Abstract
BACKGROUND: Based on the hypothesis that IgGs are potent tolerogens and that immature lymphohematopoietic antigen-presenting cells (APC), and even mature peripheral B cells, may be effective APC for tolerance induction, we designed an immunoglobulin fusion protein retroviral expression vector to test the role of B cells in a novel gene therapy strategy for the transfer of immune tolerance. METHODS: An immunodominant epitope (residues 12-26 of the lambda repressor cI protein) was fused in frame to an IgG heavy chain in a retroviral vector, which was used to infect either bone marrow cells or activated peripheral B lymphocytes. These cells were transferred into syngeneic recipients, who were subsequently challenged with the 12-26 peptide in adjuvant. RESULTS: Bone marrow (BM) chimeras generated with retrovirally transduced bone marrow were shown to be profoundly unresponsive to the 12-26 peptide at both the humoral and cellular levels, but were competent to respond to an unrelated protein (lysozyme or PPD). Importantly, we also show that immunocompetent adult recipients infused with transduced mature, activated B lymphocytes, are rendered unresponsive by this treatment. Surprisingly, lymphoid-deficient BM progenitors from syngeneic SCID donors could also be transduced to produce tolerogenic APC. CONCLUSIONS: Our data suggest that activated B cells are sufficient to be effective