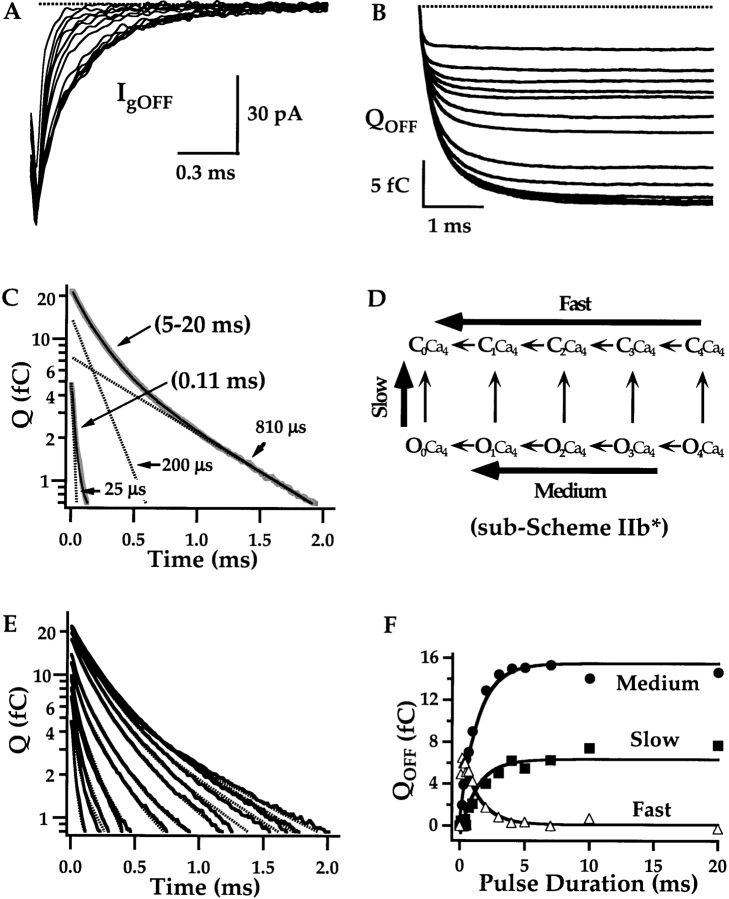
Figure 5.
Effects of Ca2+ on gating currents (A) Gating currents evoked by 1-ms pulses to 160 mV in 0 and 70 μM Ca2+. Ig was recorded in the absence of K+ and the presence of 125 mM external TEA to eliminate ionic currents. (B) Families of Ig evoked by depolarizations to 160 mV of different duration (0.1–20 ms) in 0 and 70 μM Ca2+ from two different patches. Total OFF charge for each pulse (Qp) was determined by integrating IgOFF for 5 ms and is plotted against pulse duration in (C). Results were fit with double exponential functions (0 Ca2+: τ1 = 63 μs, A1 = 11.7 fC, τ2 = 4.2 ms, A2 = 8.7 fC; 70 Ca2+: τ1 = 68 μs, A1 = 14.6 fC, τ2 = 0.85 ms, A2 = 12.8 fC). (D) The derivative of the fits to QP (solid lines) are superimposed with IgON at 160 mV. Dashed lines represent the slow component of the fits.
Figure 7.
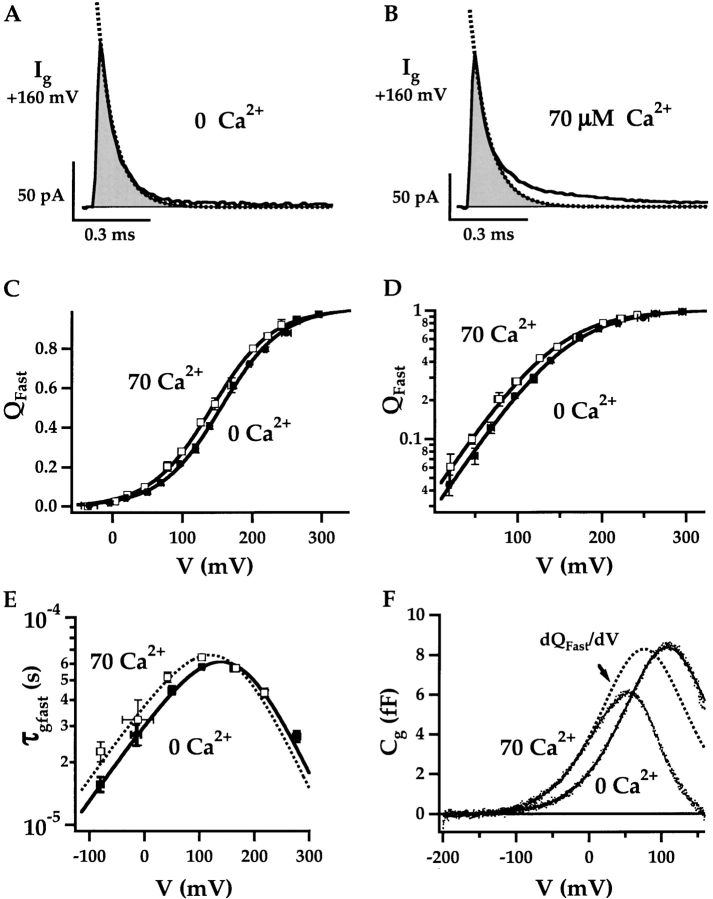
Ca2+ has little effect on fast gating charge movement. The fast components of IgON evoked at 160 mV in 0 Ca2+ (A) and 70 μM Ca2+ (B) are determined by fitting the first 100 μs of the decay with exponential functions (dashed lines; 0 Ca: τgfast = 69.7 μs, 70 μM Ca: τgfast = 73.6 μs). Fast charge movement (Qfast) was estimated by integrating under the exponential fits (shaded areas in A and B). Qfast-V relationships (mean ± SEM) are plotted on linear (C) and semilog (D) scales for 0 Ca2+ (solid symbols, n = 10) and 70 μM Ca2+ (open symbol, n = 7). To determine mean Qfast, normalized Qfast-V relationships from individual experiments were shifted along the voltage-axis by ΔVh = <Vh>-Vh to align their half-activation voltages (Vh) to the mean (0 Ca2+: <Vh> = 155 mV; 70 μM Ca: <Vh> = 135 mV). Then the shifted data were averaged in 25 mV bins (see materials and methods). The mean Qfast-Vs are fit by Boltzmann functions (lines) where the valence z = 0.58 e was constrained to the mean voltage sensor charge determined from fits to individual Qfast-Vs in 0 Ca2+ and 70 μM Ca2+. (E) Mean τgfast-V relationships were determined in 60 mV bins after individual τgfast-V relationships were normalized to peak τgfast and shifted by ΔVh (as determined from Qfast-V relationships in C). The curves are fit with functions of the form τgfast = [α0e−zα/kT + β0e−zβ/kT]−1 where zα= 0.33 e, zβ = −0.22 e and 0 Ca: α0 = 1,020 s−1, β0 = 32,500 s−1; 70 Ca: α0 = 1,220 s−1, β0 = 25,500 s−1. (F) Cg-V relationships measured with admittance analysis in 0 and 70 μM Ca2+ for a single patch indicate a Ca2+-dependent shift in the voltage dependence of fast charge movement. The membrane was clamped with a sinusoidal voltage command (868 Hz, 60 mV peak to peak) superimposed on a 1-s voltage-ramp from −200 to 160 mV. Cg was determined for each cycle of the sin wave. The Cg-V relationships were fit with the derivative of Boltzmann functions: Cg = A*z((1 + e−z(V − Vh)/kT)/(kTez(V − Vh)/kT))2 over voltage intervals where most channels are closed (0 Ca, −160 to 100 mV; 70 Ca, −160 to −10 mV). The 0 Ca2+ data were fit first (A = 1,390, z = 0.61 e, Vh = 108 mV) and the 70 Ca2+ data were fit with identical amplitude and charge (A, z) while Vh was reduced to 75 mV.
Ig evoked by 1-ms pulses to 160 mV in 0 or 70 μM Ca2+ are superimposed in Fig. 5 A. ON gating currents (IgON) decay with a similar but not identical time course in the presence or absence of Ca2+. However, the 0 Ca2+ record can be approximated by a single exponential function (Horrigan and Aldrich, 1999), whereas the 70 μM Ca2+ trace has an obvious slow component (see also Fig. 5 D). In addition, OFF gating currents (IgOFF) recorded at −80 mV after the pulse are slowed substantially in the presence of Ca2+.
Since Ca2+ affects gating current and shifts the voltage dependence of GK and τ(IK), it might appear reasonable to conclude that Ca2+ acts by promoting voltage sensor activation (Diaz et al., 1998). However, a closer examination of gating charge movement reveals that this is not the case. Although gating currents provide a direct assay of voltage sensor activation, they are also influenced by channel opening. Indeed, we have shown previously that mSlo1 Ig exhibits multiple kinetic components reflecting C-C, O-O, and C-O transitions in Scheme I* (Horrigan and Aldrich, 1999). These components must be isolated before conclusions can be drawn about the mechanism of Ca2+ action.
The Ca2+ Dependence of Slow Gating Charge Movement
IgON evoked during a pulse to 160 mV in 0 Ca2+ (Fig. 5 A) consists of a prominent fast component, representing voltage sensor activation while channels are closed, and an additional component that is ∼100-fold slower. The relaxation of the slow component, like IK activation, is limited by the speed of channel opening (Horrigan and Aldrich, 1999) and therefore should exhibit a Ca2+ and voltage dependence similar to that of τ(IK) (Fig. 4 B). To examine the slow component in detail, Ig was measured in response to pulses of different duration to 160 mV in 0 Ca2+ and 70 μM Ca2+ (Fig. 5 B). The total charge moved during each pulse (QP) was determined by integrating IgOFF and is plotted versus pulse duration in Fig. 5 C. These QP time courses, which indicate the kinetics of ON charge movement, are fit with double exponential functions (solid lines). Prominent slow components are observed in both 0 and 70 μM Ca2+ representing 43% and 47% of the steady-state charge movement, respectively. Time-derivatives of the fits to Qp superimpose with IgON (Fig. 5 D) demonstrating that Ig kinetics reflect large slow components of ON charge movement.
Slow charge movement is evident as a distinct component of IgON in 70 μM Ca2+ (dashed line – Fig. 5 D) but not in 0 Ca2+ because its time constant (τgSLOW) is decreased from 4.2 ms in 0 Ca2+ to 0.85 ms in 70 μM Ca2+ (Fig. 5 C). This fivefold change in kinetics produces a fivefold increase in slow gating current amplitude in 70 μM Ca2+. The marked Ca2+-sensitivity of τgSLOW is consistent with the ability of Ca2+ to speed IK activation (Fig. 4 A).
The Voltage Dependence of Slow Gating Charge Movement in 70 μM Ca2+
The properties of slow charge movement in 70 μM Ca2+ are examined in more detail in Fig. 6. Families of Ig evoked after pulses of different duration to various voltages are shown in Fig. 6 A. Time courses of ON charge movement (Qp) determined from these data are plotted in Fig. 6 B and fit with double exponential functions. The time constant of the slow component is plotted against voltage in Fig. 6 C together with τgSLOW at negative voltages, determined from the slow component of OFF charge movement (Horrigan and Aldrich, 1999) (see also Fig. 13 C). Also shown are the mean time constants of IK relaxation (τ[IK]) in 70 μM Ca2+. At both positive and negative voltages the voltage dependencies of τgSLOW and τ(IK) are identical (Fig. 6 C, compare solid and dashed lines), supporting the idea that slow charge movement is limited by channel opening. However, τgSLOW is approximately fourfold slower than τ(IK) at positive voltages and twofold slower at negative voltages. Differences between τ(IK) and τgSLOW were also reported previously in 0 Ca2+ and may reflect the different ionic conditions used to measure IK and Ig (Horrigan and Aldrich, 1999).
Figure 6.
Properties of gating charge movement in 70 μM Ca2+. (A) Families of Ig evoked by depolarizations of different duration to the indicated voltages in 70 μM Ca2+ (HP = −80 mV). (B) Plots of Qp versus pulse duration determined from A and fit with double exponential functions. The 160-mV trace (▾) was obtained from a different patch than the others (•) and was normalized to the 120 mV trace because the steady-state Q-V relationship is saturated for V ≥ 120 mV in 70 μM Ca2+ (see E). (C) τgSLOW-V relationships in 70 μM Ca2+ were determined at positive voltages from the slow component of Qp and at negative voltages from the slow component of QOFF (e.g., Fig. 13 C). Individual data points (Δ) and mean ± SEM (▴) are plotted. The mean τ(IK)-V relationship (○) was fit by exponential functions (solid lines) over voltage ranges corresponding to the τgSLOW data. These fits were then scaled (dashed lines) to match the τgSLOW-V relationships. (D) QP-V relationships determined following brief (0.25 ms) or prolonged (20 ms) pulses (from B) are compared with Qfast-V determined by integrating the fast component of IgON in the same patch (see Fig. 7 A). QP(20)-V and Qfast-V relationships are fit by Boltzmann functions (Qfast: z = 0.68, Vh = 123 mV, QP(20): z = 1.95, Vh = 40 mV). (E) Normalized steady-state Q-Vs (solid symbols) and G-Vs (open symbols) in 0 Ca2+ and 70 μM Ca2+. QSS was determined from 10–20-ms pulses and data from three experiments in each [Ca2+] are plotted. Individual Q-Vs were normalized based on fits to Boltzmann functions. Boltzmann fits to the cumulative data are shown (0 Ca2+: z = 0.59, Vh = 150 mV, 70 μM Ca2+: z = 1.19, Vh = 40 mV). G-Vs (mean ± SEM) were measured and fit as in Fig. 4 C.
Figure 13.
IgOFF components. (A) OFF gating currents recorded at −80 mV in 70 μM Ca2+ following pulses of different duration (0.11–20 ms) to 120 mV decay more slowly as pulse duration increases. IgOFF traces were integrated to obtain QOFF time courses in B. QOFF saturates for pulses of 5 ms or greater duration. (C) OFF kinetics following brief (0.11 ms) or prolonged (5–20 ms) pulses are compared by plotting the quantity QOFF(t)-QOFFSS on a log scale versus time where QOFFSS is the mean value of QOFF(t) for t = 4–5 ms. The 5–20 ms trace is the average of 5, 7, 10, and 20 ms records and is fit by a double exponential function (solid line, qMED = 14.1 fC, τMED = 200 μs, qSLOW = 7.4 fC, τSLOW = 810 μs) with dashed lines representing the two components. The 0.11-ms trace is fit by a triple exponential function (solid line, qFAST = 4.9; fC, τFAST = 25 μs, qMED = 0.6 fC, τMED = 200 μs, qSLOW = 0.4 fC, τSLOW = 810 μs) with a dashed line indicating the fast component. (D) The Ca2+-saturated gating scheme (sub-Scheme IIB*) indicates the origin of the three OFF components which are determined by voltage sensor deactivation when channels are closed (Fast) or open (Medium), or by channel closing (Slow). (E) OFF kinetics for all pulse durations are plotted as in C using the data in B. Dashed lines are triple exponential fits (τFast = 25 μs, τMED = 200 μs, τSLOW = 810 μs). (F) The amplitude of the three OFF components are plotted versus pulse duration and fit by exponential functions with 1.4-ms time constants representing the time course of channel opening.
Ca2+ Alters the Relationship between Q-V and G-V
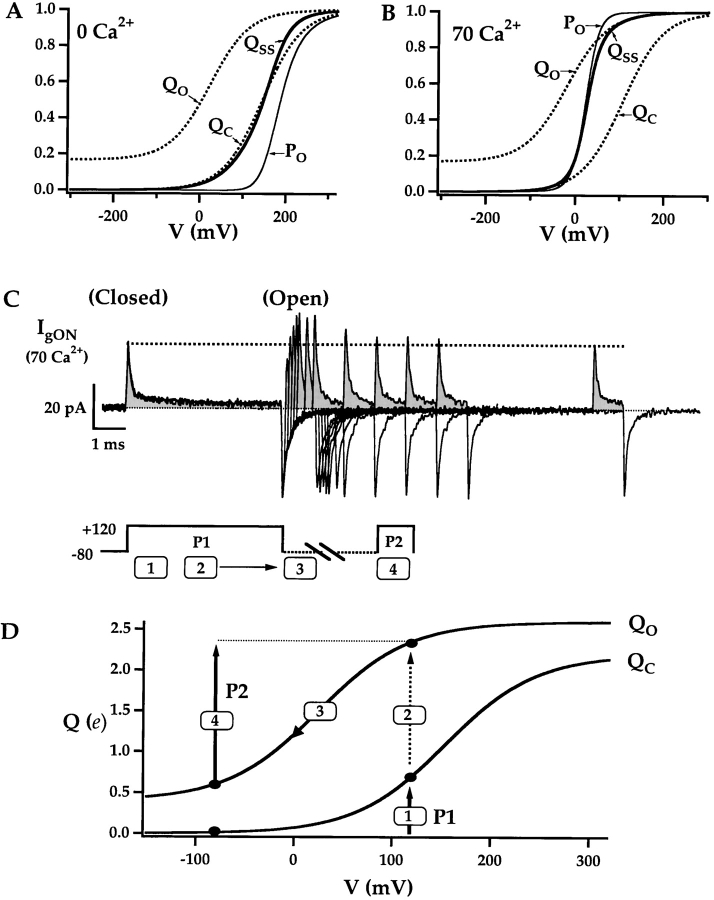
The QP-V curves in Fig. 6 D compare the voltage dependence of charge movement for pulses of different duration (from Fig. 6 B). The Q-V determined with brief 0.25 ms pulses (Qp(0.25)) reflects fast charge movement. The voltage dependence of fast charge movement was also determined over a wider voltage range by integrating the fast component of IgON (Qfast, Fig. 6 D) (Horrigan and Aldrich, 1999). The steady-state QSS-V relationship determined with a 20 ms pulse (Qp(20), Fig. 6 D) is steeper and shifted to more negative voltages than Qfast-V. This difference reflects the voltage dependence of slow charge movement and contrasts with results in 0 Ca2+ where QSS-V and Qfast-V curves are similar in shape (Horrigan and Aldrich, 1999).
The steady-state Q-V and G-V relationships in 0 and 70 μM Ca2+ are compared in Fig. 6 E and indicate that Ca2+-binding changes the relationship between charge movement and open probability. In 0 Ca2+ the Q-V is shallower and activates at more negative voltages than the G-V, exhibiting an approximate fourth-power relationship between the two, as in many voltage-dependent channels (Horrigan and Aldrich, 1999). In 70 μM Ca2+, however, the Q-V and G-V almost superimpose. Calcium has changed the coupling between charge movement and channel opening so that less charge moves, on average, at voltages where most channels are closed. That is, Ca2+ allows channels to open when fewer voltage sensors have been activated.
The Effects of Ca2+ on Voltage Sensor Activation
To determine whether Ca2+ affects voltage sensor activation directly, we compared the fast components of gating charge movement in the presence and absence of Ca2+ (Fig. 7). This analysis shows that the interaction between Ca2+ binding and voltage sensor activation is weak.
