Abstract
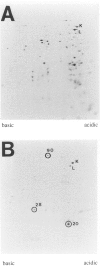
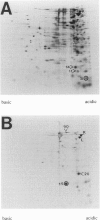
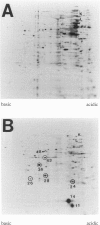
BACKGROUND: Mycobacterium tuberculosis is a significant human pathogen capable of replicating in mononuclear phagocytic cells. Exposure to reactive oxygen and nitrogen intermediates is likely to represent an important aspect of the life cycle of this organism. The response of M. tuberculosis to these agents may be of significance for its survival in the host. MATERIALS AND METHODS: Patterns of de novo proteins synthesized in M. tuberculosis H37Rv exposed to compounds that generate reactive oxygen and nitrogen intermediates were studied by metabolic labeling and two-dimensional electrophoresis. RESULTS: Menadione, a redox cycling compound which increases intracellular superoxide levels, caused enhanced synthesis of seven polypeptides, six of which appeared to be heat shock proteins. Chemical release of nitric oxide induced eight polypeptides of which only one could be identified as a heat shock protein. Nitric oxide also exhibited a mild inhibitory action on general protein synthesis in the concentration range tested. Hydrogen peroxide did not cause differential gene expression and exerted a generalized inhibition in a dose-dependent manner. Cumene hydroperoxide caused mostly inhibition but induction of two heat shock proteins was detectable. CONCLUSIONS: The presented findings indicate major differences between M. tuberculosis and the paradigms of oxidative stress response in enteric bacteria, and are consistent with the multiple lesions found in oxyR of this organism. The effect of hydrogen peroxide, which in Escherichia coli induces eight polypeptides known to be controlled by the central regulator oxyR, appears to be absent in M. tuberculosis. Superoxide and nitric oxide responses, which in E. coli overlap and are controlled by the same regulatory system soxRS, represent discrete and independent phenomena in M. tuberculosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altuvia S., Almirón M., Huisman G., Kolter R., Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994 Jul;13(2):265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Amábile-Cuevas C. F., Demple B. Molecular characterization of the soxRS genes of Escherichia coli: two genes control a superoxide stress regulon. Nucleic Acids Res. 1991 Aug 25;19(16):4479–4484. doi: 10.1093/nar/19.16.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B. Regulation of the Escherichia coli heat-shock response. Mol Microbiol. 1993 Aug;9(4):671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- Chan J., Tanaka K., Carroll D., Flynn J., Bloom B. R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995 Feb;63(2):736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J., Xing Y., Magliozzo R. S., Bloom B. R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992 Apr 1;175(4):1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman M. F., Morgan R. W., Jacobson F. S., Ames B. N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985 Jul;41(3):753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- Cooper J. B., McIntyre K., Badasso M. O., Wood S. P., Zhang Y., Garbe T. R., Young D. X-ray structure analysis of the iron-dependent superoxide dismutase from Mycobacterium tuberculosis at 2.0 Angstroms resolution reveals novel dimer-dimer interactions. J Mol Biol. 1995 Mar 3;246(4):531–544. doi: 10.1006/jmbi.1994.0105. [DOI] [PubMed] [Google Scholar]
- Demple B., Amábile-Cuevas C. F. Redox redux: the control of oxidative stress responses. Cell. 1991 Nov 29;67(5):837–839. doi: 10.1016/0092-8674(91)90355-3. [DOI] [PubMed] [Google Scholar]
- Denis M. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell Immunol. 1991 Jan;132(1):150–157. doi: 10.1016/0008-8749(91)90014-3. [DOI] [PubMed] [Google Scholar]
- Deretic V., Philipp W., Dhandayuthapani S., Mudd M. H., Curcic R., Garbe T., Heym B., Via L. E., Cole S. T. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol Microbiol. 1995 Sep;17(5):889–900. doi: 10.1111/j.1365-2958.1995.mmi_17050889.x. [DOI] [PubMed] [Google Scholar]
- Farr S. B., Kogoma T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol Rev. 1991 Dec;55(4):561–585. doi: 10.1128/mr.55.4.561-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A., Babbedge R., Brave S. R., Hart S. L., Hobbs A. J., Tucker J. F., Wallace P., Moore P. K. An investigation of some S-nitrosothiols, and of hydroxy-arginine, on the mouse anococcygeus. Br J Pharmacol. 1992 Nov;107(3):715–721. doi: 10.1111/j.1476-5381.1992.tb14512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. A global response induced in Escherichia coli by redox-cycling agents overlaps with that induced by peroxide stress. J Bacteriol. 1989 Jul;171(7):3933–3939. doi: 10.1128/jb.171.7.3933-3939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heym B., Zhang Y., Poulet S., Young D., Cole S. T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. J Bacteriol. 1993 Jul;175(13):4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994 Jan 1;13(1):138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Aber V. R., Mitchison D. A., Lowrie D. B. The contribution of hydrogen peroxide resistance to virulence of Mycobacterium tuberculosis during the first six days after intravenous infection of normal and BCG-vaccinated guinea-pigs. Br J Exp Pathol. 1981 Feb;62(1):34–40. [PMC free article] [PubMed] [Google Scholar]
- Jackett P. S., Andrew P. W., Aber V. R., Lowrie D. B. Guinea pig alveolar macrophages probably kill M. tuberculosis H37Rv and H37Ra in vivo by producing hydrogen peroxide. Adv Exp Med Biol. 1983;162:99–104. doi: 10.1007/978-1-4684-4481-0_10. [DOI] [PubMed] [Google Scholar]
- Kong T. H., Coates A. R., Butcher P. D., Hickman C. J., Shinnick T. M. Mycobacterium tuberculosis expresses two chaperonin-60 homologs. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2608–2612. doi: 10.1073/pnas.90.7.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev S. I., Fridovich I. Fumarase C, the stable fumarase of Escherichia coli, is controlled by the soxRS regulon. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5892–5896. doi: 10.1073/pnas.89.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liochev S. I., Hausladen A., Beyer W. F., Jr, Fridovich I. NADPH: ferredoxin oxidoreductase acts as a paraquat diaphorase and is a member of the soxRS regulon. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1328–1331. doi: 10.1073/pnas.91.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. F., Mills J. Pulmonary infectious complications of human immunodeficiency virus infection. Part I. Am Rev Respir Dis. 1990 May;141(5 Pt 1):1356–1372. doi: 10.1164/ajrccm/141.5_Pt_1.1356. [DOI] [PubMed] [Google Scholar]
- Nunoshiba T., DeRojas-Walker T., Tannenbaum S. R., Demple B. Roles of nitric oxide in inducible resistance of Escherichia coli to activated murine macrophages. Infect Immun. 1995 Mar;63(3):794–798. doi: 10.1128/iai.63.3.794-798.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunoshiba T., deRojas-Walker T., Wishnok J. S., Tannenbaum S. R., Demple B. Activation by nitric oxide of an oxidative-stress response that defends Escherichia coli against activated macrophages. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9993–9997. doi: 10.1073/pnas.90.21.9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Privalle C. T., Fridovich I. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. Effects of diazenedicarboxylic acid bis(N,N'-dimethylamide) (diamide). J Biol Chem. 1990 Dec 15;265(35):21966–21970. [PubMed] [Google Scholar]
- Storz G., Altuvia S. OxyR regulon. Methods Enzymol. 1994;234:217–223. doi: 10.1016/0076-6879(94)34088-9. [DOI] [PubMed] [Google Scholar]
- Tsaneva I. R., Weiss B. soxR, a locus governing a superoxide response regulon in Escherichia coli K-12. J Bacteriol. 1990 Aug;172(8):4197–4205. doi: 10.1128/jb.172.8.4197-4205.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walkup L. K., Kogoma T. Escherichia coli proteins inducible by oxidative stress mediated by the superoxide radical. J Bacteriol. 1989 Mar;171(3):1476–1484. doi: 10.1128/jb.171.3.1476-1484.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L., Kreimer D., Roth E., Silman I. Oxidative stress transforms acetylcholinesterase to a molten-globule-like state. Biochem Biophys Res Commun. 1994 Feb 15;198(3):915–922. doi: 10.1006/bbrc.1994.1130. [DOI] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Heat shock proteins and antigens of Mycobacterium tuberculosis. Infect Immun. 1991 Sep;59(9):3086–3093. doi: 10.1128/iai.59.9.3086-3093.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Zhu L., Gunn C., Beckman J. S. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]