Abstract
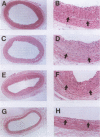
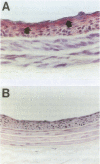
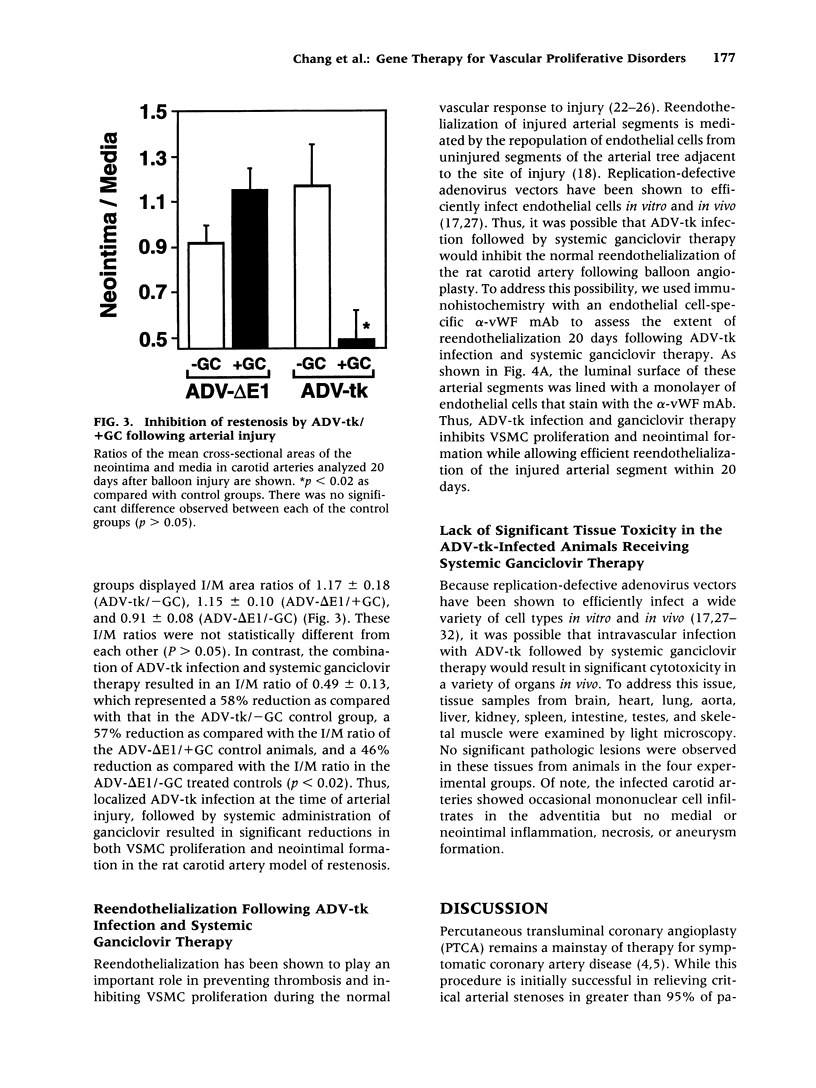
BACKGROUND: Vascular smooth muscle cell (VSMC) proliferation following arterial injury plays a critical role in a variety of vascular proliferative disorders, including atherosclerosis and restenosis after balloon angioplasty. In this study, we tested the hypothesis that localized arterial infection at the time of balloon angioplasty with an adenovirus (ADV-tk) encoding the herpes simplex virus thymidine kinase gene (HSV-tk), followed by systemic ganciclovir administration, can inhibit VSMC proliferation and neointima formation in a well-characterized model of arterial injury and restenosis. MATERIALS AND METHODS: The left carotid arteries of 31 male Sprague-Dawley rats were subjected to balloon angioplasty and immediately infected with 2 x 10(9) pfu of either ADV-tk or a control adenovirus that does not encode a recombinant protein (ADV-delta E1). Twenty-four hours after injury, animals from each experimental group were randomized to receive a course of systemic ganciclovir (ADV-tk/+GC, ADV delta E1/+GC) or saline (ADV-tk/-GC, ADV-delta E1/-GC). VSMC DNA synthesis was measured by 5'-bromodeoxuridine (BrdU) incorporation 2-4 days after balloon injury. The extent of restenosis, expressed as the neointima to media (I/M) area ratio was determined by digital planimetry 20 days after balloon injury in each of the four treatment groups. Immunohistochemistry using a mAb to von Willebrand factor (vWF) was used to determine the effects of ADV-tk infection and ganciclovir treatment on re-endothelialization of the carotid arteries 20 days following balloon angioplasty. RESULTS: Forty-one percent of the medial VSMCs in the ADV-tk/-GC arteries were labeled with BrdU 4 days after balloon injury. In contrast, ADV-tk infected animals that were treated with systemic ganciclovir (ADV-tk/+GC) displayed a 40% reduction in BrdU-staining medial VSMCs (p < 0.03). I/M area ratios of the three control groups were 1.17 +/- 0.18 (ADV-tk/-GC, n = 5), 1.15 +/- 0.10 (ADV-delta E1/+GC, n = 6), and 0.91 +/- 0.08 (ADV-delta E1/-GC, n = 6). These differences were not statistically significant (p > 0.05). In contrast, the ADV-tk/+GC animals (n = 6) displayed an I/M area ratio of 0.49 +/- 0.13 which was significantly lower than that seen in each of the three control groups (p < 0.02). None of the treated animals showed evidence of significant organ toxicity at autopsy. A regenerated endothelium was observed in the ADV-tk/+GC animals 20 days after balloon injury. CONCLUSIONS: Localized arterial infection with ADV-tk at the time of balloon angioplasty followed by systemic ganciclovir therapy reduces VSMC proliferation and neointimal expansion in the rat carotid artery injury model. Moreover, combined treatment with ADV-tk and systemic ganciclovir does not result in systemic toxicity and appears to selectively eliminate proliferating VSMCs, while preserving the capacity of the injured arterial segments to re-endothelialize within 3 weeks of injury. Taken together, these results support the feasibility of using this gene therapy approach for the treatment of human vascular proliferative disorders.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr E., Carroll J., Kalynych A. M., Tripathy S. K., Kozarsky K., Wilson J. M., Leiden J. M. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994 Jan;1(1):51–58. [PubMed] [Google Scholar]
- Baumgartner H. R., Muggli R., Tschopp T. B., Turitto V. T. Platelet adhesion, release and aggregation in flowing blood: effects of surface properties and platelet function. Thromb Haemost. 1976 Feb 29;35(1):124–138. [PubMed] [Google Scholar]
- Borrelli E., Heyman R., Hsi M., Evans R. M. Targeting of an inducible toxic phenotype in animal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7572–7576. doi: 10.1073/pnas.85.20.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield X. O., DeLuca N. A. Herpes simplex virus for gene delivery to neurons. New Biol. 1991 Mar;3(3):203–218. [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Van Gilder J., Link C. J., Carlstrom T., Buroker T., Yuh W., Koch K., Schabold K., Doornbas S., Wetjen B. Gene therapy for the treatment of malignant brain tumors with in vivo tumor transduction with the herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther. 1994 Mar;5(3):343–379. doi: 10.1089/hum.1994.5.3-343. [DOI] [PubMed] [Google Scholar]
- Ellis S. G., Roubin G. S., Wilentz J., Douglas J. S., Jr, King S. B., 3rd Effect of 18- to 24-hour heparin administration for prevention of restenosis after uncomplicated coronary angioplasty. Am Heart J. 1989 Apr;117(4):777–782. doi: 10.1016/0002-8703(89)90612-1. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Simon R. H., Yang Y., Zepeda M., Weber-Pendleton S., Doranz B., Grossman M., Wilson J. M. Adenovirus-mediated transfer of the CFTR gene to lung of nonhuman primates: biological efficacy study. Hum Gene Ther. 1993 Dec;4(6):759–769. doi: 10.1089/hum.1993.4.6-759. [DOI] [PubMed] [Google Scholar]
- Forrester J. S., Fishbein M., Helfant R., Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991 Mar 1;17(3):758–769. doi: 10.1016/s0735-1097(10)80196-2. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983 Nov;53(5):557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Guzman R. J., Lemarchand P., Crystal R. G., Epstein S. E., Finkel T. Efficient and selective adenovirus-mediated gene transfer into vascular neointima. Circulation. 1993 Dec;88(6):2838–2848. doi: 10.1161/01.cir.88.6.2838. [DOI] [PubMed] [Google Scholar]
- Heyman R. A., Borrelli E., Lesley J., Anderson D., Richman D. D., Baird S. M., Hyman R., Evans R. M. Thymidine kinase obliteration: creation of transgenic mice with controlled immune deficiency. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2698–2702. doi: 10.1073/pnas.86.8.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip J. H., Fuster V., Badimon L., Badimon J., Taubman M. B., Chesebro J. H. Syndromes of accelerated atherosclerosis: role of vascular injury and smooth muscle cell proliferation. J Am Coll Cardiol. 1990 Jun;15(7):1667–1687. doi: 10.1016/0735-1097(90)92845-s. [DOI] [PubMed] [Google Scholar]
- Kass-Eisler A., Falck-Pedersen E., Alvira M., Rivera J., Buttrick P. M., Wittenberg B. A., Cipriani L., Leinwand L. A. Quantitative determination of adenovirus-mediated gene delivery to rat cardiac myocytes in vitro and in vivo. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11498–11502. doi: 10.1073/pnas.90.24.11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau C., Lange R. A., Hillis L. D. Percutaneous transluminal coronary angioplasty. N Engl J Med. 1994 Apr 7;330(14):981–993. doi: 10.1056/NEJM199404073301407. [DOI] [PubMed] [Google Scholar]
- Lindner V., Olson N. E., Clowes A. W., Reidy M. A. Inhibition of smooth muscle cell proliferation in injured rat arteries. Interaction of heparin with basic fibroblast growth factor. J Clin Invest. 1992 Nov;90(5):2044–2049. doi: 10.1172/JCI116085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Moncada S., Herman A. G., Higgs E. A., Vane J. R. Differential formation of prostacyclin (PGX or PGI2) by layers of the arterial wall. An explanation for the anti-thrombotic properties of vascular endothelium. Thromb Res. 1977 Sep;11(3):323–344. doi: 10.1016/0049-3848(77)90185-2. [DOI] [PubMed] [Google Scholar]
- Moolten F. L., Wells J. M. Curability of tumors bearing herpes thymidine kinase genes transferred by retroviral vectors. J Natl Cancer Inst. 1990 Feb 21;82(4):297–300. doi: 10.1093/jnci/82.4.297. [DOI] [PubMed] [Google Scholar]
- Moolten F. L., Wells J. M., Heyman R. A., Evans R. M. Lymphoma regression induced by ganciclovir in mice bearing a herpes thymidine kinase transgene. Hum Gene Ther. 1990 Summer;1(2):125–134. doi: 10.1089/hum.1990.1.2-125. [DOI] [PubMed] [Google Scholar]
- Morishita R., Gibbons G. H., Ellison K. E., Nakajima M., Zhang L., Kaneda Y., Ogihara T., Dzau V. J. Single intraluminal delivery of antisense cdc2 kinase and proliferating-cell nuclear antigen oligonucleotides results in chronic inhibition of neointimal hyperplasia. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8474–8478. doi: 10.1073/pnas.90.18.8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. W., Ellis S. G., Topol E. J. Colchicine and antineoplastic therapy for the prevention of restenosis after percutaneous coronary interventions. J Am Coll Cardiol. 1991 May;17(6 Suppl B):126B–131B. doi: 10.1016/0735-1097(91)90948-9. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Shum L., Pompili V. J., Yang Z. Y., San H., Shu H. B., Liptay S., Gold L., Gordon D., Derynck R. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Gordon D., San H., Pompili V. J., Imperiale M. J., Nabel G. J., Nabel E. G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994 Aug 5;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Plautz G., Nabel E. G., Nabel G. J. Selective elimination of recombinant genes in vivo with a suicide retroviral vector. New Biol. 1991 Jul;3(7):709–715. [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. S., Holmes D. R., Jr, Topol E. J. The restenosis paradigm revisited: an alternative proposal for cellular mechanisms. J Am Coll Cardiol. 1992 Nov 1;20(5):1284–1293. doi: 10.1016/0735-1097(92)90389-5. [DOI] [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton M. A., Gruentzig A. R., Hollman J., King S. B., 3rd, Douglas J. S. Coumadin and aspirin in prevention of recurrence after transluminal coronary angioplasty: a randomized study. Circulation. 1984 Apr;69(4):721–727. doi: 10.1161/01.cir.69.4.721. [DOI] [PubMed] [Google Scholar]
- Völker W., Faber V. Aspirin reduces the growth of medial and neointimal thickenings in balloon-injured rat carotid arteries. Stroke. 1990 Dec;21(12 Suppl):IV44–IV45. [PubMed] [Google Scholar]
- Yang Y., Ertl H. C., Wilson J. M. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity. 1994 Aug;1(5):433–442. doi: 10.1016/1074-7613(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Petersen D. M., Puga A. P., Graham S. M., Couture L. A., Keyes L. D., Lukason M. J., St George J. A., Gregory R. J., Smith A. E. Safety and efficacy of repetitive adenovirus-mediated transfer of CFTR cDNA to airway epithelia of primates and cotton rats. Nat Genet. 1994 Jan;6(1):75–83. doi: 10.1038/ng0194-75. [DOI] [PubMed] [Google Scholar]