Because voltage sensor movement in BK channels is rapid compared with channel opening and closing, the fast component of ON or OFF gating current (Igfast) assays voltage sensor movement while channels remain in either a closed or open conformation (Horrigan and Aldrich, 1999) corresponding to sub-Schemes IIe and IIf, respectively (Fig. 3). For example, IK activates with a delay of ∼100 μs after a voltage step (0 Ca2+, 20°C) (Horrigan et al., 1999). During this period, Ig reflects voltage sensor activation while channels are closed. If voltage sensor activation is a two state process as in Scheme II, then the initial decay of Ig should be exponential, reflecting the R to A transition (Horrigan and Aldrich, 1999). Therefore, Igfast at positive voltages was isolated by fitting the first 100 μs of IgON with an exponential function (Fig. 7, A and B, dashed lines). Because channels are closed, the Ca2+ dependence of Igfast must reflect a direct interaction between Ca2+ binding and voltage sensor movement that is independent of the ability of Ca2+ to alter channel opening.
Ca2+Increases the Equilibrium Constant for Voltage Sensor Activation
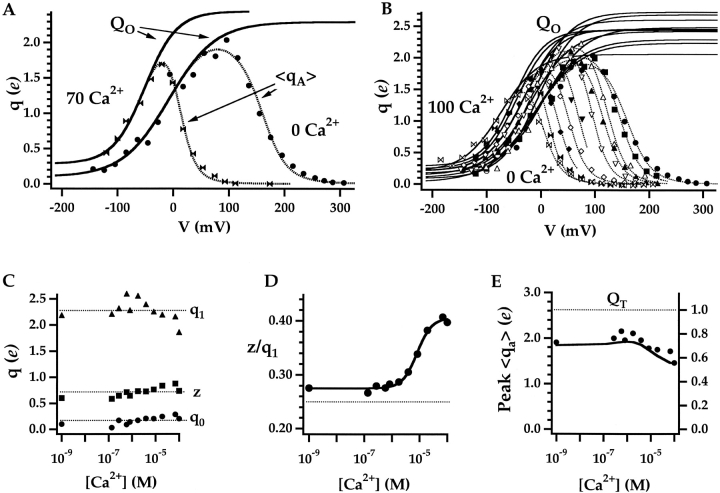
The amount of fast charge movement (Qfast) was estimated by integrating the area under the exponential fit to Igfast, corresponding to the shaded regions in Fig. 7, A and B. Normalized Qfast (mean ± SEM) is plotted against voltage on linear and log scales in Fig. 7, C and D, respectively for 0 Ca2+ and 70 μM Ca2+. The mean half-activation voltage (<Vh(Qfast)>) determined by fitting Boltzmann functions to individual Q-V records was shifted by −20 mV from 155 ± 7 mV in 0 Ca2+ (n = 10) to 135 ± 8 mV in 70 μM Ca2+ (n = 8), whereas the shape of the curves represented by the voltage sensor charge zJ was not appreciably altered (0 Ca2+: zJ = 0.59 ± 0.03 e; 70 μM Ca2+: zJ = 0.57 ± 0.03 e).
Because of the considerable patch-to-patch variation in Vh(Qfast), individual Qfast-V curves were aligned to the mean half-activation voltage in 0 or 70 μM Ca2+ before averaging (Horrigan and Aldrich, 1999)(see materials and methods). The resulting plots (Fig. 7, C and D) are well fit by Boltzmann functions (solid lines) with identical valence (0.58 e) but different half-activation voltages, implying a small increase in the equilibrium constant for voltage sensor activation upon Ca2+ binding, as predicted by Scheme II when E > 1. The good fit by Boltzmann functions is also consistent with the assumption that voltage sensor activation can be described by a single R-A transition and that voltage sensors act independently when channels are closed.
Ca2+ Slows Voltage Sensor Deactivation
The kinetics of fast charge movement are altered slightly by Ca2+, consistent with a small increase in the equilibrium constant for voltage sensor activation. The mean Igfast time constants (τgfast) from Fig. 7, A and B, and similar experiments are plotted versus voltage in Fig. 7 E. At negative voltages, τgfast was determined by fitting the fast component of IgOFF evoked after brief pulses (0.06–0.25 ms) that activate voltage sensors while most channels remain closed. The τgfast-V relationships were fit (Fig. 7 E, solid lines) with bell-shaped functions τgfast = (α + β)−1 as predicted for a two-state model of voltage sensor activation where α = α0exp(−zα/kT) and β = β0exp(−zβ/kT) represent the forward and backward rate constants for voltage sensor activation when channels are closed. The partial charges associated with these rate constants (zα , zβ) were determined from the best fit to the 0 Ca2+data when zL = |zα| + |zβ| was constrained to 0.55 e (Horrigan and Aldrich, 1999). Attempts to constrain zL = 0.58 e as for the Qfast-V fits produced τgfast-V fits that were too steep (unpublished data). Ca2+ shifts the peak of the τgfast-V fit by −20 mV, consistent with the shift in Qfast-V. Ca2+ mainly reduces the backward rate for voltage sensor activation, and therefore increases τgfast at negative voltages, but has little effect on τgfast at positive voltages where the forward rate predominates (e.g., Fig. 7, A and B, at 160 mV).
Quantifying the Interaction between Ca2+-binding and Voltage Sensor Activation
Fig. 7, A–E, show that Ca2+ has a small direct effect on voltage sensor movement. However, Qfast-V relationships in 0 and 70 μM Ca2+ were rarely obtained from the same patch because Ig is small and extensive signal averaging was required. Thus, the change in mean Vh(Qfast) (Δ<Vh(Qfast)> = (<Vh(Qfast)[0 Ca]> − <Vh(Qfast)[70 Ca]>) = 20 mV) may not represent accurately the Qfast-V shift in individual experiments.
A more accurate estimate of the Qfast-V shift (−33 mV) was obtained using admittance analysis. Admittance analysis provides an alternative method for selectively measuring fast gating charge movement (Fernandez et al., 1982). When the membrane is clamped with a sinusoidal voltage command, a nonlinear gating capacitance (Cg) proportional to dQfast/dV can be determined (Horrigan and Aldrich, 1999) (see materials and methods). This technique provides a rapid assay of Qfast(V) such that the effects of 0 Ca2+ and 70 μM Ca2+ can be compared in the same patch. Fig. 7 F shows the Cg-V relationship is altered by Ca2+. To estimate the Qfast-V relationships for closed channels, the Cg-V traces were fit by the derivative of Boltzmann functions with respect to voltage over a voltage range where PO is small (Horrigan and Aldrich, 1999). The 0 Ca2+ fit determined for V < 100 mV (solid line) was shifted along the voltage-axis by −32 mV to fit the 70 μM Ca2+ data from the same patch for V < −10 mV. Similar analysis in several different experiments indicate a mean Qfast-V shift of −33 ± 4 mV (mean ± SEM, n = 6).
The Energetic Relationship between Ca2+-binding and Voltage Sensor Activation
A −33 mV shift in Qfast(V) upon Ca2+ binding can be accounted for by Scheme II if the allosteric factor E is assigned a value of 2.1 (zJ = 0.58 e). Thus, Ca2+-binding increases the R-A equilibrium constant 2.1-fold, altering the energetics of voltage sensor activation by 0.45 kcal mole. This represents a lower limit for the interaction energy because 70 μM Ca2+ may not be sufficient to completely saturate Ca2+ binding sites and the saturating shift in Qfast(V) may therefore be underestimated.
Effects of Ca2+ on Voltage Sensor Activation Cannot Account for the Shift in PO-V
To determine whether the Ca2+-dependent shift in the half-activation voltage of the PO-V relationship (ΔVh[PO]) can be accounted for by an effect of Ca2+ on voltage sensor activation, we examined the behavior of Scheme II when there is no direct interaction between Ca2+ binding and channel opening (i.e., C = 1). If we set E = 2.1 to account for the −33 mV shift in the Qfast-V relationship produced by 70 μM Ca2+ then the model predicts ΔVh(PO) = −19 mV, only 11% of the observed value (ΔVh[PO] = −166 mV, Fig. 4 C). Even if E is infinite, Scheme II cannot reproduce a −166 mV shift when C = 1. If E is large then Ca2+ binding will effectively lock voltage sensors in the activated conformation (i.e., JE >> 1) such that in saturating [Ca2+] the channel can only occupy a single closed and open state with a C-O equilibrium constant of LC4D4, hence:
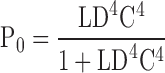 |
and
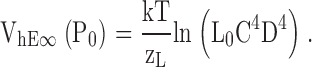 |
(2) |
VhE∝ (PO) represents, for any zL, L0,D, and C in saturating Ca2+, the lower limit of Vh(PO) as E becomes large. Given the parameters (zL, L0,D) used previously (0.4 e, 2 × 10−6, 17) (Horrigan et al., 1999) or in the present study (0.3 e, 0.98 × 10−6, 25), Eq. 2 predicts VhE∝ (PO) = 81 or 113 mV, respectively, when C = 1 as compared with the observed Vh(PO) = 20 ± 8 mV in 70 μM Ca2+. Thus, interaction of Ca2+ binding with voltage sensor activation is insufficient to account for the observed ΔVh(PO), even if E is large. We conclude that Ca2+ binding must affect the C-O transition directly (i.e., C > 1).
Effects of Ca2+ on the C-O Transition
The weak Ca2+-sensitivity of fast charge movement and data presented in Figs. 8 and 9 show that the primary mechanism of Ca2+ action involves direct interaction between Ca2+-binding and the C-O transition.
Figure 8.

The Ca2+ dependence of PO. (A) Scheme II predicts Ca2+ binding may affect the C-O transition directly (solid arrow) or indirectly (dashed arrow) by altering voltage sensor activation. (B) At low voltages Scheme II specifies an MWC-type gating scheme (Sub-Scheme IIc*) that is independent of voltage sensor activation. (C) Inward potassium currents recorded at −120 mV and filtered at 20 kHz from a macro patch in the indicated [Ca2+] demonstrate that PO increases in a Ca2+-dependent manner when voltage sensors are not activated. The corresponding all-point amplitude histograms are plotted in (D) on a semi-log scale and were constructed from 10-s recordings. (E) Similar histograms from another experiment at −160 mV over a wider [Ca2+] range (0, 0.8, 8.2, 102, 1,030 μM) reveal saturation of PO near 100 μM Ca2+.
The ability of Ca2+ to speed IK activation, slow deactivation, increase open probability (Fig. 4), and produce analogous effects on the kinetics and amplitude of slow charge movement (Figs. 5 and 6) are consistent with Ca2+ causing a change in the rate-limiting C-O transition. However, Ca2+ may alter this transition through two different pathways: a direct interaction between Ca2+ binding and channel opening (Fig. 8 A, solid arrow) (C-factor), or an indirect interaction (Fig. 8 A, dashed arrows) involving a Ca2+-dependent increase in voltage sensor activation (E-factor) that, in turn, promotes channel opening (D-factor). To isolate the direct interaction we measured open probability (PO) at very negative voltages in the presence and absence of Ca2+.
Ca2+ Increases PO when Voltage Sensors Are Not Activated
At sufficiently negative voltages, voltage sensors should remain in the resting (R) state even when Ca2+ is bound. The Qfast-V relationships in Fig. 7, C and D, show that the fraction of activated voltage sensors is small (<10−2) for V < 0 mV in 0 or 70 μM Ca2+. With voltage sensors effectively locked in the R conformation, Scheme II reduces to sub-Scheme IIc (Fig. 3), which specifies a 10 state Monod-Wyman-Changeux (MWC)-type gating scheme (Fig. 8 B, sub-Scheme IIc*). Under this extreme condition, channels can open in a Ca2+-dependent manner only through direct interaction between Ca2+ binding and the C-O transition. At negative voltages PO is highly Ca2+ sensitive (Figs. 8 and 9), confirming that a strong interaction exists between Ca2+ binding and channel opening and providing a direct estimate of the allosteric factor C that embodies this interaction.
Fig. 8 C compares IK recorded at −120 mV from a single patch in various [Ca2+]. The corresponding amplitude histograms are superimposed in Fig. 8 D. Although the patch contains hundreds of mSlo1 channels, PO is low in the absence of Ca2+ and activity is observed as the infrequent and brief opening of single channels. Application of Ca2+ causes a large increase in open probability, resulting in multichannel openings. NPO increases 2,130-fold, from 6.1 × 10−4 in 0 Ca2+ to 1.3 in 100 μM Ca2+. Histograms in Fig. 8 E demonstrate for a different patch at −160 mV that the increase in NPO saturates above 100 μM Ca2+.
To characterize the interaction between Ca2+-binding and channel opening in detail, and to confirm that the PO increase in Fig. 8 C can be described by sub-Scheme IIc, channel activity was measured over a range of negative voltages and [Ca2+]. Fig. 9 A plots NPO versus voltage for the patch in Fig. 8 C at many [Ca2+]. The NPO-V relations become weakly voltage dependent at negative potentials, consistent with the assumption that voltage sensors are not activated under these conditions (Horrigan et al., 1999). This limiting behavior is also seen in Fig. 9 B, which plots log(PO) over a wider voltage range at different [Ca2+] from many experiments (mean ± SEM).
Fig. 9, A and B, show that PO increases >1,000-fold in response to Ca2+ at negative potentials. Thus Ca2+ binding strongly affects channel opening through a pathway that does not involve voltage sensor activation. Sub-Scheme IIc predicts:
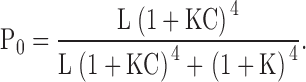 |
Because PO at extreme negative voltages is small (<10−2), even in saturating [Ca2+], this expression can be approximated:
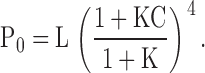 |
(3) |
Since the C-O equilibrium constant (L) is the only voltage-dependent parameter in sub-Scheme IIc, the voltage dependence of PO should be identical to that of L and independent of [Ca2+]. Consistent with this prediction, the NPO-V relations in Fig. 9 A at all [Ca2+] can be fit reasonably by exponential functions (dashed lines) with identical voltage dependencies (e-fold per 84 mV) reflecting the partial charge of L (zL = 0.3 e).
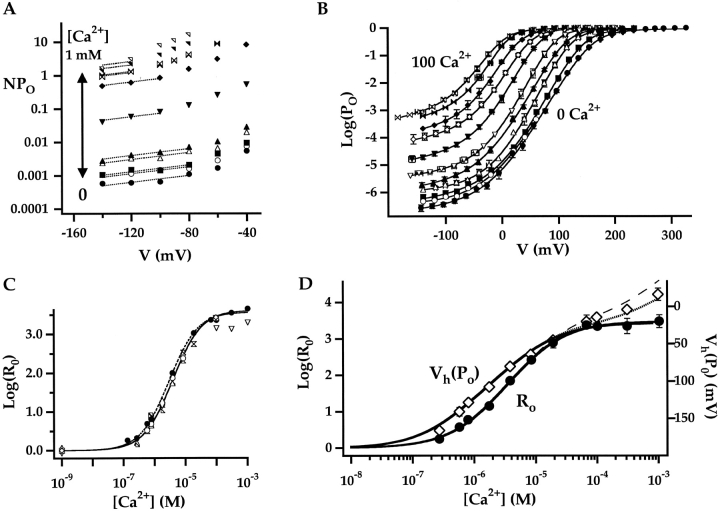
Determining the Parameters Associated with Ca2+-dependent Activation (KD, C)
The parameters that define sub-Scheme IIc (L, KD, C) are well determined by the properties of PO at extreme negative voltages. L can be measured directly in 0 Ca2+ where Eq. 3 reduces to PO = L. Then KD and C can be determined from the Ca2+ dependence of PO (Eq. 3). However, for some experiments (e.g., Fig. 9 A), PO could not be determined because macroscopic currents were large and the number of channels in the patch (N) could not be estimated accurately. Errors in the estimation of N in other experiments may contribute to the variability in PO at negative voltages in Fig. 9 B. Therefore, to evaluate KD and C independent of N, we determined the ratio (RO) of NPO in the presence and absence of Ca2+ where (based on Eq. 3):
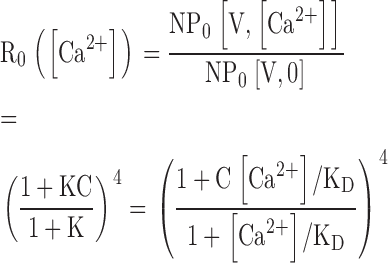 |
(4) |
RO is independent of L as well as N and therefore depends only on Ca2+ binding (KD) and its interaction with channel opening (C).
Fig. 9 C plots RO on a log scale versus [Ca2+] for several experiments. RO is highly reproducible and well fit by Eq. 4 (solid line). According to Eq. 4, RO increases from a minimum of 1 in 0 Ca2+ to a maximum of C4 in saturating Ca2+([Ca2+]sat). Thus, C can be determined from the saturating value of RO (C = [RO([Ca2+]sat)]−4). The dashed line in Fig. 9 C is the fit (C = 7.8 ± 0.1, KD = 8.2 ± 0.5 μM) to the data from Fig. 9 A (solid symbols), representing the only single experiment to span the entire [Ca2+] range. A solid line represents the parameters used in the final model (C = 8, KD = 11 μM). The best fit to mean log(RO) yielded C = 7.4 ± 0.1 and KD = 9.3 ± 0.4 μM (Fig. 9 D, solid line).
The Energetic Relationship between Ca2+ Binding and Channel Opening
The ability of Ca2+ to increase PO at extreme negative voltages shows that Ca2+ binding affects channel opening directly and provides an estimate of the interaction energy between these two processes. A C-fold increase in C-O equilibrium constant where C = 8 indicates that the free energy difference between closed and open increases 1.25 kcal mol− 1 for each Ca2+ bound (ΔΔGCO = 0.6 ln[C]) or 5.0 kcal mol−1 for the Ca2+-saturated condition.
The Ca2+ Dependence of Vh(PO)
Another measure of BK channel gating commonly used to characterize Ca2+ sensitivity is the half-activation voltage of the PO-V relationship (Vh[PO]) (Moczydlowski and Latorre, 1983). Fig. 9 D plots mean values of Vh(PO) and log(RO) against [Ca2+]. For certain gating schemes, a simple relationship between Vh(PO) and log(RO) is expected. For instance, a voltage-dependent MWC scheme (sub-Scheme IIc) predicts ΔVh(PO[Ca2+]) = −(kT/zL) ln(RO) where ΔVh(PO[Ca2+]) = Vh(PO[Ca2+]) − Vh(PO[0]). That is, changes in Vh(PO) and log(RO) should be directly proportional. However, this prediction is not generally valid for Scheme II. And, although the two dose-response curves in Fig. 9 D are similar in shape, differences are evident. First, Vh(PO) is more Ca2+-sensitive than log(RO). That is, when the two curves are scaled as in Fig. 9 D to give identical Y-axis excursions between 0 and 100 μM Ca2+, Vh(PO)-[Ca2+] is left-shifted relative to log(RO)-[Ca2+]. Such a difference is predicted by Scheme II (Fig. 9 D, solid line) because Vh(PO), unlike RO, is measured under conditions where voltage sensors are activated, thereby producing an E-fold increase in Ca2+affinity. Another difference between the dose-response curves in Fig. 9 D is that log(RO) saturates for [Ca2+] ≥ 100 μM, as predicted by Eq. 4, whereas Vh(PO) has a biphasic appearance, beginning to saturate around 100 μM Ca2+ but continuing to change by more than 30 mV between 100 and 1,000 μM Ca2+. The failure of Vh(PO) to saturate has been noted previously (Moczydlowski and Latorre, 1983; Cox et al., 1997a; Cui et al., 1997; Schreiber and Salkoff, 1997) and can be accounted for by the action of Ca2+ at distinct high affinity (micromolar) and low affinity (millimolar) binding sites (dotted line Fig. 9 D) (Shi and Cui, 2001; Zhang et al., 2001).
