Abstract
BACKGROUND: Heme oxygenase (HO; EC 1.14.99.3) catalyzes the conversion of heme to biliverdin, which is reduced enzymatically to bilirubin. Since bilirubin is a potent antioxidant and heme a pro-oxidant, HO may protect cells against oxidative damage. HO-1 is highly inducible by diverse chemical agents, resembling those evoking induction of phase 2 enzymes (i.e., Michael reaction acceptors, heavy metals, trivalent arsenicals, and sulfhydryl reagents). Phase 2 enzymes (glutathione transferases; NAD (P)H:quinone reductase; glucuronosyltransferases) are regulated by antioxidant-responsive elements (ARE), and their induction protects against chemical carcinogenesis. Is HO-1 regulated by chemical agents and enhancer elements similar to those controlling phase 2 enzymes? MATERIALS AND METHODS: Induction of HO-1 by phorbol ester and heavy metals is transcriptionally controlled through a 268-bp SX2 fragment, containing two phorbol ester-responsive (TRE) sites (TGAC/GT C/AA) which overlap ARE consensus sequences (TGACNNNGC). Therefore, mutations of the SX2 element designed to distinguish ARE from TRE were inserted into chloramphenicol acetyltransferase (CAT) reporter plasmids, and the response of the CAT activity of murine hepatoma cells stably transfected with these constructs was examined with a wide range of inducers of phase 2 enzymes. RESULTS: All compounds raised HO-1 mRNA and CAT expression constructs containing wild-type SX2. When the SX2 region was mutated to alter TRE consensus sequences without destroying the ARE consensus, full inducibility was preserved. Conversely, when the ARE consensus was disturbed, inducibility was abolished. CONCLUSION: Induction of heme oxygenase-1 is regulated by several chemically distinct classes of inducers (mostly electrophiles), which also induce phase 2 enzymes, and these inductions are mediated by similar AREs. These findings support the importance of HO-1 as a protector against oxidative damage and suggest that HO-1 induction is part of a more generalized protective cellular response that involves phase 2 enzymes.
Full text
PDF



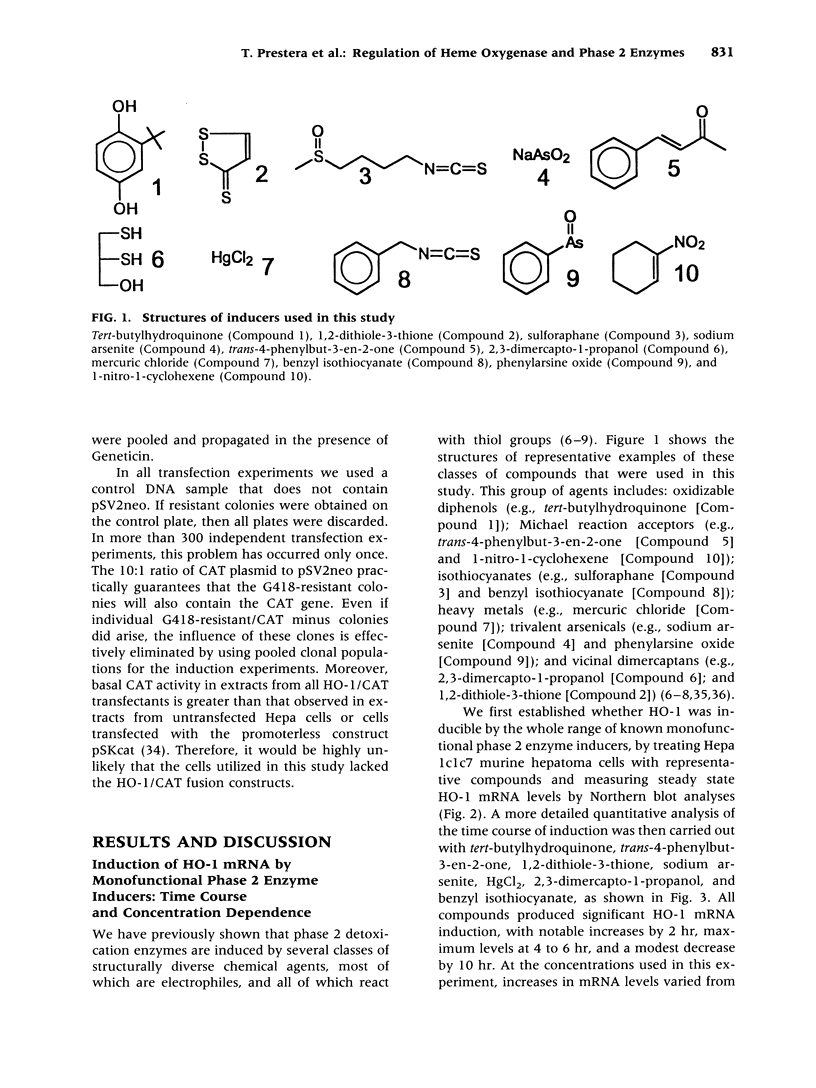
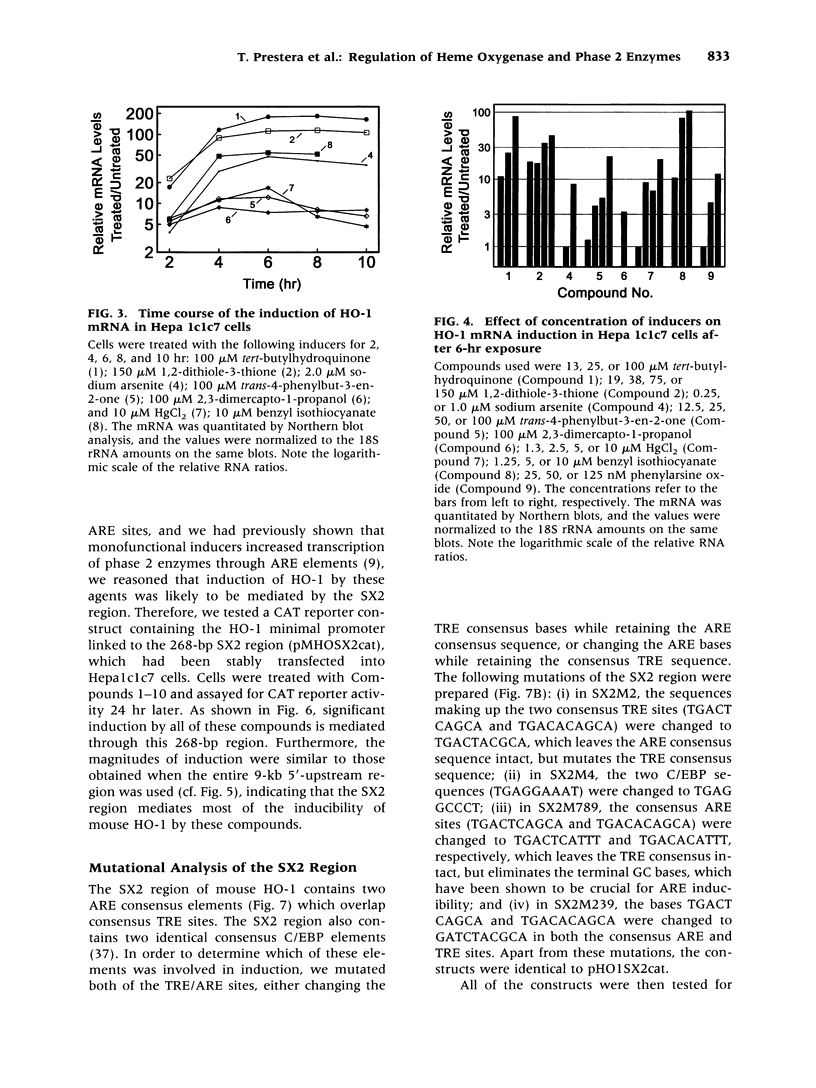


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Cai J., Smith A. Isolation and characterization of the mouse heme oxygenase-1 gene. Distal 5' sequences are required for induction by heme or heavy metals. J Biol Chem. 1994 Jan 14;269(2):1001–1009. [PubMed] [Google Scholar]
- Alam J., Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992 Oct 25;267(30):21894–21900. [PubMed] [Google Scholar]
- Alam J. Multiple elements within the 5' distal enhancer of the mouse heme oxygenase-1 gene mediate induction by heavy metals. J Biol Chem. 1994 Oct 7;269(40):25049–25056. [PubMed] [Google Scholar]
- Alam J., Yu N., Irias S., Cook J. L., Vig E. Reduced chloramphenicol acetyltransferase activity observed with vectors containing an upstream SphI recognition sequence. Biotechniques. 1991 Apr;10(4):422, 424-5. [PubMed] [Google Scholar]
- Applegate L. A., Luscher P., Tyrrell R. M. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991 Feb 1;51(3):974–978. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. P., Nickoloff J. A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992 Jan;200(1):81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- Egner P. A., Kensler T. W., Prestera T., Talalay P., Libby A. H., Joyner H. H., Curphey T. J. Regulation of phase 2 enzyme induction by oltipraz and other dithiolethiones. Carcinogenesis. 1994 Feb;15(2):177–181. doi: 10.1093/carcin/15.2.177. [DOI] [PubMed] [Google Scholar]
- Favreau L. V., Pickett C. B. Transcriptional regulation of the rat NAD(P)H:quinone reductase gene. Identification of regulatory elements controlling basal level expression and inducible expression by planar aromatic compounds and phenolic antioxidants. J Biol Chem. 1991 Mar 5;266(7):4556–4561. [PubMed] [Google Scholar]
- Friling R. S., Bensimon A., Tichauer Y., Daniel V. Xenobiotic-inducible expression of murine glutathione S-transferase Ya subunit gene is controlled by an electrophile-responsive element. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6258–6262. doi: 10.1073/pnas.87.16.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friling R. S., Bergelson S., Daniel V. Two adjacent AP-1-like binding sites form the electrophile-responsive element of the murine glutathione S-transferase Ya subunit gene. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):668–672. doi: 10.1073/pnas.89.2.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. K. Human NAD(P)H:quinone oxidoreductase (NQO1) gene structure and induction by dioxin. Biochemistry. 1991 Nov 5;30(44):10647–10653. doi: 10.1021/bi00108a007. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ziegler D. M. The enzymes of detoxication. J Biol Chem. 1990 Dec 5;265(34):20715–20718. [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lee T. C., Ho I. C. Expression of heme oxygenase in arsenic-resistant human lung adenocarcinoma cells. Cancer Res. 1994 Apr 1;54(7):1660–1664. [PubMed] [Google Scholar]
- Nguyen T., Rushmore T. H., Pickett C. B. Transcriptional regulation of a rat liver glutathione S-transferase Ya subunit gene. Analysis of the antioxidant response element and its activation by the phorbol ester 12-O-tetradecanoylphorbol-13-acetate. J Biol Chem. 1994 May 6;269(18):13656–13662. [PubMed] [Google Scholar]
- Prestera T., Holtzclaw W. D., Zhang Y., Talalay P. Chemical and molecular regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2965–2969. doi: 10.1073/pnas.90.7.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T., Talalay P. Electrophile and antioxidant regulation of enzymes that detoxify carcinogens. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8965–8969. doi: 10.1073/pnas.92.19.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestera T., Zhang Y., Spencer S. R., Wilczak C. A., Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul. 1993;33:281–296. doi: 10.1016/0065-2571(93)90024-8. [DOI] [PubMed] [Google Scholar]
- Prochaska H. J., Talalay P. Regulatory mechanisms of monofunctional and bifunctional anticarcinogenic enzyme inducers in murine liver. Cancer Res. 1988 Sep 1;48(17):4776–4782. [PubMed] [Google Scholar]
- Putzer R. R., Zhang Y., Prestera T., Holtzclaw W. D., Wade K. L., Talalay P. Mercurials and dimercaptans: synergism in the induction of chemoprotective enzymes. Chem Res Toxicol. 1995 Jan-Feb;8(1):103–110. doi: 10.1021/tx00043a014. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Morton M. R., Pickett C. B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J Biol Chem. 1991 Jun 25;266(18):11632–11639. [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stocker R., Glazer A. N., Ames B. N. Antioxidant activity of albumin-bound bilirubin. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Talalay P., De Long M. J., Prochaska H. J. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8261–8265. doi: 10.1073/pnas.85.21.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talalay P. Mechanisms of induction of enzymes that protect against chemical carcinogenesis. Adv Enzyme Regul. 1989;28:237–250. doi: 10.1016/0065-2571(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Basu-Modak S., Waltner C., Tyrrell R. M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Williamson G. Detection of a nuclear protein which binds specifically to the antioxidant responsive element (ARE) of the human NAD(P) H:quinone oxidoreductase gene. Biochim Biophys Acta. 1994 Nov 22;1219(3):645–652. doi: 10.1016/0167-4781(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Yoshioka K., Deng T., Cavigelli M., Karin M. Antitumor promotion by phenolic antioxidants: inhibition of AP-1 activity through induction of Fra expression. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4972–4976. doi: 10.1073/pnas.92.11.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Talalay P., Cho C. G., Posner G. H. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]