tolerogenic APC in immunocompetent adult mice, but that nonlymphoid cells may also induce tolerance in reconstituted hosts. This approach for gene-transferred tolerogenesis has the potential to be maintained indefinitely, and it requires only knowledge of cDNA sequences of target antigens.
Full text
PDF



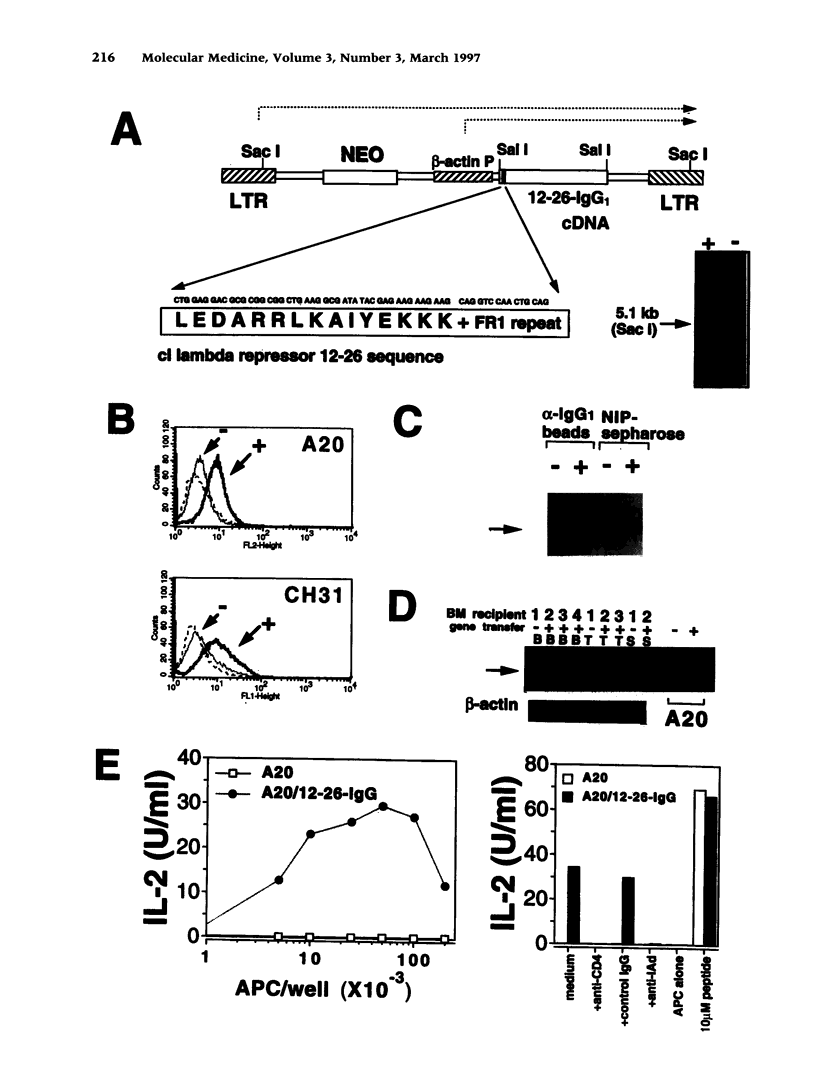

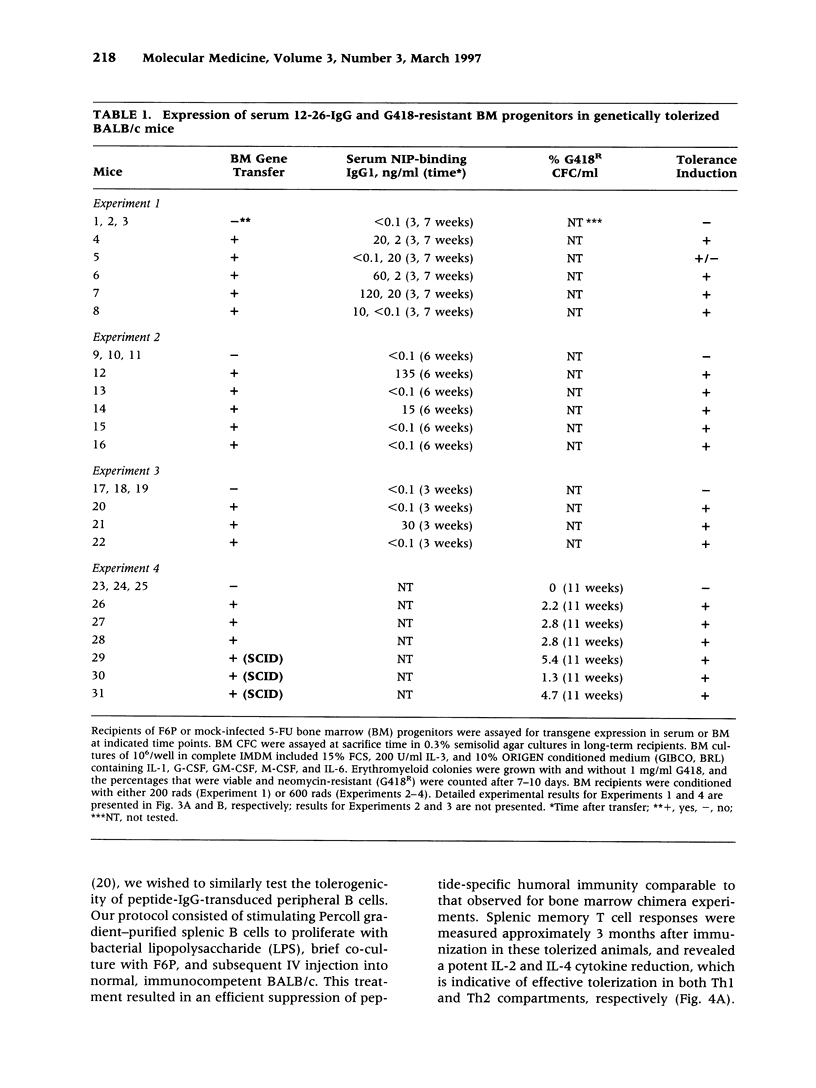
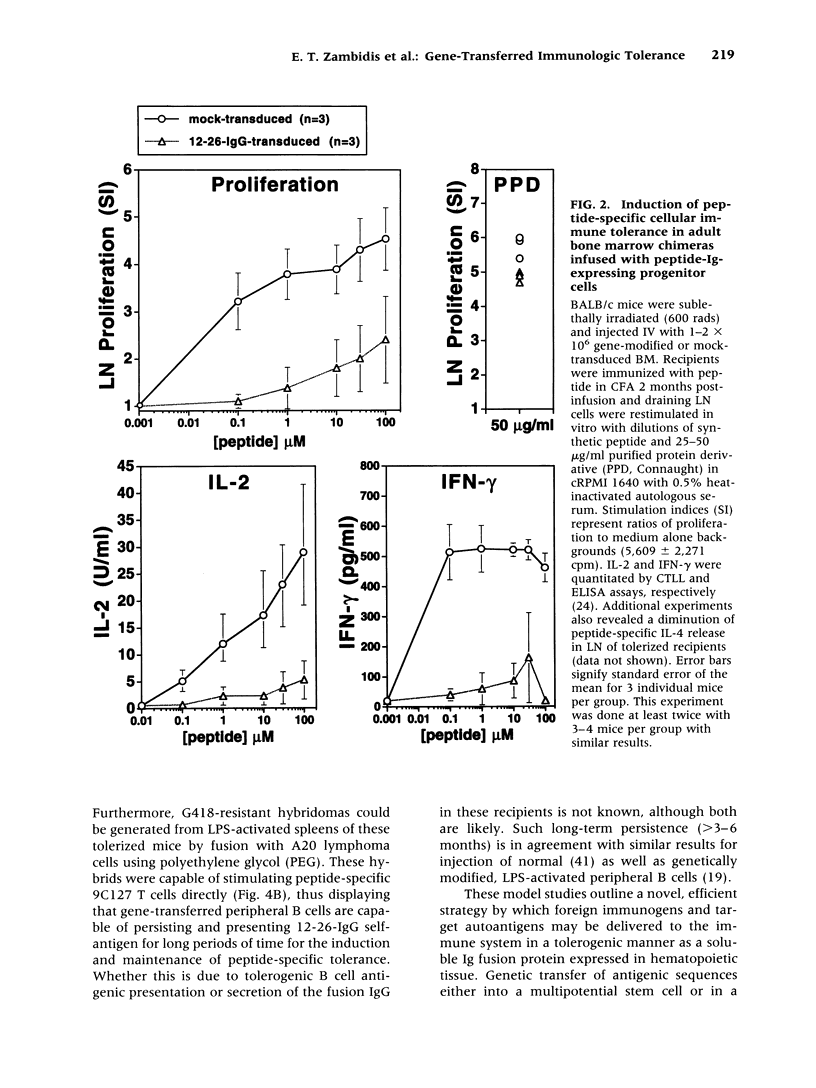
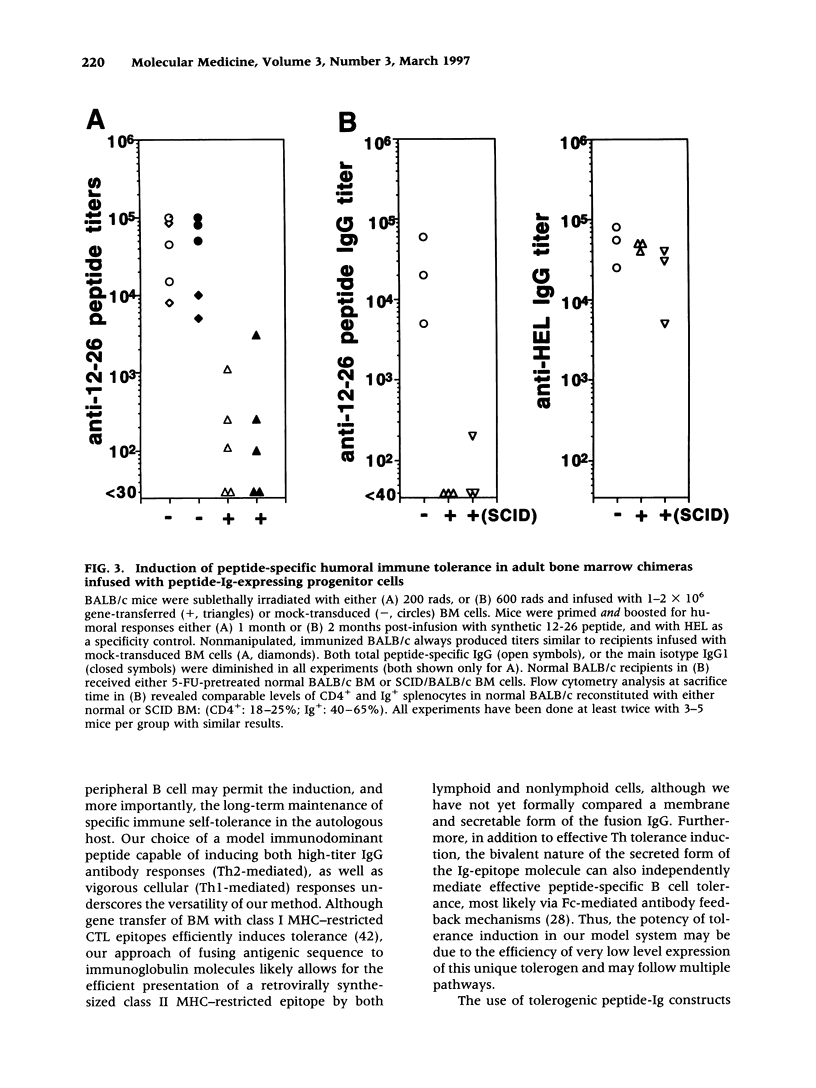



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allain J. P., Frommel D. Antibodies to factor VIII. V. Patterns of immune response to factor VIII in hemophilia A. Blood. 1976 Jun;47(6):973–982. [PubMed] [Google Scholar]
- Ally B. A., Hawley T. S., McKall-Faienza K. J., Kündig T. M., Oehen S. U., Pircher H., Hawley R. G., Ohashi P. S. Prevention of autoimmune disease by retroviral-mediated gene therapy. J Immunol. 1995 Dec 1;155(11):5404–5408. [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Bikoff E., Birshtein B. K. T cell clones specific for IgG2a of the a allotype: direct evidence for presentation of endogenous antigen. J Immunol. 1986 Jul 1;137(1):28–34. [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borel Y., Lewis R. M., Stollar B. D. Prevention of murine lupus nephritis by carrier-dependent induction of immunologic tolerance to denatured DNA. Science. 1973 Oct 5;182(4107):76–78. doi: 10.1126/science.182.4107.76. [DOI] [PubMed] [Google Scholar]
- Buhlmann J. E., Foy T. M., Aruffo A., Crassi K. M., Ledbetter J. A., Green W. R., Xu J. C., Shultz L. D., Roopesian D., Flavell R. A. In the absence of a CD40 signal, B cells are tolerogenic. Immunity. 1995 Jun;2(6):645–653. doi: 10.1016/1074-7613(95)90009-8. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Habicht G. S., Weigle W. O. Cellular sites of immunologic unresponsiveness. Proc Natl Acad Sci U S A. 1970 Mar;65(3):551–556. doi: 10.1073/pnas.65.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold S. P., Jayasuriya A., Nash A., Prospero T. D., Waldmann H. Therapy with monoclonal antibodies by elimination of T-cell subsets in vivo. Nature. 1984 Dec 6;312(5994):548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- Crispe I. N. Fatal interactions: Fas-induced apoptosis of mature T cells. Immunity. 1994 Aug;1(5):347–349. doi: 10.1016/1074-7613(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Dick J. E., Magli M. C., Huszar D., Phillips R. A., Bernstein A. Introduction of a selectable gene into primitive stem cells capable of long-term reconstitution of the hemopoietic system of W/Wv mice. Cell. 1985 Aug;42(1):71–79. doi: 10.1016/s0092-8674(85)80102-1. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Keller G. M., Phillips R. A., Miller R. G., Bosma G. C., O'Toole M., Bosma M. J. Functional status of cells from lymphoid and myeloid tissues in mice with severe combined immunodeficiency disease. J Immunol. 1984 Apr;132(4):1804–1808. [PubMed] [Google Scholar]
- Eynon E. E., Parker D. C. Small B cells as antigen-presenting cells in the induction of tolerance to soluble protein antigens. J Exp Med. 1992 Jan 1;175(1):131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. J., Matzinger P. B cells turn off virgin but not memory T cells. Science. 1992 Nov 13;258(5085):1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- Gao E. K., Lo D., Sprent J. Strong T cell tolerance in parent----F1 bone marrow chimeras prepared with supralethal irradiation. Evidence for clonal deletion and anergy. J Exp Med. 1990 Apr 1;171(4):1101–1121. doi: 10.1084/jem.171.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert K. M., Weigle W. O. Tolerogenicity of resting and activated B cells. J Exp Med. 1994 Jan 1;179(1):249–258. doi: 10.1084/jem.179.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Hathcock K. S., Laszlo G., Pucillo C., Linsley P., Hodes R. J. Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med. 1994 Aug 1;180(2):631–640. doi: 10.1084/jem.180.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins P. J., Weiner H. L. Suppression of experimental autoimmune encephalomyelitis by oral administration of myelin basic protein and its fragments. J Immunol. 1988 Jan 15;140(2):440–445. [PubMed] [Google Scholar]
- Hori S., Sato S., