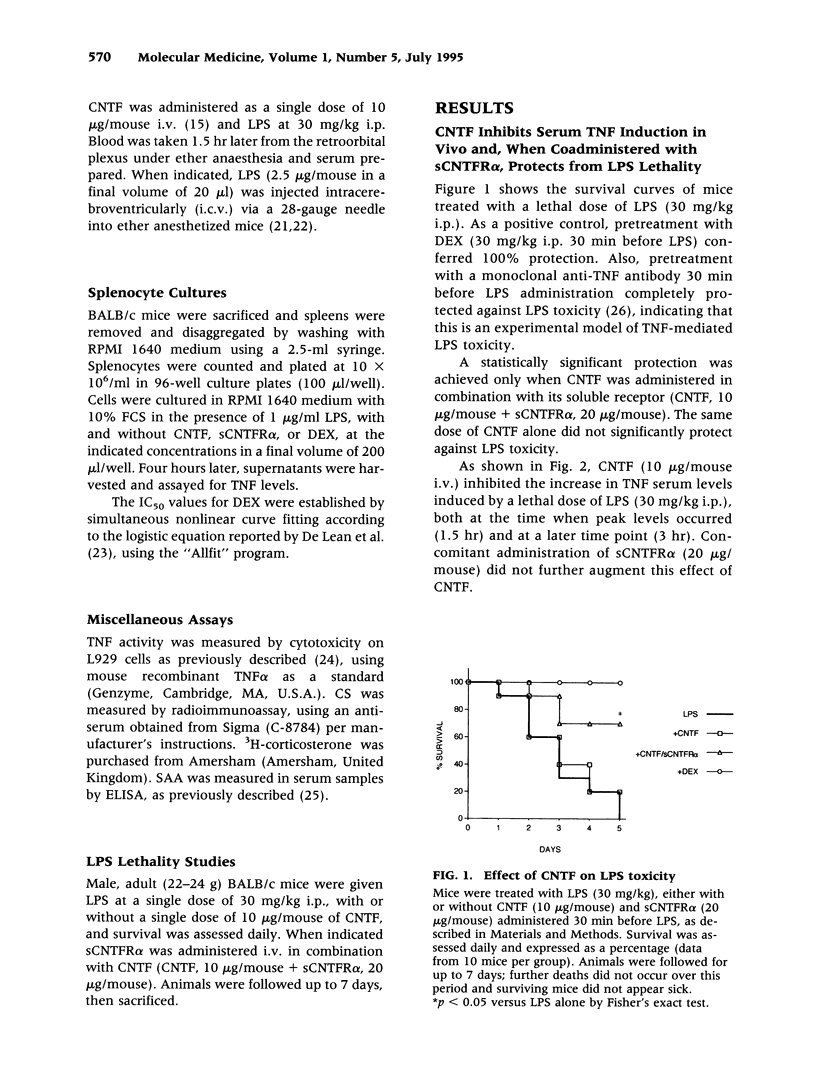
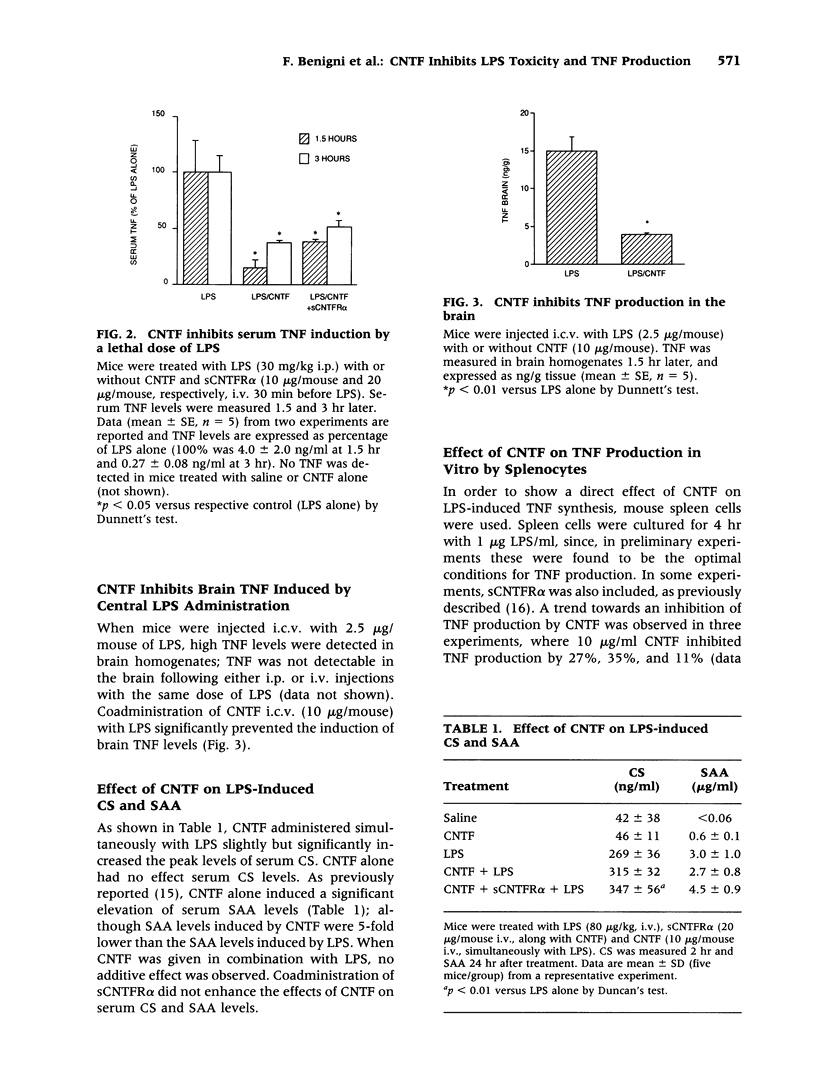
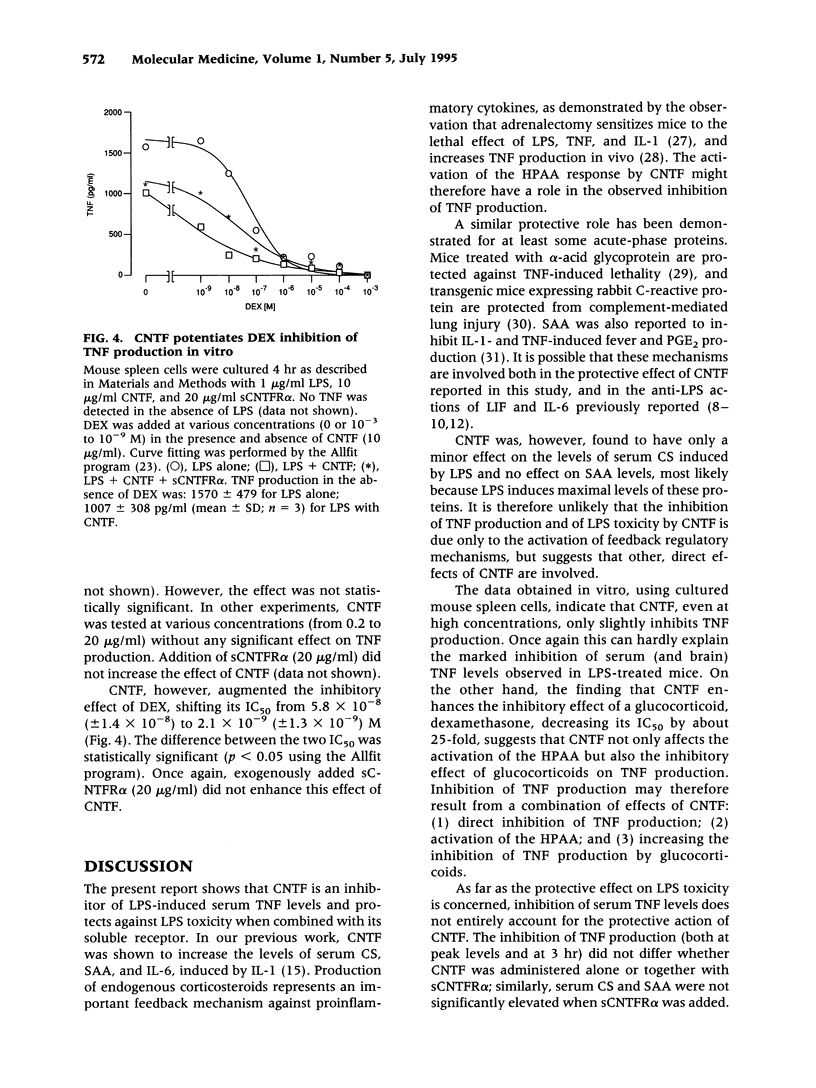
Abstract
BACKGROUND: The receptor of ciliary neurotrophic factor (CNTF) contains the signal transduction protein gp130, which is also a component of the receptors of cytokines such as interleukin (IL)-6, leukemia-inhibitory factor (LIF), IL-11, and oncostatin M. This suggests that these cytokines might share common signaling pathways. We previously reported that CNTF augments the levels of corticosterone (CS) and of IL-6 induced by IL-1 and induces the production of the acute-phase protein serum amyloid A (SAA). Since the elevation of serum CS is an important feedback mechanism to limit the synthesis of proinflammatory cytokines, particularly tumor necrosis factor (TNF), we have investigated the effect of CNTF on both TNF production and lipopolysaccharide (LPS) toxicity. MATERIALS AND METHODS: To induce serum TNF levels, LPS was administered to mice at 30 mg/kg i.p. and CNTF was administered as a single dose of 10 micrograms/mouse i.v., either alone or in combination with its soluble receptor sCNTFR alpha at 20 micrograms/mouse. Serum TNF levels were the measured by cytotoxicity on L929 cells. In order to measure the effects of CNTF on LPS-induced TNF production in the brain, mice were injected intracerebroventricularly (i.c.v.) with 2.5 micrograms/kg LPS. Mouse spleen cells cultured for 4 hr with 1 microgram LPS/ml, with or without 10 micrograms CNTF/ml, were also analyzed for TNF production. RESULTS: CNTF, administered either alone or in combination with its soluble receptor, inhibited the induction of serum TNF levels by LPS. This inhibition was also observed in the brain when CNTF and LPS were administered centrally. In vitro, CNTF only marginally affected TNF production by LPS-stimulated mouse splenocytes, but it acted synergistically with dexamethasone (DEX) in inhibiting TNF production. Most importantly, CNTF administered together with sCNTFR alpha protected mice against LPS-induced mortality. CONCLUSIONS: These data suggest that CNTF might act as a protective cytokine against TNF-mediated pathologies both in the brain and in the periphery.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Le J. M., Vilcek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989 Dec 1;143(11):3517–3523. [PubMed] [Google Scholar]
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Alexander H. R., Wong G. G., Doherty G. M., Venzon D. J., Fraker D. L., Norton J. A. Differentiation factor/leukemia inhibitory factor protection against lethal endotoxemia in mice: synergistic effect with interleukin 1 and tumor necrosis factor. J Exp Med. 1992 Apr 1;175(4):1139–1142. doi: 10.1084/jem.175.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Wong G. G. Hepatocyte-stimulating factor III shares structural and functional identity with leukemia-inhibitory factor. J Immunol. 1989 Aug 15;143(4):1163–1167. [PubMed] [Google Scholar]
- Bertini R., Bianchi M., Ghezzi P. Adrenalectomy sensitizes mice to the lethal effects of interleukin 1 and tumor necrosis factor. J Exp Med. 1988 May 1;167(5):1708–1712. doi: 10.1084/jem.167.5.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besedovsky H., del Rey A., Sorkin E., Dinarello C. A. Immunoregulatory feedback between interleukin-1 and glucocorticoid hormones. Science. 1986 Aug 8;233(4764):652–654. doi: 10.1126/science.3014662. [DOI] [PubMed] [Google Scholar]
- Davis S., Aldrich T. H., Ip N. Y., Stahl N., Scherer S., Farruggella T., DiStefano P. S., Curtis R., Panayotatos N., Gascan H. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993 Mar 19;259(5102):1736–1739. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mancilla J., Bishai I., Lees J., Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 1991 Oct 25;562(2):199–206. doi: 10.1016/0006-8993(91)90622-3. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G., Benigni F., Sironi M., Conni M., Carelli M., Cantoni L., Shapiro L., Dinarello C. A., Sipe J. D., Ghezzi P. Ciliary neurotrophic factor (CNTF) induces serum amyloid A, hypoglycaemia and anorexia, and potentiates IL-1 induced corticosterone and IL-6 production in mice. Cytokine. 1995 Feb;7(2):150–156. doi: 10.1006/cyto.1995.1020. [DOI] [PubMed] [Google Scholar]
- Fattori E., Cappelletti M., Costa P., Sellitto C., Cantoni L., Carelli M., Faggioni R., Fantuzzi G., Ghezzi P., Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994 Oct 1;180(4):1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti S., Faggioni R., Echtenacher B., Ghezzi P. Role of tumour necrosis factor and reactive oxygen intermediates in lipopolysaccharide-induced pulmonary oedema and lethality. Clin Exp Immunol. 1993 Mar;91(3):456–461. doi: 10.1111/j.1365-2249.1993.tb05924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALEY T. J., MCCORMICK W. G. Pharmacological effects produced by intracerebral injection of drugs in the conscious mouse. Br J Pharmacol Chemother. 1957 Mar;12(1):12–15. doi: 10.1111/j.1476-5381.1957.tb01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heremans H., Dillen C., Put W., Van Damme J., Billiau A. Protective effect of anti-interleukin (IL)-6 antibody against endotoxin, associated with paradoxically increased IL-6 levels. Eur J Immunol. 1992 Sep;22(9):2395–2401. doi: 10.1002/eji.1830220932. [DOI] [PubMed] [Google Scholar]
- Ip N. Y., Nye S. H., Boulton T. G., Davis S., Taga T., Li Y., Birren S. J., Yasukawa K., Kishimoto T., Anderson D. J. CNTF and LIF act on neuronal cells via shared signaling pathways that involve the IL-6 signal transducing receptor component gp130. Cell. 1992 Jun 26;69(7):1121–1132. doi: 10.1016/0092-8674(92)90634-o. [DOI] [PubMed] [Google Scholar]
- Libert C., Brouckaert P., Fiers W. Protection by alpha 1-acid glycoprotein against tumor necrosis factor-induced lethality. J Exp Med. 1994 Oct 1;180(4):1571–1575. doi: 10.1084/jem.180.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton J. M., Macaluso A., Hiltz M. E., Catania A. Central administration of the peptide alpha-MSH inhibits inflammation in the skin. Peptides. 1991 Jul-Aug;12(4):795–798. doi: 10.1016/0196-9781(91)90135-c. [DOI] [PubMed] [Google Scholar]
- MacPhee I. A., Antoni F. A., Mason D. W. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989 Feb 1;169(2):431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiakowski P., Liu H. X., Radziejewski C., Lottspeich F., Oberthuer W., Wong V., Lindsay R. M., Furth M. E., Panayotatos N. Recombinant human and rat ciliary neurotrophic factors. J Neurochem. 1991 Sep;57(3):1003–1012. doi: 10.1111/j.1471-4159.1991.tb08250.x. [DOI] [PubMed] [Google Scholar]
- Mason D. Genetic variation in the stress response: susceptibility to experimental allergic encephalomyelitis and implications for human inflammatory disease. Immunol Today. 1991 Feb;12(2):57–60. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- Mayer M., Noble M. N-acetyl-L-cysteine is a pluripotent protector against cell death and enhancer of trophic factor-mediated cell survival in vitro. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengozzi M., Fantuzzi G., Faggioni R., Marchant A., Goldman M., Orencole S., Clark B. D., Sironi M., Benigni F., Ghezzi P. Chlorpromazine specifically inhibits peripheral and brain TNF production, and up-regulates IL-10 production, in mice. Immunology. 1994 Jun;82(2):207–210. [PMC free article] [PubMed] [Google Scholar]
- Panayotatos N., Everdeen D., Liten A., Somogyi R., Acheson A. Recombinant human CNTF receptor alpha: production, binding stoichiometry, and characterization of its activity as a diffusible factor. Biochemistry. 1994 May 17;33(19):5813–5818. doi: 10.1021/bi00185a020. [DOI] [PubMed] [Google Scholar]
- Parant M., Le Contel C., Parant F., Chedid L. Influence of endogenous glucocorticoid on endotoxin-induced production of circulating TNF-alpha. Lymphokine Cytokine Res. 1991 Aug;10(4):265–271. [PubMed] [Google Scholar]
- Pennica D., King K. L., Shaw K. J., Luis E., Rullamas J., Luoh S. M., Darbonne W. C., Knutzon D. S., Yen R., Chien K. R. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci U S A. 1995 Feb 14;92(4):1142–1146. doi: 10.1073/pnas.92.4.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein R. S., Mougey E. H., Jackson W. E., Neta R. Interleukin-1 and interleukin-6 act synergistically to stimulate the release of adrenocorticotropic hormone in vivo. Lymphokine Cytokine Res. 1991 Apr;10(1-2):141–146. [PubMed] [Google Scholar]
- Richards C. D., Brown T. J., Shoyab M., Baumann H., Gauldie J. Recombinant oncostatin M stimulates the production of acute phase proteins in HepG2 cells and rat primary hepatocytes in vitro. J Immunol. 1992 Mar 15;148(6):1731–1736. [PubMed] [Google Scholar]
- Rodriguez M., Pavelko K. D., McKinney C. W., Leibowitz J. L. Recombinant human IL-6 suppresses demyelination in a viral model of multiple sclerosis. J Immunol. 1994 Oct 15;153(8):3811–3821. [PubMed] [Google Scholar]
- Shainkin-Kestenbaum R., Berlyne G., Zimlichman S., Sorin H. R., Nyska M., Danon A. Acute phase protein, serum amyloid A, inhibits IL-1- and TNF-induced fever and hypothalamic PGE2 in mice. Scand J Immunol. 1991 Aug;34(2):179–183. doi: 10.1111/j.1365-3083.1991.tb01535.x. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Panayotatos N., Meydani S. N., Wu D., Dinarello C. A. Ciliary neurotrophic factor combined with soluble receptor inhibits synthesis of proinflammatory cytokines and prostaglandin-E2 in vitro. Exp Cell Res. 1994 Nov;215(1):51–56. doi: 10.1006/excr.1994.1313. [DOI] [PubMed] [Google Scholar]
- Sipe J. D., Gonnerman W. A., Loose L. D., Knapschaefer G., Xie W. J., Franzblau C. Direct binding enzyme-linked immunosorbent assay (ELISA) for serum amyloid A (SAA). J Immunol Methods. 1989 Dec 20;125(1-2):125–135. doi: 10.1016/0022-1759(89)90085-9. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Cerami A. Tumor necrosis factor, other cytokines and disease. Annu Rev Cell Biol. 1993;9:317–343. doi: 10.1146/annurev.cb.09.110193.001533. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Fann M. J., Patterson P. H., Williams J. H., Samal B., Del Castillo J., Yin S., Guo K., Remick D. G. Intratracheal injection of LPS and cytokines. V. LPS induces expression of LIF and LIF inhibits acute inflammation. Am J Physiol. 1994 Oct;267(4 Pt 1):L442–L446. doi: 10.1152/ajplung.1994.267.4.L442. [DOI] [PubMed] [Google Scholar]