The apparent saturation of log(RO) in 100–1,000 μM Ca2+ is consistent with RO reflecting the action of Ca2+ at high affinity binding sites. The differential effect of 100–1,000 μM Ca2+ on Vh(PO) and log(RO) has implications for the mechanism of Ca2+ action at low affinity binding sites that will be discussed below.
Scheme II Reproduces the Ca2+ and Voltage Dependence of PO
Thus far we have examined the gating of mSlo1 channels under extreme conditions to isolate interactions involved in Ca2+-dependent activation and to test a general allosteric gating mechanism (Scheme II). Our results show that the interaction between Ca2+ binding and channel opening is strong and independent of voltage sensor activation. The interaction between Ca2+ binding and voltage sensor activation is weak and independent of channel opening.
Together with previous analysis of the voltage-dependent gating of unliganded channels (Horrigan and Aldrich, 1999; Horrigan et al., 1999) these results support Scheme II. However, an important test of the model is its ability to describe the combined effects of Ca2+ and voltage over a broad range of conditions. In general, the Ca2+ and voltage dependence of PO will depend on all the parameters in Scheme II (Eq. 1). Thus, by fitting PO(V,[Ca2+]) it is theoretically possible to test the model and define its parameters. Excellent fits to the PO-V relationships for [Ca2+] ≤ 100 μM were obtained (Fig. 10 A) when L0 and zL were set to 2 × 10−6 and 0.4 e, respectively, based on previous estimates (Horrigan et al., 1999) and all other parameters were allowed to vary (Table II , Fit A). However several parameters in addition to L0 and zL are poorly constrained by this procedure and the ability of Eq. 1 to fit PO is an inadequate test of Scheme II. Although the values of zJ (0.55 e), Vh(J) (154 mV), and D (18.4) obtained from the fit are similar to those estimated previously from 0 Ca2+ data (zJ = 0.55 e, Vh(J) = 145 mV, and D = 17) (Horrigan et al., 1999), the values of C (2.4) and KD (32 μM) are inconsistent with the log(RO)-[Ca2+] relationship (Fig. 10 B, Fit A). And the value of E (31) predicts a −158 mV shift in the QFast-V relationship upon Ca2+ binding, much larger than the observed −33 mV shift in 70 μM Ca2+.
Figure 10.
Fitting steady-state data with Scheme II. (A) Mean PO-V relationships in different [Ca2+](in μM: 0 (•), 0.27 (▪), 0.58 (▵), 0.81 (▴), 1.8 (▿), 3.8 (▾), 8.2 (⋄), 19 (♦), 68 ( ), 99 (
), 99 ( )) were fit by Scheme II by holding L0, zL constant and allowing the other parameters to vary (Fit A, Table II). (B) Mean log(RO)-[Ca2+] relationship (symbols) is compared with predictions of Scheme II based on different fits to the PO data (Table II, Fits A, B, and C). (C) Mean PO (C1) and log(PO) (C2) are plotted versus voltage for different [Ca2+] (symbols) and are fit by Scheme II (Table II, Fit B). The linear and log transformed data were fit simultaneously using a weighting function to compensate for the greater amplitude range of log(PO). (D) Scheme II reproduces (lines) the observed change in relationship between mean steady-state Q-V and PO-V relationships (Table II, Fit B parameters). (E) Gating schemes for unliganded and Ca2+-saturated channels illustrate the changes in equilibria induced by Ca2+ binding. By increasing the C-O equilibrium constants Ca2+ has the effect of changing the primary activation pathway, accounting in part for the altered relationship between Q-V and G-V.
)) were fit by Scheme II by holding L0, zL constant and allowing the other parameters to vary (Fit A, Table II). (B) Mean log(RO)-[Ca2+] relationship (symbols) is compared with predictions of Scheme II based on different fits to the PO data (Table II, Fits A, B, and C). (C) Mean PO (C1) and log(PO) (C2) are plotted versus voltage for different [Ca2+] (symbols) and are fit by Scheme II (Table II, Fit B). The linear and log transformed data were fit simultaneously using a weighting function to compensate for the greater amplitude range of log(PO). (D) Scheme II reproduces (lines) the observed change in relationship between mean steady-state Q-V and PO-V relationships (Table II, Fit B parameters). (E) Gating schemes for unliganded and Ca2+-saturated channels illustrate the changes in equilibria induced by Ca2+ binding. By increasing the C-O equilibrium constants Ca2+ has the effect of changing the primary activation pathway, accounting in part for the altered relationship between Q-V and G-V.
TABLE II.
Steady-State Parameters
| L0 | zL | zJ | Vh(J) | KD | C | D | E | |
|---|---|---|---|---|---|---|---|---|
| e | e | mV | μM | |||||
| Fit A | 2 × 10−6 | 0.4 | 0.55 | 154 | 32 | 2.4 | 18.4 | 31 |
| Fit B | 9.8 × 10−7 | 0.3 | 0.58 | 150 | 11 | 8 | 25 | 2.4 |
| Fit C | 9.8 × 10−7 | 0.3 | 0.58 | 150 | 11 | 11 | 25 | 1 |
One reason why the PO fit fails to constrain L0, zL, C, KD is that it places little weight on the data at extreme negative voltages where PO is small. These parameters are better constrained by fitting log(PO). Obviously the fit also excludes information available from gating currents. The final parameters assigned to Scheme II (Table II, Fit B) are a compromise determined by constraining many of the parameters within a restricted range and then fitting both PO and log(PO) simultaneously in an attempt to weigh equally the data at extreme negative voltage and near Vh(PO). Fig. 10 C plots PO (Fig. 10 C1) and log(PO) (Fig. 10 C2) together with the predictions of Scheme II (lines). The parameters were restricted as follows:
Vh(J), the voltage where J = 1 (Vh(J) = (kT/zJ) ln(J0)), was restricted to a range from 145 to 155 mV. The upper limit corresponds to the half activation voltage of Qfast-V determined from gating currents in 0 Ca2+. The lower limit is based on previous analysis that suggests Vh(J) may be reduced slightly under the ionic conditions used to measure PO (Horrigan and Aldrich, 1999).
zJ, the voltage sensor charge was set to 0.58 e representing the mean value determined from Boltzmann fits to Qfast-V relationships in 0 and 70 μM Ca2+.
C and KD were set to 8 and 11 μM, respectively, based on a fit to the RO-[Ca2+] data where C was fixed and KD allowed to vary (Fig. 9 C). C was chosen as the maximum value judged consistent with the amplitude range of RO because higher C consistently produced better fits to PO(V,[Ca2+]) by least-squares criteria. In addition, since C and E both contribute to the Ca2+-dependent shift in Vh(PO), higher values of C allowed E to be reduced to a value (2.4) consistent with gating current measurements (see below). Allowing KD to vary during the PO-V fits consistently produced higher values (15–16 μM) than indicated by the RO-[Ca2+] data (11 μM). This may reflect the contribution of low affinity Ca2+-binding sites estimated by Zhang et al. (2001) to shift Vh(PO) by −7 mV in 100 μM Ca2+. When PO-V data in 100, 70, 21, and 10 μM Ca2+ were shifted by −7, −5, −2, −1 mV to correct for the effect of low affinity sites, a lower KD of 12 μM was obtained from PO-V fits, consistent with the RO data.
E was constrained to a range of 2.1–2.7 indicated by a 33–43 mV shift in the charge distribution for closed (QC) and open (QO) channels, respectively, in 70 μM Ca2+. QC was determined from gating currents when channels are closed (i.e., Qfast, Fig. 7). QO was estimated from the limiting voltage dependence of log(PO) as described later (see Fig. 12) .
Figure 12.
Estimating QO from the limiting voltage dependence of PO. (A) <qa>-V relationships representing the derivative with respect to voltage of mean log(PO) (Eq. 7) are plotted for 0 and 70 μM Ca2+. Dashed lines are smoothing functions determined from fits to mean log(PO) in Fig. 9 B. QO-V relationships (solid lines) were estimated by fitting the smoothing functions with Eq. 12 at voltages where QC and PO are small (0 Ca2+: V ≤ 0 mV; 70 μM Ca2+: V ≤ −50 mV). (B) QO is estimated as in A for all Ca2+ (0 to 100 μM). <qa> was determined from mean log(PO) in Fig. 9 B after excluding some data points that represent single measurements at the most positive or negative voltages. (C) Parameters for the QO-V fits are plotted versus [Ca2+]. Dashed lines indicate mean values. (D) The ratio of the valence (z) and amplitude (q1) of the voltage-dependent component of QO is plotted versus [Ca2+] and fit by a Hill equation (n = 1.6, K1/2 = 8.1 μM). (E) Peak <qa> determined from smoothing functions in (B) (symbols) is reproduced by Scheme II (solid line; Table II, Fit B parameters). Peak <qa> decreases with increasing [Ca2+] and underestimates total gating charge assigned to Scheme II (QT, dashed line).
zL, the partial charge associated with the C-O transition was adjusted to 0.3 e based on the limiting slope of log(PO) and the voltage dependence of τ(IK). Larger values of zL were clearly inconsistent with the weak voltage dependence of log(PO) at extreme negative voltages, whereas smaller values, as discussed later, appear inconsistent with the voltage dependence of τ(IK) at extreme negative and positive voltages.
D,L0 were allowed to vary freely. L0 is highly constrained by PO at negative voltages in 0 Ca2+ where Eq. 3 reduces to  . D, as discussed later, is constrained by the shape of the log(PO)-V relationship.
. D, as discussed later, is constrained by the shape of the log(PO)-V relationship.
Using the above constraints Scheme II does a reasonable job of reproducing PO over a broad range of conditions while also reproducing results obtained under extreme conditions. Log(PO) is well fit at all [Ca2+] (Fig. 10 C2) although fits to PO tend to be too steep in intermediate [Ca2+] (e.g., 10 μM). The allosteric factors C = 8 and E = 2.4 demonstrate that a combination of strong direct and weak indirect interactions between Ca2+ binding and channel opening is sufficient to account for the Ca2+-dependent shift in Vh(PO) (Fig. 9 D).
We also attempted to fit the data with E = 1 (Table II, Fit C) to determine whether weak interaction between Ca2+ binding sites and voltage sensors is necessary for obtaining an adequate fit. In this case, increasing C from 8 to 11 was sufficient to reproduce the Ca2+-dependent shift in Vh(PO) (unpublished data). However, such an increase in C is inconsistent with the Ca2+ dependence of RO, predicting a larger increase in RO than is actually observed (Fig. 10 B, Fit C). Thus, a small but significant interaction between Ca2+ binding and voltage sensor activation is required to account for the Ca2+ dependence of PO.
The parameters used to fit steady-state PO (Table II, Fit B) also account for the change in shape and shift of the steady-state Q-V relation along the voltage axis in response to 70 μM Ca2+ as shown in Fig. 10 D. Ca2+ alters the relationship between open probability and charge movement by increasing the C-O equilibria, thus increasing the likelihood that channels will open at voltages where voltage sensors are not activated. Thus, the predominant activation pathway is altered by Ca2+ as illustrated in Fig. 10 E.
In the following section (Figs. 11–15) we test Scheme II further and refine the parameters by fitting the model to additional features of the ionic and gating current data.
Figure 11.
Charge distribution of open and closed channels. Normalized plots of QC, QO, PO, and QSS versus voltage predicted by Scheme II (Table II, Fit B parameters) are compared in (A) 0 Ca2+ and (B) 70 μM Ca2+. (C) A double pulse experiment in 70 μM Ca2+ shows that peak Ig evoked by the second pulse (P2) is larger than that evoked by the first pulse (P1) when the interpulse interval is brief. This behavior is consistent with the predicted difference between QC and QO as illustrated by the numbered arrows in (D) corresponding to the pulse intervals in C. Arrows 1 and 4 represent the amount of charge that should move in response to a step from −80 to 120 mV if channels are closed or open, respectively.
Figure 15.
The Ca2+ and voltage dependence of C-O rate constants. (A) Mean τ(IK)-V relationships in 0 and 68 μM Ca2+ are fit by Scheme I* (unliganded, Fig. 1 B) and sub-Scheme IIb* (Ca2+ saturated, Fig. 4 E), respectively, with the assumption the voltage sensors are equilibrated (Eq. 13). Each model contains five C-O transitions whose equilibrium constants are defined by the steady-state parameters (Table II, Fit B), increasing D-fold (25-fold) for each voltage sensor activated. (B) The backward rate constants for the C-O transitions (γij) determined from the fits are plotted (symbols) versus the number of activated voltage sensors (i). The forward rate constants (δij) are specified by γij and the equilibrium constants. Dashed lines in A and open symbols B represent fits where γij was allowed to vary freely. Similar fits (solid lines [A] and closed symbols [B]) were obtained when γij was constrained to decrease by at most D−1-fold when a voltage sensor activates such that δij increases monotonically. (C) Mean τ(IK)-V at all [Ca2+] (0–100 μM) are fit by Scheme II using the steady-state parameters in Table II (Fit B) and a two-barrier transition state model to describe the C-O rate constants (dashed lines, Eq. 20; solid lines, Eq. 21). The corresponding values of γij are plotted in B (lines). (D) τ(IK)-V relationships (mean ± SEM) in 0, 0.81, and 68 μM Ca2+ are compared with fits (Eq. 21). (E) Transition state diagrams illustrate how a model with two transition states (T1, T2) can account for a complex relationship between Ca2+, voltage, and closing rates. Free energy (ΔG) relative to the open state is plotted against the C-O reaction coordinate for the unliganded (E1) and Ca2+-saturated (E2) case. Solid lines represent the transition when voltage sensors are not activated (i = 0). Dashed lines indicate the additive perturbations produced by activation of 1–4 voltage sensors. The relative energies of C versus O and T1 versus T2 and the perturbations to each of these states produced by Ca2+ binding and voltage sensor activation were determined from the fits in (C) using Eq. 21. The ΔG between the open and transition states is not determined by the data and was adjusted arbitrarily. (E1) In the unliganded state when voltage sensors are not activated (solid line) the transition rate is dominated by a single barrier (T1). Voltage sensor activation produces a small perturbation to T1 ( = 0.041) such that γij is not sensitive to voltage sensor activation for i = 1–3. However, T2 is strongly perturbed (
= 0.041) such that γij is not sensitive to voltage sensor activation for i = 1–3. However, T2 is strongly perturbed ( = 1) such that it becomes rate-determining when i = 4 (starred barrier). (E2) Ca2+ binding also perturbs T2 more than T1 (
= 1) such that it becomes rate-determining when i = 4 (starred barrier). (E2) Ca2+ binding also perturbs T2 more than T1 ( = 0.7;
= 0.7;  = 0.089) such that when the channel is Ca2+ saturated (thick solid line) T2 becomes rate determining when i = 2–4 (starred traces).
= 0.089) such that when the channel is Ca2+ saturated (thick solid line) T2 becomes rate determining when i = 2–4 (starred traces).
The Reciprocity of Allosteric Interactions
A fundamental prediction of an allosteric mechanism (Scheme II) is the reciprocal nature of sensor–gate interactions. That is, if Ca2+ and voltage affect channel opening then channel opening should also affect Ca2+ binding and voltage sensor movement. Specifically, opening should increase the affinity of binding sites for Ca2+ C-fold, and increase the equilibrium constant for voltage sensor activation D-fold (Fig. 3, compare sub-Schemes IIe and IIf). The first prediction cannot be tested directly because we observe the binding of Ca2+ indirectly via its effects on channel function. As discussed later, the differential sensitivity of τ(IK) to Ca2+ observed at extreme positive and negative voltages is consistent with an increase in Ca2+ binding affinity upon channel opening. Technical factors prevent detailed comparison of gating charge movement in open versus closed channels. However several lines of evidence (below) confirm that channel opening affects voltage sensor movement in a manner predicted by Scheme II.
Channel Opening Promotes Voltage Sensor Activation
The change in relationship between QSS-V and PO-V (Fig. 10 D) reflects in large part a marked steepening of QSS-V in 70 μM Ca2+. This increase in apparent voltage dependence seems surprising given that the shape of the PO-V and Qfast-V relationships are relatively unaffected by Ca2+ (Figs. 4 C2 and 7 C). However, the steepening of QSS-V is explained by the change in voltage sensor activation that is predicted to accompany channel opening.
According to Scheme II, the steady-state charge distribution for closed channels (QC; approximated by Qfast in Fig. 7) is determined by the R-A equilibrium constant (J in 0 Ca2+; JE in saturating Ca2+) and by the charge associated with voltage sensor activation (zJ):
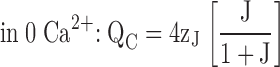 |
 |
When channels open, charge associated with the C-O transition (zL) moves and the R-A equilibrium constant increases D-fold, producing an open channel charge distribution:
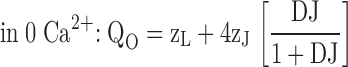 |
(5a) |
 |
(5b) |
Channel opening increases the R-A equilibrium constant, thus shifting the QO-V to more negative voltages than QC-V with little change in shape (Fig. 11, A and B).
The steady-state charge distribution (QSS) can be expressed in general as a sum of QC and QO, weighted by open probability:
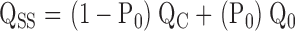 |
(6) |
In 0 Ca2+, PO is small at voltages where QC and QO increase, so Eq. 6 can be approximated:
 |
QSS deviates from QC only at high voltages where PO becomes significant and, even then, the QSS-V relationship is only slightly steeper than QC-V (Fig. 11 A). In the presence of 70 μM Ca2+, however, the relationship between QC, QO, and PO changes (Fig. 11 B). The PO-V relation shifts to more negative voltages while QC-V and QO-V are relatively unaffected. Consequently, PO increases at voltages where QC is small (QC << 1) and Eq. 6 can be approximated:
 |
In addition, Scheme II predicts QO will increase at more negative voltages than PO and is relatively constant over the voltage-range where PO increases most steeply. Thus QSS is roughly proportional to PO. That QSS in 0 and 70 μM Ca2+ is proportional to QC and PO respectively accounts for the marked change in shape of the QSS-V relationship upon application of Ca2+.
Although the QSS-V relationship does not reveal the voltage dependence of QO in detail, the observation that QSS-V and PO-V superimpose in 70 μM Ca2+ (Fig. 10 D) is consistent with QO increasing over a more negative voltage range than PO, as predicted by Scheme II. Additional evidence presented below defines the QO-V relationship in more detail.
Channel Opening Produces “Charge Mobilization”
A double pulse gating current experiment in Fig. 11 C provides additional evidence that gating charge distribution differs for open and closed channels. In the presence of 70 μM Ca2+, the membrane voltage was stepped to 120 mV for 5 ms, returned to the holding potential (−80 mV) for various durations (0.1–5 ms), and then stepped again to 120 mV. The amplitude of IgON evoked by the first and second pulse (P1, P2) are equal when the interpulse interval is ≥3 ms (Fig. 11 C, dashed line). However, with shorter intervals (0.25–2 ms) peak IgON increases significantly during P2. A maximal increase in peak IgON of 55% is observed together with a 43% increase in Qfast using an interpulse interval of 0.5 ms.
The increase in Qfast observed during the second pulse can be understood in terms of the relationship between QO and QC illustrated in Fig. 11 D. Initially, channels are closed at −80 mV, so Qfast during P1 is determined by the voltage dependence of QC, and a pulse to 120 mV moves less than half of the total charge rapidly (Fig. 11 D, arrow 1). However, a 5-ms pulse to 120 mV in 70 μM Ca2+ is sufficient to activate mSlo1 channels maximally (Fig. 4 C) and to saturate the QSS-V relationship (Fig. 6 E). Therefore, additional charge must move as channels open. This slow component of QON can be described in terms of a transition between QC and QO (Fig. 11 D, arrow 2). After P1, repolarization to −80 mV moves voltage sensors rapidly back to their resting state while many channels remain open (Fig. 11 D, arrow 3) and channels subsequently close. With an interpulse interval of 3 ms or more, most channels are closed at the beginning of P2, so Ig evoked by P1 and P2 are identical. However, as the interpulse interval is reduced, some channels remain open at the beginning of P2 and an increase in Qfast is observed because a step from −80 to 120 mV produces a greater increase in QO (Fig. 11 D, arrow 4) than it does in QC (arrow 1). That is, P2 evokes charge movement among open states (O-O), whereas P1 only evokes charge movement among closed states (C-C).
If all channels remain open and all voltage sensors are deactivated during the interpulse interval, then a 2.5-fold increase in Qfast would be expected during P2 (Fig. 11 D, compare arrows 1 and 4). However, with a 0.5-ms interpulse interval only 37% of channels should remain open (PO = 0.375) based on IK deactivation kinetics (τ[IK] = 0.51 ± 0.04 ms at −80 mV). In addition, 92% of the voltage sensors will reach the resting state (PR = 0.92) based on the time constant of voltage sensor deactivation in open channels (τ = 0.2 ms at −80 mV, see Fig. 13). Therefore, a 49% increase in Qfast is predicted for P2 similar to the observed 43% increase (Qfast[P2] = [QO(+120) − QO(−80)] PO PR + [QC(+120) − QC(−80))] (1 − PO) = [(2.5)(0.375)(0.92) + (1 − 0.375)]Qfast[P1] = 1.49 Qfast[P1]).
The increase in charge mobilization revealed by the double pulse experiment is consistent with QO being shifted to more negative voltages than QC and further demonstrates that QO increases from −80 to 120 mV. This finding is completely consistent with the proposed allosteric relationship between voltage sensor activation (charge movement) and channel opening.
The Limiting Voltage Dependence of PO Reveals the QO-V Relationship
To determine the voltage dependence of QO in more detail we analyzed the voltage dependence of PO. The mean activation charge displacement <qa> can be defined in terms of the logarithmic slope of the PO-V relationship (Sigg and Bezanilla, 1997):
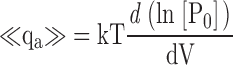 |
(7) |
Sigg and Bezanilla (1997) derived a general expression for <qa> in terms of the free energy and conductance of each state in an arbitrary gating scheme:
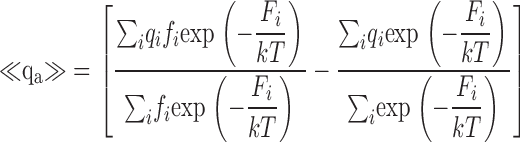 |
(8) |
where Fi = Gi + qiV, and qi, Gi, and fi are the charge displacement, free energy at V = 0, and fractional conductance (0 ≤ fi ≤ 1) respectively, of state i.
Rearrangement of Eq. 8 yields an expression for QO in terms of <qa>, PO, and QC (Eq. 9, below) provided that all open states have the same conductance (i.e., fi = 1 for open states and fi = 0 for closed states). This appears to be a reasonable assumption for BK channels, since subconductance events are rare (Barrett et al., 1982; Rae et al., 1990) or brief (Ferguson et al., 1993). Thus,
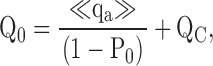 |
(9) |
where
 |
Combining Eqs. 7 and 9 yields an expression for QO in terms of PO and QC:
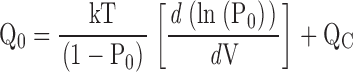 |
(10) |
Thus, in theory, QO can be determined in a model-independent manner at any voltage and [Ca2+] by measuring PO and QC (i.e., Qfast). However, several experimental factors limit the conditions under which Eq. 10 can be evaluated accurately. First, at high voltages and/or [Ca2+] the PO of BK channels approaches unity (Rothberg and Magleby, 2000), such that the first term of Eq. 10 becomes large and sensitive to errors in PO. That our experiments measure normalized open probability (PO/POMAX) rather than absolute PO introduces an additional systematic error when PO approaches POMAX. Second, the method of estimating QC is model dependent. The voltage dependence of QC can be determined from the Qfast-V relationship (Fig. 7), if we assume, as in Scheme II, that Qfast represents the movement of four independent and identical voltage sensors. In this case the maximal amplitude of QC is 4zJ, where zJ, the voltage sensor charge, can be determined by fitting the Qfast-V relationship to the Boltzmann distribution.
QO can be determined in a model-independent manner that is insensitive to the use of normalized PO if we restrict our analysis to conditions where both PO and QC are small. If PO << 1, Eq. 10 reduces to:
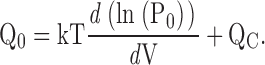 |
If, in addition, QC << QO, then QO can be approximated by the mean activation charge displacement:
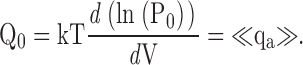 |
(11) |
According to Scheme II, QO should increase to roughly half of its maximal value before QC or PO become significant (i.e., >10−1)(see Fig. 11, A and B). Thus, over a range of negative voltages, the QO-V relationship can be determined solely from the voltage dependence of PO. Since Eq. 11 is insensitive to the scaling of PO, normalized PO or NPO can be used without introducing additional errors. Since Eq. 11 is independent of QC, evaluation of QO is not restricted to those [Ca2+] where gating currents have been measured.
Fig. 12 A plots <qa> determined from mean PO in 0 and 70 μM Ca2+ (Fig. 9 B). The <qa>-V relations are bell-shaped and are expected to approximate the QO-V relation only at negative voltages where QC and PO are small and Eq. 11 is valid (i.e., V < ∼0 in 0 Ca2+, V < −50 in 70 μM Ca). The QO-V relationships are estimated (Fig. 12 A, solid lines) by fitting the foot of the <qa>-V curves with functions of the form:
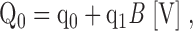 |
(12) |
where 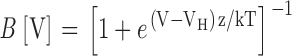 is a Boltzmann function characterized by a half-activation voltage (Vh) and gating valence (z), whereas q0 and q1 represent the amplitudes of the voltage-independent and voltage-dependent components of QO, respectively. The prediction of Scheme II (Eq. 5) can be expressed in terms of Eq. 12 when q0 = zL, q1 = 4zJ, z = zJ and Vh = (kT/zJ)ln(DJ0) in 0 Ca2+ or Vh = (kT/zJ)ln(DEJ0) in saturating Ca2+.
is a Boltzmann function characterized by a half-activation voltage (Vh) and gating valence (z), whereas q0 and q1 represent the amplitudes of the voltage-independent and voltage-dependent components of QO, respectively. The prediction of Scheme II (Eq. 5) can be expressed in terms of Eq. 12 when q0 = zL, q1 = 4zJ, z = zJ and Vh = (kT/zJ)ln(DJ0) in 0 Ca2+ or Vh = (kT/zJ)ln(DEJ0) in saturating Ca2+.
<qa> is very sensitive to experimental discontinuities in the PO-V relationship. Consequently, increased variation in <qa> is observed near the potassium reversal potential (0 mV), where PO was not measured, and near the peak of the <qa>-V relationship corresponding to the transition between microscopic and macroscopic PO measurements. These local regions of increased variability can strongly influence QO-V fits, especially the estimated amplitude of QO (q1 in Eq. 12). Therefore, to limit the effects of local variation, the <qa>-V relationships were fit first with approximating functions (Fig. 12, A and B, dashed lines) to provide smoothly varying curves through the data. The QO-V relationships were then determined from the foot of these curves rather than from individual data points. The approximating functions represent the derivative with respect to voltage of fits to the mean log(PO)-V data (Fig. 9 B, solid lines) using a 10-state gating scheme (Scheme I*, Fig. 1 B) where all the parameters (L0, zL, J0, zJ, D) were allowed to vary freely at each [Ca2+]. In this case, Scheme I was used simply to reproduce the shapes of the log(PO)-V and <qa>-V relations; the parameters have no mechanistic meaning. Although fits produced in this way appear to be excellent, it is possible that the use of a particular approximating function biases the QO-V fits. Similar QO-V curves were obtained when the approximation function was determined by smoothing spline interpolation (Reinsch, 1967)(unpublished data). However, this alternative method required subjective adjustment of smoothing parameters at each [Ca2+], thus fits to Scheme I* based on least squares criteria were more consistent.
QO Confirms That Channel Opening and Ca2+ Binding Increase the Voltage Sensor Equilibrium
As predicted by Scheme II, the QO-V relationship is similar in shape to the QC-V relationship but shifted to more negative voltages, consistent with an increase in the R-A equilibrium constant upon channel opening. In 0 Ca2+, QO-V is characterized by a valence z = 0.60 e (Fig. 12 A) indistinguishable from that estimated for the QC-V relationship (zJ = 0.59 ± 0.03 e, 0 Ca2+; zJ = 0.57 ± 0.03 e, 70 Ca2+). The amplitude of the voltage-dependent component of QO (q1 =2.19 e, 0 Ca; q1 = 2.16 e, 70 μM Ca) is also similar to that predicted by Scheme II (4zJ = 2.32 e). Finally, Vh(QO) shifts by −43 mV in 70 μM Ca2+, similar to the −33 mV shift in QC determined from admittance analysis (Fig. 7 F). Thus, the limiting voltage dependence of PO confirms that Ca2+ binding has a small direct effect on voltage sensor movement.
To examine further the Ca2+ dependence of QO, <qa> was plotted in different [Ca2+] from 0 to 100 μM (Fig. 12 B) and QO-V was determined (solid lines) as in Fig. 12 A. The QO-V relations shift along the voltage axis in a Ca2+-dependent manner while maintaining similar shapes and amplitudes. The parameters q0, q1, and z derived from the QO-V fits are plotted against [Ca2+] in Fig. 12 C. Mean values of each parameter are indicated by dashed lines (q0 = 0.17 ± 0.02, q1 = 2.28 ± 0.06, z = 0.72 ± 0.03).
Ca2+ Alters the Apparent Voltage Dependence of Channel Opening and Voltage Sensor Activation
Although the results in Fig. 12, A and B, are generally consistent with Scheme II, several details are not. Ca2+ appears to increase both the steepness of the QO-V relationship (z) and the amplitude of the voltage-independent component (q0) (Fig. 12 C). According to Scheme II these parameters, reflecting the voltage dependence of channel opening (zL) and voltage sensor activation (zJ), respectively, should be Ca2+ independent (compare Eqs. 5a and b). Below, we consider refinements to Scheme II that could conceivably account for these results. However, given the possibilities for measurement error in QO and the failure of other types of data to confirm a Ca-dependent change in zL or zJ, the necessity of altering Scheme II is questionable.
Z or q0 might increase with Ca2+ if Ca2+ binding sites are displaced through the membrane electric field when mSlo1 channels change conformation. That is, Ca2+ ions might contribute to the gating charge associated with voltage sensor activation (zJ) or channel opening (zL). However, several lines of evidence argue against this possibility. In the case of voltage sensor activation, Qfast-V relationships in 0 and 70 μM Ca2+ (Fig. 7 C) are characterized by similar valence (0.59 e, 0.57 e), indicating that zJ in closed channels is not altered by Ca2+-binding. Similarly, the voltage-dependent component of QO (q1), representing the total voltage sensor charge when channels are open, is relatively unaffected by Ca2+ (Fig. 12 C). In the case of the C-O transition, IK kinetics appear inconsistent with a large Ca2+-dependent change in zL, suggesting that q0 in low Ca2+ may be underestimated (see below).
Although the QO-V relationships in Fig. 12 B are similar to one another, they are derived from PO measurements that differ by three orders of magnitude (Fig. 9 B). It is conceivable that the apparent Ca2+-dependence of z and q0 reflects systematic measurement errors correlated with a large Ca2+-dependent increase in PO. Although a particular source of error is not evident, the variance of log(PO) is greatest in low Ca2+ and it is likely that sources of error that contribute to PO in low Ca2+ are negligible in high Ca2+. One potential source of error in q0, evident from Fig. 12, A and B, is that the data do not in general extend to sufficiently negative voltages to achieve saturation of <qa>. Thus, q0 is extrapolated from a Boltzmann fit to <qa>-V and is not measured directly.
A possible mechanism for the Ca2+ dependence of z is that Ca2+ binding increases the cooperativity of voltage sensor activation. Cooperativity could increase the steepness of QO-V (z) without altering the total open channel charge (q1). In the extreme case that voltage sensor activation is fully cooperative (i.e., concerted), we expect z/q1 = 1; in contrast, an independent mechanism such as Scheme II predicts z/q1 = 0.25. Fig. 12 D plots the ratio z/q1 against [Ca2+]. From 0 to 1 μM Ca2+, z/q1 remains relatively constant with a mean value 0.28 ± 0.003, consistent with an independent mechanism (Fig. 12 D, dashed line). But z/q1 increases with [Ca2+] to a maximum of 0.41. The data are fit by a Hill equation (solid line) with n = 1.6 and K1/2 = 8.1 μM, similar to the KD determined from the RO-[Ca2+] relationship. These results are consistent with the idea that Ca2+ binding increases the cooperativity of voltage sensor activation. Cooperativity might also explain why the PO-V relationships in high Ca2+ tend to be steeper than predicted by Scheme II (Fig. 10 C).
The Maximum Voltage Dependence of Po Underestimates Total Gating Charge in a Ca2+-dependent Manner
For channels containing a single open state, <qa> is expected to attain a maximum at limiting negative voltages. This “limiting slope” indicates the total gating charge (QT) associated with channel activation representing the sum of charges associated with voltage sensor activation and channel opening (Almers, 1978; Sigg and Bezanilla, 1997). However, in mSlo1 channels the limiting and maximal values of <qa> are different and neither provides a direct measure of QT. The limiting slope merely indicates the charge associated with the C-O transition (zL). And QT, representing the maximum amplitude of QO, is always greater than peak <qa> (Fig. 12, A and B). Since QT must be estimated in a model-dependent manner by fitting QO-V to the foot of the <qa>-V relationship it is of interest to determine to what extent peak <qa> underestimates QT. Fig. 12 E plots peak <qa> (solid symbols) determined from Fig. 12 B against [Ca2+] and demonstrates that the maximal voltage dependence of PO varies with [Ca2+], attaining a maximum (∼2.1 e) at intermediate [Ca2+] and a minimum (∼1.5 e) at high [Ca2+]. The allosteric model (Scheme II) reproduces this behavior (Fig. 12 E, solid line) and shows that peak <qa> underestimates the value of QT in the model (dashed line, QT = 4zJ + zL= 2.62 e) by 26–44%, depending on [Ca2+].
Effects of Channel Opening on the Kinetics of OFF Charge Movement
The voltage and Ca2+ dependence of QC and QO indicate that channel opening strongly affects voltage sensor activation, whereas Ca2+ binding does not (i.e., D >> E in Scheme II). That 70 μM Ca2+ greatly slows the decay of IgOFF following a 1-ms pulse to 160 mV (Fig. 5 A) may appear to contradict the conclusion that direct interaction between Ca2+ binding sites and voltage sensors is weak. However, the Ca2+-sensitivity of OFF charge movement can be understood in terms of an indirect interaction, mediated by the allosteric factors C and D in Scheme II, whereby Ca2+ promotes channel opening and channel opening affects voltage sensor movement.
In the presence of 70 μM Ca2+, OFF currents evoked after pulses to 160 mV decrease in amplitude and decay more slowly as pulse duration is increased (Figs. 5 B and 13 A). This phenomenon is also evident in the absence of Ca2+ at more positive pulse voltages, is associated with the time course of channel opening, and is predicted by an allosteric voltage-gating mechanism (Horrigan and Aldrich, 1999). Channel opening biases the R-A equilibrium toward the activated state, with the result that voltage sensor deactivation, and therefore OFF charge movement, is slowed and peak IgOFF is reduced.
The effect of pulse duration on IgOFF in Fig. 5 B is more prominent in 70 μM Ca2+ than in 0 Ca2+ because Ca2+ promotes channel opening. OFF currents evoked after brief pulses, when channels remain closed, decay rapidly in the presence or absence of Ca2+ (Fig. 5 B) because Ca2+ has little direct effect on voltage sensor deactivation kinetics (Fig. 7 E, compare τgfast at −80 mV in 0 and 70 μM Ca2+). The marked slowing of IgOFF in 70 μM Ca2+ after a 1-ms pulse (Fig. 5 A) reflects that channel opening is both more complete and more rapid in the presence of Ca2+. Therefore, the effect of Ca2+ on IgOFF in Fig. 5 A reflects a difference in voltage sensor deactivation for open and closed channels, rather than a direct effect of Ca2+ on voltage sensor movement.
Multiple Components of OFF Gating Charge Movement
The effects of channel opening on OFF charge movement in 70 μM Ca2+ are examined in more detail in Fig. 13. OFF currents evoked at −80 mV following pulses to 120 mV of different duration (0.11–20 ms) are superimposed on an expanded timescale in Fig. 13 A and exhibit a slowing in the decay of IgOFF that saturates for pulses of ≥5 ms duration. The integrals of these traces (QOFF, Fig. 13 B) reveal an additional slow component of charge relaxation after prolonged depolarizations that is not obvious in the IgOFF records and requires ∼4 ms to reach a steady-state.
The kinetics of QOFF relaxation were analyzed by plotting the quantity QOFF(t)-QOFFSS on a semi-log scale (Fig. 13, C and E), where QOFFSS is the steady-state value of QOFF(t). Fig. 13 C compares OFF kinetics after the most brief (0.11 ms) and prolonged (5–20 ms) depolarizations. The 5–20-ms trace is fit by a double exponential function (solid line) with time constants of 200 and 810 μs (dashed lines) termed “Medium” and “Slow” components. The 0.11-ms record is fit by a triple-exponential function with a primary “Fast” 25-μs component (dashed line) in addition to the 200- and 810-μs components. Qualitatively similar results were reported previously in the absence of Ca2+(Horrigan and Aldrich, 1999).
These kinetics can be understood in terms of sub-Scheme IIb* (Fig. 13 D). A prolonged depolarization to 120 mV in 70 μM Ca2+ is sufficient to activate mSlo1 channels maximally. Therefore, the biexponential decay of QOFF after a 5–20-ms pulse represents the relaxation of open channels back to their resting state and is determined by voltage sensor deactivation while channels are open (O-O transitions labeled Medium in Fig. 13 D) and by channel closing (Slow). A brief depolarization is sufficient to activate voltage sensors but does not allow many channels to open. Thus, the decay of QOFF after a 0.11-ms pulse is dominated by a Fast component representing the deactivation of voltage sensors while channels are closed (C-C transitions labeled Fast in Fig. 13 D).
Sub-Scheme IIb* predicts that QOFF measured after pulses of intermediate duration will be best fit by a combination of all three time constants, since some channels will be open and some will be closed. Fig. 13 E shows that QOFF(t)-QOFFSS derived from the OFF currents in Fig. 13 A for all pulse durations can be fit by triple-exponential functions with 25-, 200-, and 810-μs time constants. The amplitudes of the different exponential components are plotted versus pulse duration in Fig. 13 F. The fast component increases rapidly as voltage sensors are activated and then decreases as channels leave the closed state. At the same time, the Medium and Slow components increase in parallel reflecting the increased occupancy of open states. The time courses of the amplitudes of all three components are fit by exponential functions with a time constant of 1.4 ms, representing the kinetics of channel opening.
The Kinetics of IK Relaxation
In response to a voltage step, IK activates or deactivates with an exponential time course after a brief delay (Fig. 4 B) (Horrigan et al., 1999). The τ(IK)-V relationship characterizing this relaxation exhibits a complex voltage dependence. Increasing Ca2+ from 0 to 70 μM shifts the relation with little effect on its shape (Fig. 4 D). A similar pattern is observed at intermediate [Ca2+] (Fig. 14 A).
Figure 14.

IK kinetics. (A) Mean τ(IK)-V relationships for different [Ca2+] (in μM: 0 (•), 0.27 (▪), 0.58 (▵), 0.81 (▴), 1.8 (▿), 3.8 (▾), 8.2 (⋄), 19 (♦), 68 ( ), 99 (
), 99 ( ). Dashed lines are fits to exponential functions at extreme positive voltages (V ≥ 200 mV in 0 Ca2+ or for V ≥ 140 mV in high Ca2+ (68 and 99 μM)). (B) Comparison of τ(IK)-V (mean ± SEM) in 0, 0.81, and 68 μM. Dashed lines are fits to exponential functions between −300 and −150 mV, indicating that the voltage dependence of τ(IK) at extreme negative voltages is not appreciably Ca2+ sensitive. However, the voltages where τ(IK) begins to deviate from these fits in 0 and 68 μM Ca2+ differ by approximately −150 mV (arrows). (C) The partial charges determined from exponential fits to τ(IK) at extreme positive (zP) and negative (zN) voltages as in A and B, respectively, are plotted against [Ca2+] and fit by lines (zP = 0.203–0.0044 log[Ca2+], zN = −0.161–0.0042 log[Ca2+]). Individual data points (open symbols) and mean ± SEM (solid symbols) are shown. The fits in A and B were constrained to the mean values of zP and zN respectively for each [Ca2+].
). Dashed lines are fits to exponential functions at extreme positive voltages (V ≥ 200 mV in 0 Ca2+ or for V ≥ 140 mV in high Ca2+ (68 and 99 μM)). (B) Comparison of τ(IK)-V (mean ± SEM) in 0, 0.81, and 68 μM. Dashed lines are fits to exponential functions between −300 and −150 mV, indicating that the voltage dependence of τ(IK) at extreme negative voltages is not appreciably Ca2+ sensitive. However, the voltages where τ(IK) begins to deviate from these fits in 0 and 68 μM Ca2+ differ by approximately −150 mV (arrows). (C) The partial charges determined from exponential fits to τ(IK) at extreme positive (zP) and negative (zN) voltages as in A and B, respectively, are plotted against [Ca2+] and fit by lines (zP = 0.203–0.0044 log[Ca2+], zN = −0.161–0.0042 log[Ca2+]). Individual data points (open symbols) and mean ± SEM (solid symbols) are shown. The fits in A and B were constrained to the mean values of zP and zN respectively for each [Ca2+].
IK kinetics were characterized primarily in 0 Ca2+ or high Ca2+ (70–100 μM) and the data at intermediate [Ca2+] often do not include the most positive voltages. Nonetheless, trends in the behavior of τ(IK) are evident that help to constrain the parameters in Scheme II and provide important information about the Ca2+and voltage dependence of gating. In particular, the response of τ(IK) to Ca2+ at negative voltages suggests that a complex relationship exists between the Ca2+- and voltage dependence of the rate limiting C-O transition.
The Voltage Dependence of τ(IK)
If voltage sensor activation and Ca2+ binding are fast and assumed to equilibrate on the timescale of channel opening, then τ(IK) can be written (Cox et al., 1997a; Horrigan et al., 1999):
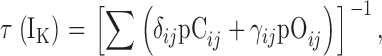 |
(13) |
where i and j represent the number of activated voltage sensors and occupied Ca2+-binding sites, respectively, δij and γij are forward and backward rate constants for the Cij-Oij transitions and pCij, pOij are conditional occupancies of the open and closed states (pCij = p(Cij|C), pOij = p(Oij|O)). Comparison of Ig and IK kinetics in 0 and 70 μM Ca2+ supports the notion that voltage sensor activation is always faster than channel opening. The exponential relaxation of IK and the similar voltage dependence of τ(IK) in the presence and absence of Ca2+ are consistent with the assumption that Ca2+ binding is fast and that τ(IK) is not limited by Ca2+-binding transitions (Cox et al., 1997a).
The similar shape of τ(IK)-V relations in different [Ca2+] is not surprising since, in the presence or absence of Ca2+, τ(IK) is governed by voltage sensor activation and by voltage-dependent changes in the rate constants for the C-O transition. These two processes define four regions in the τ(IK)-V relationship (Eqs. 14–17 below).
At negative voltages where forward rate constants (δij) are small compared with backward rates constants (γij) Eq. 13 reduces to:
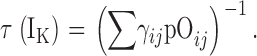 |
(14) |
Conversely, at positive voltages the backward rates are small, and Eq. 13 reduces to:
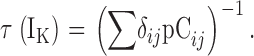 |
(15) |
The equilibrium constants and rate constants for the C-O transitions vary with the number of activated voltage sensors. Consequently, Eqs. 14 and 15 define regions to either side of the peak in the τ(IK)-V relationship where the voltage dependence of τ(IK) is steepest, reflecting that different C-O transitions dominate IK relaxation at different voltages (Horrigan et al., 1999). At more extreme potentials, by contrast, voltage sensor activation is saturated, and τ(IK) reflects only the weak voltage dependence of the C-O rate constants (Eqs. 16 and 17 below).
At extreme negative voltages where voltage sensors are not activated (i = 0, Fig. 3 sub-Scheme IIC), Eq. 14 simplifies to:
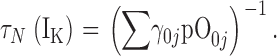 |
(16) |
Thus, τN depends only on the closing rate constants and the distribution of Ca2+-bound open states. Eq. 16 is valid when voltage sensors in the open channel are not activated and QO is therefore at a minimum. Based on Fig. 12 B, this condition is satisfied for all [Ca2+] at voltages less than approximately −150 mV. Consistent with this prediction, τ(IK) in all [Ca2+] exhibits a similar exponential voltage dependence for V < −150 mV (Fig. 14 A), presumably reflecting the voltage dependence of the closing rates (γ
0j). In Fig. 14 B the mean τ(IK)-V relationships in 0, 1, and 70 μM Ca2+ are fit (dashed lines) by exponential functions (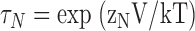 ) from −300 to −150 mV. The exponential voltage dependence of τN was shown previously to extend to at least −500 mV in 0 Ca2+ (Horrigan et al., 1999). The partial charges, zN, determined from fits to individual τ(IK)-V relationships are plotted versus [Ca2+] in Fig. 14 C (open circles) and exhibit little Ca2+-dependence, with mean values (solid circles) of −0.124 ±0.003 e in 0 Ca2+ (n = 21) and −0.145 ± 0.007 e in high (70–100 μM) Ca2+ (n = 5).
) from −300 to −150 mV. The exponential voltage dependence of τN was shown previously to extend to at least −500 mV in 0 Ca2+ (Horrigan et al., 1999). The partial charges, zN, determined from fits to individual τ(IK)-V relationships are plotted versus [Ca2+] in Fig. 14 C (open circles) and exhibit little Ca2+-dependence, with mean values (solid circles) of −0.124 ±0.003 e in 0 Ca2+ (n = 21) and −0.145 ± 0.007 e in high (70–100 μM) Ca2+ (n = 5).
At extreme positive voltages, where all voltage sensors are activated (i = 4, Fig. 3, sub-Scheme IID), Eq. 15 simplifies to:
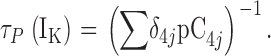 |
(17) |
Thus, τP depends only on the opening rate constants and the distribution of Ca2+-bound closed states. Eq. 17 is valid when voltage sensors in closed channels are activated such that QC is maximal. Because QC saturates at ∼250 mV (Fig. 7 C), τP cannot be measured over a large voltage range. Therefore, the voltage dependence of τP was estimated by fitting the τ(IK)-V relationships in 0, 70, and 100 μM Ca2+ with exponential functions (dashed lines Fig. 14 A) at voltages where QC is not completely saturated (>200 mV, 0 Ca2+, >140 mV, 70–100 μM Ca2+). These fits are consistent with τ(IK) at the most positive voltages and are characterized by similar partial charges in different [Ca2+] (zP, Fig. 14 C), reflecting the charge associated with opening rate constants. However, zP may be overestimated slightly because QC is not completely saturated.
Ca2+ Mainly Affects Opening Rates at Limiting Negative Voltages
Fig. 14 B shows that τN(IK) at −150 mV increases approximately twofold as [Ca2+] increases from 0 to 70 μM Ca2+, implying that the closing rate (γ 0j) decreases only twofold when Ca2+ is bound (i.e., γ00/γ04 = 2). By contrast, PO at extreme negative voltages (−140 to −120 mV) increases ∼1,000-fold (Fig. 9 A). Therefore, the main effect of Ca2+ on the C-O transition at negative voltages must be to increase the forward (opening) rates.
τ(IK) Reflects the Differential Affinities of Ca2+ for Open and Closed Channels
Although the change in τ(IK) at negative voltages is small, it is remarkably Ca2+ sensitive. Fig. 14 B shows that 1 μM Ca2+ produces an almost maximal increase in τN(IK), similar to that in 70 μM Ca2+. By contrast, 1 μM Ca2+ has almost no effect on τ(IK) measured at extreme positive voltages (Fig. 14 B). The Ca2+ dependence of τ(IK) depends on the C-O rate constants as well as the distribution of Ca2+-bound states (Eq. 13) and therefore does not provide a direct measure of Ca2+ binding. However, the differential Ca2+ sensitivities of τN(IK) and τP(IK) are qualitatively consistent with an allosteric effect of channel opening on Ca2+ binding affinity. τN(IK) depends on the distribution of Ca2+-bound open states (Eq. 16) and should therefore reflect the higher Ca2+ affinity of open channels, whereas τP(IK) will reflect the lower affinity of closed channels (Eq. 17).
The Voltage Dependence of τN(IK) and τP(IK) Suggest zL Is Ca2+ Independent and KD Is Voltage Independent
The charge qO estimated from the limiting voltage dependence of PO increases with [Ca2+] (Fig. 12 C), suggesting that bound Ca2+ ions may contribute to gating charge associated with the C-O equilibrium (zL) and, conversely, that Ca2+ binding may be voltage dependent. However, the voltage dependence of τN(IK) and τP(IK) in different [Ca2+] do not support these possibilities and suggest instead that qO in low Ca2+ may underestimate zL.
Fig. 14 C plots the partial charges (zN, zP) determined from exponential fits to τN(IK) and τP(IK) at different [Ca2+]. According to Eqs. 16 and 17, zN and zP reflect the voltage dependence of the backward and forward rate constants, respectively, for the C-O transition. In 0 Ca2+ and saturating Ca2+, zN and zP should be determined by single rate-constants (0 Ca2+: zN = z[γ00] and zP = z[δ40], saturating Ca2+: zN = z[γ04] and zP=z[δ40]).
Fig. 14 C shows that neither zN nor zP are strongly Ca2+ sensitive. zN increases in magnitude 1.16-fold from −0.124 ± 0.003 e in 0 Ca2+ (n = 21) to an average of −0.145 ± 0.007 e in high (70–100 μM) Ca2+ (n = 5). zP decreases 1.1-fold from 0.24 ± 0.02 e (0 Ca2+, n=12) to 0.22 ± 0.005 e in high Ca2+ (n = 6). The sum zT = |zN| + |zP| is identical in 0 Ca2+ and high Ca2+ (zT = 0.37 e), suggesting that zL is not Ca2+ dependent. zT is expected to equal zL only in the simple case that the C-O transition can be represented as single energy barrier; it is formally possible for zT to be greater or less than zL. Furthermore, zT may be overestimated if zP was not measured at sufficiently positive voltages. Nonetheless, the weak Ca2+-sensitivity of zN, zP, and zT argue against the idea that the partial charge for channel opening (zL) depends on Ca2+.
If zL is not Ca2+ dependent, then qO, determined from the limiting slope of PO, is probably underestimated in low Ca2+. qO in high Ca2+ (∼0.3 e) is comparable to zT (0.37 e) and may therefore represent a reasonable estimate of zL. But qO in low Ca2+ (∼0.1 e) appears underestimated since it is much smaller than zT and smaller even that zN. As noted above, factors that might contribute to errors in determining qO include failure to measure PO at sufficiently negative voltages and large Ca2+-dependent changes in the magnitude of PO. In contrast, measurements of zN and zP are less subject to these types of errors. zN, in particular, should be accurate since τN(IK) was routinely measured over a large voltage range (−300 to −150 mV) where voltage sensors are not activated and τ(IK)-V is exponential. Moreover, systematic Ca2+-dependent errors in the measurement of zN should be small because the amplitude of τN(IK) changes only twofold in response to Ca2+.
The Ca2+ and Voltage Dependence of C-O Transition Kinetics
The effects of Ca2+ on steady-state activation and IK kinetics superficially resemble each other in that the PO-V and τ(IK)-V relationships are shifted similarly along the voltage axis with little change in shape (Fig. 4, C and D). Although the shift in PO-V can be reproduced by assuming Ca2+and voltage act almost independently to alter the C-O equilibrium (Scheme II), the shift in τ(IK)-V implies that a more complex relationship exists between the Ca2+ and V dependence of the C-O transition kinetics. We can show that this is the case by focusing on the foot of the τ(IK)-V relation.
As membrane potential increases at negative voltages, τ(IK) deviates from its limiting exponential voltage dependence (τN(V)), implying that τ(IK) can no longer be described by Eq. 16. However, this deviation occurs at voltages where PO is small and closing rates are presumably much greater than opening rates. Therefore, Eq. 14 should be valid. Comparing Eqs. 14 and 16, it is evident that two conditions must be satisfied for a deviation from exponential voltage dependence to occur. First, voltage sensors must activate so that open states other than O0j are occupied. That is, the open channel charge distribution QO must increase from its minimum level. Second, voltage sensor activation must reduce the closing rates. The voltage (VdN) where deviation from τN(V) occurs reflects both the voltage dependence of QO and the effect of voltage sensor activation on the O to C transition rates.
VdN decreases by almost 150 mV as [Ca2+] increases from 0 to 70 μM (arrows, Fig. 14 B). This shift is not accounted for entirely by a Ca2+-dependent change in voltage sensor activation, because QO shifts by only ∼40 mV over the same Ca2+-range (Fig. 12 B). Therefore, the change in VdN must reflect a Ca2+-dependent change in the sensitivity of closing rates to voltage sensor activation. To test this hypothesis, we fit the τ(IK)-V relationships in 0 Ca2+and 70 μM Ca2+to Scheme II (Fig. 15 A).
Ca2+ Alters the Sensitivity of Closing Rates to Voltage Sensor Activation
Scheme II reduces to a 10-state gating scheme containing five C-O transitions in either 0 Ca2+ (Scheme I*, Fig. 1 B) or saturating Ca2+ (sub-Scheme IIb*, Fig. 4 E). The equilibrium constants for the C-O transitions are determined by the steady-state parameters, leaving five additional kinetic parameters to specify the opening and closing rate constants. The mean τ(IK)-V relationships in 0 Ca2+ and 70 μM Ca2+ are plotted in Fig. 15 A together with fits to the unliganded and Ca2+-saturated schemes respectively. The log closing rate constants are plotted in Fig. 15 B (symbols) against the number of voltage sensors activated (i). Similar fits were obtained when the closing rates were allowed to vary freely (Fig. 15 A, dashed lines, and B, open symbols) or constrained such that opening and closing rates change monotonically with voltage sensor activation (Fig. 15 A, solid lines, and B, filled symbols).
The closing rates (Fig. 15 B) reveal a different response to voltage sensor activation in 0 Ca2+ and 70 μM Ca2+. In 0 Ca2+, closing rates remain relatively constant until the fourth voltage sensor is activated. In 70 μM Ca2+, however, closing rates begin to decrease when one voltage sensor is activated and decrease more sharply when three or four are activated. This change in the pattern of closing rates is necessary to explain the large shift in the foot of the τ(IK)-V relationship with Ca2+. In high Ca2+, VdN coincides roughly with the foot of the QO-V relationship, whereas, in 0 Ca2+, VdN occurs at more positive voltages where QO has already increased substantially and several voltage sensors have been activated.
τ(IK) in Intermediate [Ca2+]
τ(IK)-V relationships in intermediate [Ca2+] are similar in shape to those in 0 Ca2+ and 70 μM Ca2+ and can also be fit by 10-state gating schemes (unpublished data). However, such fits are of limited value since many more than 10 states are accessible at intermediate [Ca2+]. Even with the simplifying assumption that Ca2+-binding and voltage sensor transitions are equilibrated, the 70-state gating scheme specified by Scheme II contains 35 C-O transitions that can influence τ(IK). The equilibrium constants for these transitions are specified by Scheme II and constrained by the steady-state data. Yet the forward and backward rate constants are, in principal, free to vary within this constraint, adding 35 kinetic parameters.
Fitting τ(IK) in 0 Ca2+ and high Ca2+ and at extreme voltages helps to constrain subsets of the kinetic parameters in Scheme II. Nonetheless, attempts to fit the entire τ(IK) dataset to Eq. 13 were problematic. The τ(IK) data were not sufficient to constrain the large number of free kinetic parameters. Fits often failed to converge or did not converge to unique solutions. In some cases, the model traces “oscillated” about the τ(IK)-V plots, exhibiting periodic changes in voltage dependence caused when neighboring C-O transitions in Scheme II were assigned rate constants of different orders of magnitude (unpublished data). Such fits fail to capture the gradual changes in voltage dependence that are evident in the data. This suggests that neighboring rate constants in the model are not completely free to vary but are to some extent correlated.
A Transition State Model for the C-O Conformational Change
To reduce the number of free parameters in Scheme II and to better reproduce the voltage dependence of τ(IK) by imposing a correlation among rate constants, we attempted to devise a model to account for the Ca2+ and voltage dependence of the rate constants. It is likely that C-O transition kinetics might be described in terms of a simple mechanism since the steady-state data are reproduced reasonably well by Scheme II. That is, the effect of Ca2+ and voltage on the C-O equilibrium is accounted for with the assumption that perturbations in ΔGCO caused by Ca2+ binding ( ) or by voltage sensor activation (
) or by voltage sensor activation (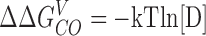 ) are additive and independent (Cui and Aldrich, 2000). Thus, a linear relationship exists between ΔGCO and the number of bound Ca2+ (j) or activated voltage sensors (i). Given this simple relationship, it seems plausible that C-O rate constants might also be altered systematically by Ca2+ binding and voltage sensor activation.
) are additive and independent (Cui and Aldrich, 2000). Thus, a linear relationship exists between ΔGCO and the number of bound Ca2+ (j) or activated voltage sensors (i). Given this simple relationship, it seems plausible that C-O rate constants might also be altered systematically by Ca2+ binding and voltage sensor activation.
Although perturbations in equilibria and kinetics need not be related, simple correlations are often observed for protein conformational changes, including the opening of acetylcholine receptor channels (Grosman et al., 2000), the allosteric transition of hemoglobin (Eaton et al., 1991; Hofrichter et al., 1991), and protein folding reactions (Fersht, 1995). A simple example in the case of mSlo1 is that τ(IK) and PO both exhibit exponential voltage dependencies at extreme negative voltages (Figs. 9 A and 14 B). Thus, a linear relationship exists between the log closing rate constant (ln[γ]) and log C-O equilibrium constant (ln[κ]) in response to voltage perturbation when voltage sensors are not activated. Such relationships can be explained in terms of transition state theory.
According to transition state theory, the C-O conformational change may be described as a reaction whose kinetics are dominated by an energy barrier, represented as a transiently occupied state (T). The C-O equilibrium constant is determined by the free energy difference (ΔGCO) between C and O states ( ), whereas the closing rate is determined by the difference (ΔGOT) between O and T (
), whereas the closing rate is determined by the difference (ΔGOT) between O and T ( ). Thus, proportional changes in ln(γ) and ln(κ) imply proportional changes in ΔGCO and ΔGOT (i.e., ΔΔGOT = Ψ ΔΔGCO). In the case of a voltage-dependent transition, such a rate-equilibrium linear-free energy relationship could arise if the gating charge moved during the O-T transition is a constant fraction of that moved during the O-C transition. In a more general sense, proportional changes in ΔGCO and ΔGOT are thought to occur when the transition state has structural features intermediate between the C and O conformations and is therefore sensitive to perturbations that affect C and O (Leffler, 1953; Hammond, 1955).
). Thus, proportional changes in ln(γ) and ln(κ) imply proportional changes in ΔGCO and ΔGOT (i.e., ΔΔGOT = Ψ ΔΔGCO). In the case of a voltage-dependent transition, such a rate-equilibrium linear-free energy relationship could arise if the gating charge moved during the O-T transition is a constant fraction of that moved during the O-C transition. In a more general sense, proportional changes in ΔGCO and ΔGOT are thought to occur when the transition state has structural features intermediate between the C and O conformations and is therefore sensitive to perturbations that affect C and O (Leffler, 1953; Hammond, 1955).
A Single Barrier Model Cannot Account for the Voltage Dependence of τ(IK)
To describe the rate constants in Scheme II we first considered the possibility that the C-O conformational change can be represented by a single energy barrier with a transition state (T) and that perturbations in the barrier energy produced by Ca2+-binding ( ) or voltage sensor activation (
) or voltage sensor activation (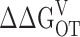 ) are additive and proportional to perturbations in ΔGCO where
) are additive and proportional to perturbations in ΔGCO where 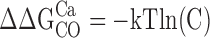 and
and 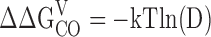 . In this case three parameters, in addition to the equilibrium parameters in Scheme II, are required to specify the rate constants:
. In this case three parameters, in addition to the equilibrium parameters in Scheme II, are required to specify the rate constants:
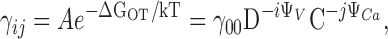 |
(18) |
where γ00 is the closing rate for the unliganded channel (j = 0) with no voltage sensors activated (i = 0). ΨV and ΨCa represent the fractional perturbations of ΔGOT produced by voltage sensor activation or Ca2+-binding, respectively ( ).
).
Although Eq. 18 only specifies 25 rate constants, this is sufficient to characterize the 35 C-O transitions in Scheme II, provided we make the simplifying assumption that transitions with identical equilibrium constants have identical rate constants. Scheme II predicts that multiple C-O transitions exist for some combinations of i and j, owing to direct interactions between voltage sensors and Ca2+ binding sites represented by the E-factor in Scheme II. However, these interactions do not alter the C-O equilibrium constants which are identical for any one combination of i and j.
The single barrier model predicts a linear relationship between log closing rate and the number of activated voltage sensors (i) or bound Ca2+ (j):
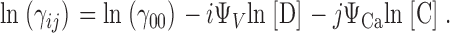 |
This prediction is inconsistent with the observed relationships between log(γij) and i in 0 Ca2+ or 70 μM Ca2+ (Fig. 15 B). Thus, a single barrier model is inadequate to describe the observed voltage dependence of τ(IK).
A Two Barrier Model Reproduces the Apparent Ca2+ and Voltage Dependence of C-O Transition Kinetics
The relationship between log(γij) and voltage sensor activation in 0 Ca2+ (Fig. 15 B) suggests that perturbations in ΔGCO by activation of each voltage sensor produce small ΔΔGOT when the first three voltage sensors activate, but a larger ΔΔGOT for the fourth voltage sensor. This biphasic pattern might arise if the C-O transition is characterized by two barriers that become rate limiting over different ranges of ΔGCO. It seems reasonable that a complex protein conformational change such as channel opening might be characterized by a complex energy landscape rather than a single uniform barrier (Fersht, 1995; Sigg et al., 1999). Thus, we considered a model in which the C-O transition is represented by two transition states (T1,T2); Ca2+ and voltage are assumed to perturb the energy of the transition states to different extents as determined by the perturbation factors 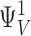 ,
,  ,
, 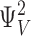 ,
, 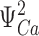 .
.
Modeling the C-O transition rates in terms of a complex energy barrier using Eyring rate theory is a simplification and requires additional assumptions about the energy landscape. A two-barrier model suggests that an energy well defining an intermediate state (SI) might exist between C and O. However, a variety of macroscopic and single channel kinetics data imply that the C-O transition is concerted (i.e., highly cooperative), such that any intermediate states must have a low equilibrium occupancy and brief dwell time (see discussion). Thus, we make the simplifying assumption that the free energy of any intermediate is high relative to both C and O such that the sum of the rate constants for leaving SI are fast relative to those for entering. If this general condition is satisfied then it is not necessary to include intermediate states explicitly in our kinetic scheme. The overall closing rate constant from O to C (γ) is determined by the product of the rate constant from O to SI and the conditional probability that a channel in S1 will exit to C rather than O. Consequently, the closing rate can be expressed in terms of the free energy differences between the open state and the two transition states (ΔGOT1, ΔGOT2):
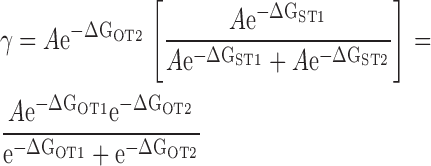 |
(19) |
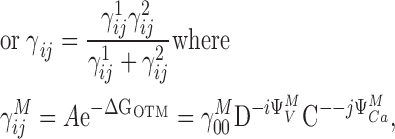 |
(20) |
where M = 1,2 and  and
and  represent the closing rates determined by T1 and T2, respectively.
represent the closing rates determined by T1 and T2, respectively.
When the free energies of T1 and T2 are comparable, Eq. 19 depends on both barriers. But as the difference between barriers increases, the rate constant becomes limited by the highest barrier. Alternatively, the closing rate may be determined by the highest barrier under all conditions if there is no energy well (intermediate state) between the two barriers when they are of similar height:
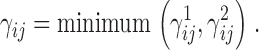 |
(21) |
In Fig. 15 C the τ(IK)-V relationships for all Ca2+ were fit simultaneously using the alternative 2-barrier models described by Eqs. 20 (dashed lines) and 21 (solid lines). The steady-state parameters were held constant (Table II, Fit B) while the kinetic parameters were allowed to vary (Table III) .
TABLE III.
Scheme II: Two-barrier Model Kinetic Parameters
| γ1if | γ2if | Ψ1V | Ψ1Ca | Ψ2V | Ψ2Ca | |
|---|---|---|---|---|---|---|
| s−1 | s−1 | |||||
| Eq. 20 | 4,193 | 1.90 × 108 | 0.0 | 0.083 | 1.0 | 0.70 |
| Eq. 21 | 4,322 | 1.74 × 108 | 0.041 | 0.089 | 1.0 | 0.70 |
The steady-state parameter were from Table II, Fit B: L0 = 9.8 × 10−7, zL = 0.3 e, zJ = 0.58 e, Vh(J) = 150 mV, KD = 11 mM, C = 8, D = 25, E = 2.4.
The two-barrier models provide reasonable fits to the 0 Ca2+ and 70 μM Ca2+ data, and reproduce the large shift in VdN (Fig. 15 D). The models also approximate the τ(IK)-V relationship in 1 μM Ca2+ (Fig. 15 D) and capture the envelope of time constants observed over a range of intermediate Ca2+ (Fig. 15 C). The model lacking an intermediate state (Eq. 21, Fig. 15, C and D, solid lines) provided slightly better fits than Eq. 20 (dashed lines) based on chi-squared criteria. Improved fits at intermediate Ca2+ were obtained if KD was reduced (unpublished data). However, a reduction in KD is not consistent with the RO-[Ca2+] relationship. The poorest fits were obtained in 10 μM Ca2+, which is not surprising since the steady-state model underestimates the steepness of the PO-V relationship in 10 Ca2+ (Fig. 10 C1).
The closing rate constants predicted by the two barrier models are plotted in Fig. 15 B (Eq. 20, dashed lines; Eq. 21, solid lines) for different numbers of bound Ca2+ (j). The models not only reproduce the biphasic pattern of closing rates in 0 Ca2+ but also the modification of this pattern that occurs in saturating Ca2+.
The mechanism by which the two barrier model reproduces the different patterns of backward rates observed in 0 Ca2+ and 70 μM Ca2+ is illustrated by the transition state diagrams in Fig. 15 E. In 0 Ca2+ at extreme negative voltages the closing rate (γ00) is determined by the highest barrier (T1). Voltage sensor activation produces little change in ΔGOT1 (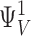 is small) such that γij is not greatly altered by activation of one or two voltage sensors (Fig. 15 E1). However, the energy of the second barrier (ΔGOT2) is altered by voltage sensor activation (
is small) such that γij is not greatly altered by activation of one or two voltage sensors (Fig. 15 E1). However, the energy of the second barrier (ΔGOT2) is altered by voltage sensor activation (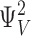 is large) such that T2 becomes rate-limiting, and γ becomes sensitive to voltage sensor activation, when more than three voltage sensors are activated (starred trace Fig. 15 E1) thereby producing a biphasic pattern in the relationship between ln(γij) and i. Ca2+ binding also increases ΔGOT2 (
is large) such that T2 becomes rate-limiting, and γ becomes sensitive to voltage sensor activation, when more than three voltage sensors are activated (starred trace Fig. 15 E1) thereby producing a biphasic pattern in the relationship between ln(γij) and i. Ca2+ binding also increases ΔGOT2 (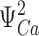 is large) such that, in 70 μM Ca2+, T2 becomes rate limiting when fewer than three voltage sensors are activated (starred traces Fig. 15 E2). By the same logic, Ca2+ exerts little effect on the closing rate when voltage sensors are not activated (
is large) such that, in 70 μM Ca2+, T2 becomes rate limiting when fewer than three voltage sensors are activated (starred traces Fig. 15 E2). By the same logic, Ca2+ exerts little effect on the closing rate when voltage sensors are not activated (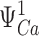 is small), but has a much bigger effect at high voltages when T2 becomes rate limiting. In this way, additive effects of Ca2+ binding and voltage sensor activation on the C-O equilibrium free energy change can produce nonlinear changes in ln(γij).
is small), but has a much bigger effect at high voltages when T2 becomes rate limiting. In this way, additive effects of Ca2+ binding and voltage sensor activation on the C-O equilibrium free energy change can produce nonlinear changes in ln(γij).
DISCUSSION
The combined effects of Ca2+ and voltage on BK channel gating are important to understand because they underlie physiological processes in many cell types, but also because they provide information about channel gating that is difficult to obtain from channels that are purely ligand or voltage gated. For example, the voltage dependence of BK channels allows us to compare the gating of liganded and unliganded channels to an extent that is not possible for most ligand-gated channels. BK channels can be activated maximally by voltage in the absence of Ca2+, allowing the detailed study of transitions among unliganded states (Cui et al., 1997; Stefani et al., 1997; Horrigan and Aldrich, 1999; Horrigan et al., 1999; Nimigean and Magleby, 2000; Talukder and Aldrich, 2000). Such openings support allosteric models where ligand-bound and unliganded channels undergo similar conformational changes, but ligand binding alters the energetics of certain transitions. Here we compare the gating of Ca2+-bound and unliganded BK channels to identify Ca2+-sensitive transitions and determine the mechanism of Ca2+ action.
Relationship to Previous Studies
Previous studies support the basic conclusion that both voltage sensor activation and Ca2+ binding act to promote BK channel opening through allosteric mechanisms (Cox et al., 1997a; Cui et al., 1997; Horrigan and Aldrich, 1999; Horrigan et al., 1999; Rothberg and Magleby, 1999, 2000; Cox and Aldrich, 2000; Cui and Aldrich, 2000; Talukder and Aldrich, 2000; Zhang et al., 2001). However, as discussed below, none of these studies have fully tested the assumptions and predictions of a particular mechanism such as Scheme II.
In general, BK channels distribute among a large number of states in a Ca2+- and voltage-dependent manner. Scheme II predicts a gating architecture (i.e., connectivity among states) and equilibrium constants that are determined by the model parameters and by assumptions about the functional properties of sensors and gates and their interactions with each other. Thus, in theory, the gating mechanism can be tested by determining the arrangement of states and the relationships among the equilibria.
Single channel IK kinetic analysis confirms that BK channels can occupy many different open and closed states consistent with the allosteric assumption that no combination of sensor and gate conformations are disallowed (Rothberg and Magleby, 1999; Talukder and Aldrich, 2000). Moreover, these kinetics can be reproduced by gating schemes with separate voltage- and Ca2+-dependent transitions (Rothberg and Magleby, 2000) consistent with a mechanism where Ca2+- and voltage sensors are capable of nonconcerted action. However, the number of accessible states is large, transitions associated with voltage and Ca2+ sensor activation are fast, and single channel recordings at extreme voltages are difficult. Hence, some states are rarely occupied or are difficult to resolve so that many rate constants, and the corresponding equilibrium constants, are poorly determined. Restricting the analysis to the unliganded or Ca2+-saturated conditions reduces the number of accessible states and improves, but does not solve, the problem of determining the relationship among equilibrium constants (Rothberg and Magleby, 1999; Talukder and Aldrich, 2000).
The Ca2+ and voltage dependence of BK channel open probability also provide information about the underlying gating mechanism. Models similar to Scheme II have been used to fit the shape and Ca2+ dependence of macroscopic G-V relationships successfully over a wide range of conditions (Cox and Aldrich, 2000; Cui and Aldrich, 2000; Zhang et al., 2001). However, some parameters in Scheme II are poorly constrained by such data and others covary, and thus are not determined uniquely (Zhang et al., 2001). As we have shown in Fig. 10 A, excellent fits can be obtained when some parameters are adjusted to unrealistic values. Thus, our ability to test many aspects of the mechanism by the G-V data alone is questionable.
Additional tests of the BK channel gating mechanism are provided by combining IK data with manipulations of channel function. The effect on G-V relationships of the accessory β1 subunit (Cox and Aldrich, 2000) or mutations in the S4 voltage sensor (Cui and Aldrich, 2000) are consistent with mechanisms similar to Scheme II. In addition the effects of voltage sensor, mutations suggest that the interaction between Ca2+ binding sites and voltage sensors are weak. However, the extent to which this conclusion is model dependent or can be quantified is unclear.
Testing the Allosteric Gating Mechanism
To test the mechanism of BK channel gating we have measured gating currents, extended IK measurements to extreme negative voltages, and employed conditions in which subsets of the processes and interactions depicted in Scheme II can be studied in isolation. Gating currents allow us to characterize voltage sensor function directly and to examine the interaction of voltage sensors with Ca2+ binding and channel opening. The limiting voltage dependence of PO provides additional information about the interaction between voltage sensor activation and channel opening that is not obtained readily from gating currents. Measurements of PO and macroscopic IK relaxation kinetics at extreme negative voltages provide direct information about the C-O transition properties and allow us to test the interaction between Ca2+ binding and channel opening in a way that is independent of voltage sensor activation. Although we do not monitor the Ca2+-bound state of the channel directly, the Ca2+ dependence of PO at extreme negative voltages provides a more direct indication of Ca2+ binding properties than previous measurements based on Ca2+ dependence of the half activation voltage. By focusing on conditions of high (70–100 μM) and low (1 nM) [Ca2+] we are able to examine gating when the state of the Ca2+ binding site is well defined (i.e., saturated or unliganded).
Another important strategy used in this investigation was to examine the reciprocal nature of interactions between sensors and gates. Such reciprocal interactions are a hallmark of allosteric mechanisms and provide alternative methods to measure each interaction factor in Scheme II.
Our results allow us to make a number of conclusions about the properties and relationships between voltage sensor activation, calcium binding, subunit interactions, and channel opening that are outlined in the following sections.
Isolation of Channel Opening, Voltage Sensor Activation, and Ca2+ Binding
A basic prediction of an allosteric gating mechanism is that all combinations of sensor and gate conformation are allowed. We have tested this hypothesis by examining mSlo1 gating under a variety of extreme conditions. Channel opening is weakly voltage dependent at extreme negative voltages in the absence of Ca2+. This shows that the C-O conformational change occurs whether or not voltage sensors or Ca2+-binding sites are activated. Similarly, gating currents are observed when channels are closed or open in the presence or absence of Ca2+. Thus, voltage sensors can activate whether channels are closed or open, unliganded or Ca2+ bound. That Ca2+ sensors activate whether or not voltage sensors are activated is indicated by the ability of Ca2+ to increase PO at extreme positive and negative voltages. Similarly, the ability of Ca2+ to shift the open and closed channel charge distributions (QO, QC) and to alter τ(IK) at both extreme positive and negative voltages indicates that Ca2+ binds whether channels are open or closed.
The Functional Properties of Sensor Activation and Channel Opening
Scheme II assumes that voltage sensor activation, Ca2+ binding, and channel opening can each be described by simple two-state processes and that voltage and Ca2+ sensors in different subunits act independently. These assumptions appear reasonable within the resolution of our measurements, although indirect evidence suggests that channel opening may not be completely concerted and that voltage sensors in different subunits may interact weakly.
The R-A Transition
Consistent with a two-state model of voltage sensor activation, gating currents recorded in the absence of Ca2+ activate without a detectable delay and decay with a rapid exponential time course while channels are closed (Horrigan and Aldrich, 1999). In the presence of 70 μM Ca2+, a slow component of IgON decay is evident, and slow components of both ON and OFF charge movement are observed. However, these observations are readily accounted for by interactions between channel opening and voltage sensor movement and do not imply a complex voltage sensor activation mechanism. Qfast-V relationships determined by fitting the fast component of IgON in either 0 or 70 Ca2+ reflect the voltage sensor equilibrium for closed channels (QC) and are fit well by single Boltzmann functions, again consistent with a two-state process and a voltage sensor charge zJ = 0.58 e. The limiting slope of the PO-V relationship that reveals the voltage sensor equilibrium for open channels (QO) is also fit reasonably by a single Boltzmann function. Although exponential kinetics and a Boltzmann equilibrium distribution are consistent with the assumption of independent voltage sensor movement, the increased slope of the QO-V relationship caused by Ca2+ is not predicted by Scheme II. This effect may indicate a small cooperative interaction among voltage sensors or may represent a systematic measurement error related to large Ca2+-dependent increases in PO.
The C-O Transition
Scheme II represents the C-O conformational change as a concerted two-state process that is rate limiting for channel activation and has a weak intrinsic voltage dependence. Consistent with a two-state C-O transition, macroscopic IK relaxation kinetics are single exponential after a brief delay, over a wide range of voltage and [Ca2+] (Cox et al., 1997a; Cui et al., 1997; Horrigan et al., 1999). The delay can be attributed to voltage sensor activation (Horrigan and Aldrich, 1999; Horrigan et al., 1999) and does not imply a complex C-O transition. The exponential voltage dependence of τ(IK) over a large range of negative voltages is also consistent with a two-state process. The weak voltage dependence of τ(IK) and PO at negative voltages demonstrates that charge movement associated with the C-O transition is small (zL = 0.3 e).
Single channel studies also support a two-state C-O conformational change. The number of detectable open and closed dwell-time components are comparable under a variety of conditions (McManus and Magleby, 1988; Rothberg and Magleby, 1999; Nimigean and Magleby, 2000; Talukder and Aldrich, 2000). This observation is consistent with an allosteric mechanism like Scheme II with a concerted C-O transition that implies gating schemes with an equal number of open and closed states directly connected in a two-tiered arrangement. Kinetic components can underestimate the accessible states and do not generally provide a precise comparison of closed versus open states. However, in 0 Ca2+ (Nimigean and Magleby, 2000; Talukder and Aldrich, 2000) and saturating (100–1,000 μM) Ca2+ (Rothberg and Magleby, 1999), where the accessible states are minimized, the number of open and closed components are less than or equal to the number of states predicted by Scheme II (five open, five closed), consistent with a concerted C-O transition. In addition, two-dimensional dwell time distributions are consistent with the connectivity of open and closed states specified by a two-tiered gating architecture (Rothberg and Magleby, 1999). And the detailed kinetics can be reproduced by models similar to Scheme II (Rothberg and Magleby, 1999, 2000).
Although the macroscopic and single channel kinetics are generally consistent with a two state C-O transition, the C-O transition maybe more complex. Brief “flicker” closings are commonly observed in single BK channel recordings. These events are not predicted by Scheme II and may represent intermediates during the C-O conformational change or closed states, such as blocking events, that lie outside of the activation pathway. Detailed single channel analysis cannot distinguish these possibilities (Rothberg and Magleby, 1998). In addition, as discussed below, the voltage and Ca2+ dependence of macroscopic τ(IK) suggest that C-O transition kinetics respond in a complex fashion to voltage sensor activation and Ca2+ binding. To account for these results we have suggested that the C-O conformational change may be characterized by a complex energy landscape.
Ca2+ Binding
It is reasonable to describe Ca2+ binding to each high affinity binding site as a two-state process. However, we do not measure Ca2+ binding directly, and the indirect analysis of Ca2+-binding properties based on the Ca2+ dependence of channel opening is complicated by the presence in Slo1 channels of additional low affinity binding sites and by effects of Ca2+ on voltage sensor activation. Here we have measured open probability at extreme negative voltages to isolate the effect of Ca2+ binding on channel opening from its effect on voltage sensor activation. Under these conditions, the ratio (RO) of NPO in the presence and absence of Ca2+ should depend only on the Ca2+ dissociation constant (KD) and the allosteric interaction between Ca2+-binding and channel opening (C). The Ca2+ dependence of RO is well fit by an MWC model (sub-Scheme IIc), consistent with independent and identical two-state binding reactions. The KDs determined in this fashion (9.3 ± 0.4 μM) or used in Scheme II (11 μM) fall within the range of previous estimates for mSlo1 (7.4–11.2 μM) (Cox et al., 1997a; Cox and Aldrich, 2000; Shi and Cui, 2001; Zhang et al., 2001).
It is important to realize that Scheme II specifies four different state-dependent Ca2+ dissociation constants for the high-affinity binding site. KD represents the dissociation constant for closed channels when voltage sensors are in the resting state (KD[CR] = 11 μM). Channel opening produces a C-fold (eightfold) increase in affinity (KD[OR] = 1.4 μM). Voltage sensor activation produces an additional E-fold (2.4-fold) increase in affinity for both closed and open channels (KD[CA] = 4.6 μM, KD[OA] = 0.57 μM).
The extent to which Ca2+ binding may be voltage dependent has yet to be defined. The gating of unliganded channels rules out that voltage gating is determined solely by Ca2+ binding (Cui et al., 1997). However, voltage-dependent binding could potentially contribute to voltage gating and provide clues as to the location of the Ca2+ binding site relative to the membrane electric field. PO or τ(IK) exhibit similar voltage dependencies in different [Ca2+], suggesting that Ca2+ binding is not strongly voltage dependent relative to voltage sensor activation (Cox et al., 1997a; Rothberg and Magleby, 2000). Assuming Ca2+ binding is not rate limiting for channel gating (Cox et al., 1997a; Cui et al., 1997), effects of voltage on Ca2+ binding should be evident only at intermediate [Ca2+] where the occupancy of binding sites can be altered by changes in binding affinity. Since PO increases with Ca2+ occupancy, one consequence of voltage-dependent binding should be to increase the voltage dependence of PO at intermediate [Ca2+] relative to saturated or unliganded conditions. Although a bell-shaped dependence on [Ca2+] is observed in the steepness of PO-V relationships (Cui et al., 1997), this effect can be reproduced by a model similar to Scheme II without voltage-dependent Ca2+ binding (Cox and Aldrich, 2000). Our results indicate an apparent increase with [Ca2+] in the voltage dependence of PO at extreme negative voltages (q0, Fig. 12 C). But this effect is not characterized by a bell-shaped Ca2+ dependence and may reflect measurement error at low open probability. τ(IK), which can be measured with greater precision than PO at extreme voltages, exhibits exponential voltage dependencies at both positive and negative voltages that are essentially Ca2+ independent (Fig. 14 C). From these kinetic results we conclude that the voltage dependence of Ca2+ binding must be weak relative to that of the C-O transition (zL = 0.3 e). Therefore, we make the simplifying assumption in Scheme II that Ca2+ binding is voltage independent.
Interactions Among Sensors and Gates
We pursued several distinct goals in characterizing the interactions among voltage sensor activation, Ca2+-binding, and channel opening. First was to quantify the interaction energies, i.e., the allosteric factors C, D, E in Scheme II. When possible, we determined these quantities by measuring the equilibrium (energetic) change in a target process produced by extreme (saturating) changes in the state of a coupled effector process. Second, we tested the allosteric prediction that such interactions are reciprocal in nature. Finally, to test the Scheme II prediction that the activation of an individual sensor has an additive effect on the C-O equilibrium constant, we fit the Ca2+ and voltage dependencies of PO.
Interaction of Ca2+ Binding and Channel Opening
Interactions between Ca2+ binding and channel opening can in theory be studied in isolation from voltage sensor activation at extreme positive or negative voltages. However, at extreme positive voltages where all voltage sensors are activated, measurement of IK is difficult and PO saturates, even in the absence of Ca2+, providing little information about the C-O equilibrium constant. The effect of Ca2+ on channel opening is best studied at extreme negative voltages where no voltage sensors are activated and PO is small. Under these conditions, PO increases in a Ca2+-dependent manner by more than three orders of magnitude, indicating a strong interaction between Ca2+ binding and channel opening. To quantify this interaction, we determined the ratio (RO) of NPO in the presence and absence of Ca2+. According to Scheme II, RO = C4 in saturating Ca2+, providing a direct measure of the interaction factor C = 7.4 ± 0.1 (1.20 kcal mol−1) based on mean RO. This is lower than values of C reported previously for mSlo1 (C = 8.4–11.6) (Cox et al., 1997a; Cox and Aldrich, 2000; Shi and Cui, 2001; Zhang et al., 2001). However, the previous estimates, based on PO at more positive voltages, reflect interactions between Ca2+ binding and voltage sensor activation and therefore represent a composite of the allosteric factors C and E in Scheme II.
Once C was determined from the saturating value of RO, the RO-Ca2+ relationship was fit well by an MWC model (sub-Scheme IIC) by simply adjusting KD. The ability of sub-Scheme IIC to fit the Ca2+ dependence of RO supports the assumption that Ca2+ sensors in different subunits have independent and identical additive effects on the free energy of the C-O transition.
The reciprocal effect of channel opening on Ca2+-binding predicted by the allosteric model was not tested directly by measuring bound Ca2+. However τ(IK) measured at extreme positive voltage (τP) or negative voltages (τN) should reflect the distribution of Ca2+-bound closed and open states respectively. That τN is sensitive to sub-micromolar Ca2+ while τP is not is therefore qualitatively consistent with the prediction that an increase in Ca2+-affinity accompanies channel opening.
Interaction of Voltage Sensor Activation and Channel Opening
Interactions between voltage sensor and gate can be studied in isolation from Ca2+ binding in the unliganded or Ca2+-saturated states. The PO-V relationships under these conditions reflect the effect of voltage sensor activation on channel opening. Specifically, the change in PO that occurs from extreme negative voltages (VN < −100 mV), where no voltage sensors are activated, to extreme positive voltages (VP > 250 mV), where all voltage sensors are activated, reflects the interaction energy and the intrinsic voltage dependence of the C-O transition. Scheme II predicts:
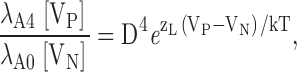 |
(22) |
where (λAι) is the C-O equilibrium constant when i voltage sensors are activated and zL is its partial charge. λA0[VN] is well determined in both 0 Ca2+ and high (70–100 μM) Ca2+ by measurements of PO over a range of negative voltages, whereas zL can be estimated from the weak voltage dependence of Po[VN]. Unfortunately λ = PO/(1 − PO) cannot be measured accurately at extreme positive voltages because PO(VP) saturates, near unity, even in 0 Ca2+. Therefore, D cannot be determined from Eq. 22. However, we can place a lower limit on D by considering that λ ≤ λA4 for any V ≤ VP. Thus:
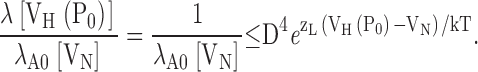 |
(23) |
In 0 Ca2+, the mean half-activation voltage Vh(PO) is 186 mV and λA0[VN] = PO(−124 mV) = 3.2 × 10−7. Therefore, Eq. 23 yields D ≥ 17 (ΔΔG = 1.7 kcal mol−1) if zL = 0.3 e.
A best value of D = 25 (ΔΔG = 1.93 kcal mol−1) was ultimately determined by fitting PO over the entire voltage range. The ability of Scheme II to fit the PO-V relationships supports the assumption that activation of each individual voltage sensor has an additive effect on channel opening.
Several lines of evidence confirm the allosteric prediction that channel opening has a reciprocal effect on voltage sensor activation. First, the decay of OFF gating current is slowed when channels are opened (Fig. 13). In addition IgON increases in amplitude in response to a double pulse protocol (Fig. 11). Finally, the open channel charge distribution (QO) estimated from the limiting voltage dependence of PO (Fig. 12 A) is shifted to more negative voltages than the closed channel distribution (QC) determined from IgON (Fig. 7 C). The similar shapes of QO and QC are consistent with the prediction that channel opening has equivalent effects on all four voltage sensors.
The shift in half-activation voltage between QC and QO (ΔVh[QCO]) provides an additional measure of the interaction factor 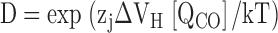 . In 0 Ca2+, the half-activation voltages for QC (155 mV) and QO (−4.3 mV) differ by 159 mV, yielding D = 38.5 (ΔΔG = 2.19 kcal mol−1) if zJ = 0.58 e. Although this estimate of D is 54% greater than that obtained by fitting the PO-V relationships (D = 25), the difference in energetic terms (ΔΔG = ln[D]) is only 13% and could be accounted for by a 19 mV overestimation of ΔVh(QCO). Several factors might contribute to an error of this magnitude, including patch-to-patch variation, the different ionic conditions used to measure QO and QC, and that QO is extrapolated from a fit to the foot of the <qa>-V relationship.
. In 0 Ca2+, the half-activation voltages for QC (155 mV) and QO (−4.3 mV) differ by 159 mV, yielding D = 38.5 (ΔΔG = 2.19 kcal mol−1) if zJ = 0.58 e. Although this estimate of D is 54% greater than that obtained by fitting the PO-V relationships (D = 25), the difference in energetic terms (ΔΔG = ln[D]) is only 13% and could be accounted for by a 19 mV overestimation of ΔVh(QCO). Several factors might contribute to an error of this magnitude, including patch-to-patch variation, the different ionic conditions used to measure QO and QC, and that QO is extrapolated from a fit to the foot of the <qa>-V relationship.
Interaction of Ca2+ Binding and Voltage Sensor Activation
We isolated the effect of Ca2+ binding on voltage sensor activation from its effect on channel opening by taking advantage of the fact that C-O transition kinetics are much slower than voltage sensor activation in Slo1 channels. Fast components of gating charge movement occurring in the first 100–200 μs after a voltage step reveal the kinetic and steady-state properties of voltage sensor activation while the open/closed state of the channel remains effectively constant (Horrigan and Aldrich, 1999). For closed channels, we find 70 μM Ca2+ slows the fast component of IgOFF but has little or no effect on IgON. Consistent with a slowing of voltage sensor deactivation, the QC-V relationship shifts by −33 mV in 70 μM Ca2+, as determined by gating-capacitance measurements. Similarly, the QO-V relationship shifts by −43 mV. According to Scheme II, the shifts in QC and QO are produced by an E-fold increase in the equilibrium constant for voltage sensor activation where  ranges from 2.1 to 2.7 (ΔΔG = 0.45 to 0.59 kcal mol−1) based on QC and QO, respectively. The shift in QC may underestimate E because Ca2+ binding sites in the closed channel, with a KD of 11 μM, are expected to be 86% occupied in 70 μM Ca2+, whereas open channels with a higher Ca2+ affinity should be fully saturated. A final value of E = 2.4 was assigned to Scheme II based on fits to the PO-V relationships over a range of [Ca2+].
ranges from 2.1 to 2.7 (ΔΔG = 0.45 to 0.59 kcal mol−1) based on QC and QO, respectively. The shift in QC may underestimate E because Ca2+ binding sites in the closed channel, with a KD of 11 μM, are expected to be 86% occupied in 70 μM Ca2+, whereas open channels with a higher Ca2+ affinity should be fully saturated. A final value of E = 2.4 was assigned to Scheme II based on fits to the PO-V relationships over a range of [Ca2+].
In Scheme II we assume that the effects of Ca2+ on voltage sensor activation and channel opening are mediated by the same Ca2+ binding sites. An alternative hypothesis is that Ca2+ can bind nonspecifically to the channel or membrane and shift the voltage dependence of voltage sensor activation through a charge screening mechanism. We cannot rule out this possibility based on gating current measurements because the dose-dependent effects of Ca2+ were not examined. However, IK measurements over a range of Ca2+ support the assumption that effects of Ca2+ on voltage sensor activation are mediated by high affinity binding sites. First, the QO-V relationship shifts even in low micromolar Ca2+ (Fig. 12 B), whereas surface charge effects are unlikely at such low concentrations. In addition, we have described a difference between the apparent Ca2+ sensitivities of Vh(PO) and PO measured at extreme negative voltages (i.e., RO), which can be accounted for by interactions between Ca2+ binding and voltage sensor activation. The discrepancy between Vh(PO) and Ro is evident even in submicromolar Ca2+ and is well described by Scheme II (Fig. 9 D) from 0 to 100 μM Ca2+, consistent with the assumption that effects of Ca2+ on voltage sensor activation and channel opening are mediated by the same binding sites.
Limitations of Limiting Slope Measurements
The maximal voltage dependence of PO (i.e., peak <qa>) does not provide a direct indication of total gating charge for channels like Slo1 that can occupy multiple open states (Sigg and Bezanilla, 1997; Horrigan et al., 1999). We have shown that peak <qa> underestimates the total gating charge of mSlo1 by 26–44% in a Ca2+-dependent manner (Fig. 12 E). The tendency of peak <qa> to decrease with increasing [Ca2+] is reproduced by Scheme II without requiring that Ca2+ binding is voltage dependent or that Ca2+ ions contribute to gating charge. These observations have consequences for the interpretation of previous studies. For example, Diaz et al. (1998) studied the effect of charge altering point mutations in the S4 voltage sensor of hSlo1. The voltage dependence of PO at low voltages was used to assess changes in total gating charge. Their conclusions concerning the contribution of different S4 residues to gating charge are difficult to evaluate given the tendency of this method to underestimate gating charge and because different mutants were studied in different [Ca2+].
Mechanism of Channel Activation by Low Affinity Ca2+ Binding Sites
Recent studies indicate that Ca2+ and Mg2+ at high concentrations (>100 μM) can act at low affinity (millimolar) binding sites to promote channel opening (Shi and Cui, 2001; Zhang et al., 2001). These studies conclude that Ca2+ binding at low affinity sites increases the C-O equilibrium constant through an allosteric mechanism analogous to that embodied by the allosteric factor C in Scheme II. An alternative hypothesis, that low affinity sites interact exclusively with voltage sensors, was rejected (Zhang et al., 2001). However, the possibility that low affinity sites interact with both C-O and R-A transition as in Scheme II was not ruled out. Indeed, our results and analysis suggest that low affinity sites must interact with voltage sensors.
When Ca2+ is increased from 100 to 1,000 μM we observe an approximately −30 mV shift in Vh(PO). This effect is consistent with previous reports (Cox et al., 1997a; Shi and Cui, 2001; Zhang et al., 2001) and can be reproduced (Fig. 9 D, dotted line) by modifying Scheme II after Zhang et al. (2001) to include additional low affinity binding sites (KDLow = 2.33 mM) that are allosterically coupled to the C-O transition (CLow = 3.53). If Ca2+ binding at these sites acts merely to increase the C-O equilibrium constant, then changes in Vh(PO) should be accompanied by proportional changes in PO at extreme negative voltages. Consequently the modified model predicts a 27% increase in log(RO) from 100 to 1,000 μM Ca2+ (Fig. 9 D, dashed line) corresponding to a large ninefold increase in PO. By contrast, we observe a 2.4% increase in mean log(RO), similar to the 3.6% increase predicted by Scheme II, and a maximum 9% increase was observed in an individual experiment (Fig. 8 E). Thus, the RO data are inconsistent with the hypothesis that Ca2+ binding to low affinity sites acts merely to increase the C-O equilibrium constant.
A discrepancy between the Ca2+ dependence of Vh(PO) and RO is observed in 0–100 μM Ca2+ and can be accounted for by interactions between high affinity Ca2+ binding sites and voltage sensors. As shown in Fig. 9 D, Scheme II predicts Vh(PO) will begin to increase over a range of Ca2+ where RO remains relatively constant. A similar mechanism involving interaction of low affinity sites with voltage sensors might account for the differential response of Vh(PO) and RO to Ca2+ in the 100–1,000 μM range. If this is the case, measurements at [Ca2+] > 1 mM may be necessary to reveal an effect of low affinity sites on RO.
If Ca2+ binding at low affinity sites increases the voltage sensor equilibrium, then the charge distribution for open channels (QO) should shift to more negative voltages in high Ca2+. Although we did not test this prediction, kinetic data reported by Zhang et. al. (2001) are consistent with a shift in QO. According to our analysis, τ(IK) achieves a weak exponential voltage dependence at extreme negative voltages and can deviate from this limiting voltage dependence only when QO begins to increase. Consistent with this prediction, τ(IK)-V in 0–100 μM Ca2+ exhibit a similar limiting voltage dependence between −300 and −150 mV (Fig. 14, B and C) where QO has apparently achieved a minimum (Fig. 12 B). Similar behavior is observed in the τ(IK)-V data reported by Zhang et al. (2001) for 0–100 μM Ca2+ with a limiting voltage dependence of e-fold per 195 mV (zN = −0.13 e) from −200 to −150 mV in 1 μM Ca2+ (measured from Fig. 10 A [Zhang et al., 2001]). By contrast, τ(IK)-V in 10–100 mM Ca2+ is approximately fourfold steeper (zN = −0.51 e, 10 mM Ca2+) over the same voltage range. This result suggests that the foot of the QO-V relationship has shifted to voltages more negative than −200 mV in 10 mM Ca2+. Thus, low affinity Ca2+ binding sites may interact with voltage sensor activation.
Kinetic Models and the Nature of the C-O Transition
Although we depict the C-O transition in Scheme II as a two-state reaction, it is likely to represent a complex quaternary conformational change involving the concerted movement of four subunits. The existence of concerted but asynchronous transitions are well recognized in the case of protein-folding reactions where multiple interactions may be broken and formed during a rapid transition between two stable conformations (Fersht, 1995). In such a case, the transition-free energy cannot be described as a smooth symmetrical barrier as might characterize a simple small molecule reaction. Rather, complex energy landscapes are observed where the barrier is often described as “broad” and may also be “rugged,” containing numerous local maxima and minima (Fersht, 1995; Oliveberg et al., 1998). A complex barrier is not incompatible with apparent two-state behavior provided that the intermediate species are unstable and do not accumulate. Moreover, a complex transition with a single dominant barrier can exhibit simple kinetic behaviors, such as linear free energy relationships, in response to small equilibrium perturbations. However, large perturbations frequently alter the position or identity of the rate limiting barrier, producing complex changes in the relationship between equilibria and kinetics (Oliveberg et al., 1998; Oliveberg, 2001). In some cases, nonlinear free energy relationships are observed that have been interpreted in terms of a gradual shift in the position of the transition state along the reaction coordinate, the so called Hammond postulate behavior (Matouschek and Fersht, 1993; Hammond, 1955; Matouschek et al., 1995; Matthews and Fersht, 1995). In other cases, sudden or large changes in the slope of linear free energy relationships are observed that are thought to represent a switch in the rate-determining barrier similar to that proposed here for the C-O transition of mSlo1 (Milla et al., 1995; Jonsson et al., 1996; Silow and Oliveberg, 1997; Oliveberg et al., 1998).
The voltage and Ca2+ dependence of τ(IK) for mSlo1 suggest that C-O transition kinetics respond in a complex fashion to voltage sensor activation and Ca2+ binding. In particular, biphasic relationships are observed between closing rate constants and voltage sensor activation in 0 Ca2+ and 70 μM Ca2+. To account for this feature of the data we propose a two-barrier transition state model for the C-O conformational change. The model reproduces the biphasic pattern of closing rates as well as the changes that occur in that pattern between 0 Ca2+ and 70 μM Ca2+ (Fig. 15 B). The τ(IK)-V relationships at intermediate Ca2+ are only approximated by this scheme and it is possible that a more complex model is required. However, this example illustrates the basic point that complex patterns of rate constants can be reproduced while maintaining the Scheme II assumptions that the C-O transition is concerted and that activation of individual voltage sensors and Ca2+ binding sites have additive effects on the C-O transition energy.
It is conceivable that the effects of Ca2+ binding and voltage sensor activation on the C-O transition energy are to some extent nonadditive (i.e., cooperative), and this might account for the tendency of Scheme II to overestimate the steepness of PO-V relationships in intermediate [Ca2+]. Since the rate constants in Fig. 15 B were determined from fits to Scheme II, the pattern of closing rates could also be affected by the assumption of energy additivity. Regardless of this possibility, the behavior of τ(IK) at negative voltages supports the general conclusion that Ca2+ binding alters the sensitivity of C-O rate constants to voltage sensor activation. τ(IK) should only deviate from a limiting exponential voltage dependence at extreme negative voltages when voltage sensors begin to activate and the O-to-C rate constant decreases. The voltage at which this deviation occurs is strongly affected by Ca2+, whereas voltage sensor activation (QO) is not. This implies that fewer voltage sensors need be activated in high Ca2+ to produce a decrease in the closing rate constant.
Our two-barrier model predicts a linear relationship between log closing rate and the free energy perturbation produced by activation of a few Ca2+ or voltage sensors, but deviation from a linear relationship as more sensors are activated. This hypothesis appears reasonable given the intrinsic voltage dependence of the C-O transition and the magnitude of the perturbations produced by voltage sensor activation and Ca2+ binding. At negative voltages in 0 Ca2+, where voltage and Ca2+ sensors are not activated, τ(IK) exhibits an exponential dependence on voltage over an ∼400 mV voltage range (−500 to –100 mV) (Horrigan et al., 1999). We expect 1/τ(IK), at extreme negative voltages, to represent the closing rate constant, and the C-O equilibrium–free energy should change in proportion to voltage. Thus, the exponential voltage dependence of τ(IK) shows that the C-O transition exhibits a rate-equilibrium linear free energy relationship over an ∼120-fold change in the C-O equilibrium constant (assuming zL = 0.3 e). Linear free energy relationships were also reported for acetylcholine receptor channels in response to a three order of magnitude change in the C-O equilibrium constant (Grosman et al., 2000). We observe similar behavior in mSlo1 in response to 2–3 order of magnitude perturbations in the C-O equilibrium constant by activation of two voltage sensors (D2 = 625) or even Ca2+-binding to all four high affinity sites (C4 = 4.1 × 103). However, we observe deviations from linearity when additional sensors are activated, since a large nine order of magnitude maximum perturbation is possible (C4D4 = 1.6 × 109, 12.7 kcal mole−1).
There is evidence in BK and other channels for activation-associated transiently occupied sub-conductance states that may support the notion of a complex C-O transition. Although stable subconductance events in BK channels are rare, brief (∼50 μs) sojourns to a subconductance level (10–35% of fully open) are frequently observed during “flicker” closings or immediately before and after transitions to a fully closed state (Ferguson et al., 1993). Similarly, Shaker K+ channels pass through two sequentially occupied substates with microsecond lifetimes during the transition between fully closed and open (Zheng et al., 2001). These brief subconductance events could represent metastable intermediates in what is an essentially concerted but complex C-O transition.
Acknowledgments
We thank Tom Middendorf, Richard Lewis, Jon Sack, Toshi Hoshi, and Merrit Maduke for helpful comments on the manuscript.
This work was supported by a grant from the National Institutes of Health (NS42901) for F.T. Horrigan and by a National Institute of Mental Health Silvio Conte Center for Neuroscience Research grant (MH48108) for R.W. Aldrich. R.W. Aldrich is an investigator with the Howard Hughes Medical Institute.
References
- Almers, W. 1978. Gating currents and charge movements in excitable membranes. Rev. Physiol. Biochem. Pharmacol. 82:96–190. [DOI] [PubMed] [Google Scholar]
- Armstrong, C.M., and F. Bezanilla. 1974. Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63:533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett, J.N., K.L. Magleby, and B.S. Pallotta. 1982. Properties of single calcium-activated potassium channels in cultured rat muscle. J. Physiol. 331:211–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler, A., S. Tsunoda, D.P. McCobb, A. Wei, and L. Salkoff. 1993. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 261:221–224. [DOI] [PubMed] [Google Scholar]
- Cox, D.H., and R.W. Aldrich. 2000. Role of the beta1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 116:411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D.H., J. Cui, and R.W. Aldrich. 1997. a. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 110:257–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D.H., J. Cui, and R.W. Aldrich. 1997. b. Separation of gating properties from permeation and block in mslo large conductance Ca2+-activated K+ channels. J. Gen. Physiol. 109:633–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, J., and R.W. Aldrich. 2000. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry. 39:15612–15619. [DOI] [PubMed] [Google Scholar]
- Cui, J., D.H. Cox, and R.W. Aldrich. 1997. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca2+-activated K+ channels. J. Gen. Physiol. 109:647–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, F., M. Wallner, E. Stefani, L. Toro, and R. Latorre. 1996. Interaction of internal Ba2+ with a cloned Ca2+-dependent K+ (hslo) channel from smooth muscle, J Gen. Physiol. 107:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, L., P. Meera, J. Amigo, E. Stefani, O. Alvarez, L. Toro, and R. Latorre. 1998. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J. Biol. Chem. 273:32430–32436. [DOI] [PubMed] [Google Scholar]
- DiChiara, T.J., and P.H. Reinhart. 1997. Redox modulation of hslo Ca2+-activated K+ channels. J. Neurosci. 17:4942–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton, W.A., E.R. Henry, and J. Hofrichter. 1991. Application of linear free energy relations to protein conformational changes: the quaternary structural change of hemoglobin. Proc. Natl. Acad. Sci. USA. 88:4472–4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, W.B., O.B. McManus, and K.L. Magleby. 1993. Opening and closing transitions for BK channels often occur in two steps via sojourns through a brief lifetime subconductance state. Biophys. J. 65:702–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez, J.M., F. Bezanilla, and R.E. Taylor. 1982. Distribution and kinetics of membrane dielectric polarization. II. Frequency domain studies of gating currents. J. Gen. Physiol. 79:41–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht, A.R. 1995. Characterizing transition states in protein folding: an essential step in the puzzle. Curr. Opin. Struct. Biol. 5:79–84. [DOI] [PubMed] [Google Scholar]
- Grosman, C., M. Zhou, and A. Auerbach. 2000. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 403:773–776. [DOI] [PubMed] [Google Scholar]
- Hamill, O.P., A. Marty, E. Neher, B. Sakmann, and F.J. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflug. Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Hammond, G.S. 1955. A correlation of reaction rates. J. Am. Chem. Soc. 77:334–338. [Google Scholar]
- Herrington, J., and R.J. Bookman. 1995. Pulse Control. University of Miami Press, Miami, FL.
- Hofrichter, J., E.R. Henry, A. Szabo, L.P. Murray, A. Ansari, C.M. Jones, M. Coletta, G. Falcioni, M. Brunori, and W.A. Eaton. 1991. Dynamics of the quaternary conformational change in trout hemoglobin. Biochemistry. 30:6583–6598. [DOI] [PubMed] [Google Scholar]
- Horrigan, F.T., and R.W. Aldrich. 1999. Allosteric voltage gating of potassium channels II. Mslo channel gating charge movement in the absence of Ca2+. J. Gen. Physiol. 114:305–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan, F.T., J. Cui, and R.W. Aldrich. 1999. Allosteric voltage gating of potassium channels I. Mslo ionic currents in the absence of Ca2+. J. Gen. Physiol. 114:277–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas, L.D., and F.J. Sigworth. 1999. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J. Gen. Physiol. 114:723–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson, T., C.D. Waldburger, and R.T. Sauer. 1996. Nonlinear free energy relationships in Arc repressor unfolding imply the existence of unstable, native-like folding intermediates. Biochemistry. 35:4795–4802. [DOI] [PubMed] [Google Scholar]
- Leffler, J.E. 1953. Parameters for the description of transition states. Science. 117:340–341. [DOI] [PubMed] [Google Scholar]
- Marks, T.N., and S.W. Jones. 1992. Calcium currents in the A7r5 smooth muscle-derived cell line. An allosteric model for calcium channel activation and dihydropyridine agonist action. J. Gen. Physiol. 99:367–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek, A., and A.R. Fersht. 1993. Application of physical organic chemistry to engineered mutants of proteins: Hammond postulate behavior in the transition state of protein folding. Proc. Natl. Acad. Sci. USA. 90:7814–7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matouschek, A., D.E. Otzen, L.S. Itzhaki, S.E. Jackson, and A.R. Fersht. 1995. Movement of the position of the transition state in protein folding. Biochemistry. 34:13656–13662. [DOI] [PubMed] [Google Scholar]
- Matthews, J.M., and A.R. Fersht. 1995. Exploring the energy surface of protein folding by structure-reactivity relationships and engineered proteins: observation of Hammond behavior for the gross structure of the transition state and anti-Hammond behavior for structural elements for unfolding/folding of barnase. Biochemistry. 34:6805–6814. [DOI] [PubMed] [Google Scholar]
- McCormack, K., W.J. Joiner, and S.H. Heinemann. 1994. A characterization of the activating structural rearrangements in voltage-dependent Shaker K+ channels [published erratum appears in Neuron 12:706]. Neuron. 12:301–315. [DOI] [PubMed] [Google Scholar]
- McManus, O.B., and K.L. Magleby. 1988. Kinetic states and modes of single large-conductance calcium-activated potassium channels in cultured rat skeletal muscle. J. Physiol. 402:79–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milla, M.E., B.M. Brown, C.D. Waldburger, and R.T. Sauer. 1995. P22 Arc repressor: transition state properties inferred from mutational effects on the rates of protein unfolding and refolding. Biochemistry. 34:13914–13919. [DOI] [PubMed] [Google Scholar]
- Moczydlowski, E., and R. Latorre. 1983. Gating kinetics of Ca2+-activated K+ channels from rat muscle incorporated into planar lipid bilayers. Evidence for two voltage-dependent Ca2+ binding reactions. J. Gen. Physiol. 82:511–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton, J. 1996. A Ba2+ chelator suppresses long shut events in fully activated high-conductance Ca2+-dependent K+ channels. Biophys. J. 71:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimigean, C.M., and K.L. Magleby. 2000. Functional coupling of the beta(1) subunit to the large conductance Ca2+-activated K(+) channel in the absence of Ca2+. Increased Ca2+ sensitivity from a Ca2+-independent mechanism. J. Gen. Physiol. 115:719–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveberg, M. 2001. Characterisation of the transition states for protein folding: towards a new level of mechanistic detail in protein engineering analysis. Curr. Opin. Struct. Biol. 11:94–100. [DOI] [PubMed] [Google Scholar]
- Oliveberg, M., Y.J. Tan, M. Silow, and A.R. Fersht. 1998. The changing nature of the protein folding transition state: implications for the shape of the free-energy profile for folding. J. Mol. Biol. 277:933–943. [DOI] [PubMed] [Google Scholar]
- Rae, J.L., J. Dewey, J.S. Rae, and K. Cooper. 1990. A maxi calcium-activated potassium channel from chick lens epithelium. Curr. Eye Res. 9:847–861. [DOI] [PubMed] [Google Scholar]
- Reinsch, C.H. 1967. Smoothing spline functions. Numeriche Mathematic. 10:177–183. [Google Scholar]
- Rios, E., M. Karhanek, J. Ma, and A. Gonzalez. 1993. An allosteric model of the molecular interactions of excitation-contraction coupling in skeletal muscle. J. Gen. Physiol. 102:449–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., and K.L. Magleby. 1998. Kinetic structure of large-conductance Ca2+-activated K+ channels suggests that the gating includes transitions through intermediate or secondary states. A mechanism for flickers. J. Gen. Physiol. 111:751–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., and K.L. Magleby. 1999. Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism. J. Gen. Physiol. 114:93–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., and K.L. Magleby. 2000. Voltage and Ca2+ activation of single large-conductance Ca2+-activated K+ channels described by a two-tiered allosteric gating mechanism. J. Gen. Physiol. 116:75–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, M., and L. Salkoff. 1997. A novel calcium-sensing domain in the BK channel. Biophys. J. 73:1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J., and J. Cui. 2001. Intracellular Mg2+ enhances the function of BK-type Ca2+-activated K(+) channels. J. Gen. Physiol. 118:589–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigg, D., and F. Bezanilla. 1997. Total charge movement per channel. The relation between gating charge displacement and the voltage sensitivity of activation. J. Gen. Physiol. 109:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigg, D., H. Qian, and F. Bezanilla. 1999. Kramers' diffusion theory applied to gating kinetics of voltage-dependent ion channels. Biophys. J. 76:782–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F.J. 1994. Voltage gating of ion channels. Q. Rev. Biophys. 27:1–40. [DOI] [PubMed] [Google Scholar]
- Silow, M., and M. Oliveberg. 1997. High-energy channeling in protein folding. Biochemistry. 36:7633–7637. [DOI] [PubMed] [Google Scholar]
- Stefani, E., M. Ottolia, F. Noceti, R. Olcese, M. Wallner, R. Latorre, and L. Toro. 1997. Voltage-controlled gating in a large conductance Ca2+-sensitive K+channel (hslo). Proc. Natl. Acad. Sci. USA. 94:5427–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder, G., and R.W. Aldrich. 2000. Complex voltage-dependent behavior of single unliganded calcium- sensitive potassium channels. Biophys. J. 78:761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X.D., H. Daggett, M. Hanner, M.L. Garcia, O.B. McManus, N. Brot, H. Weissbach, S.H. Heinemann, and T. Hoshi. 2001. Oxidative regulation of large conductance calcium-activated potassium channels. J. Gen. Physiol. 117:253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta, W.N., T. Hoshi, and R.W. Aldrich. 1994. Shaker potassium channel gating. III: Evaluation of kinetic models for activation. J. Gen. Physiol. 103:321–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., C.R. Solaro, and C.J. Lingle. 2001. Allosteric regulation of BK channel gating by Ca2+ and Mg2+ through a nonselective, low affinity divalent cation site. J. Gen. Physiol. 118:607–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J., L. Vankataramanan, and F.J. Sigworth. 2001. Hidden Markov model analysis of intermediate gating steps associated with the pore gate of shaker potassium channels. J. Gen. Physiol. 118:547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]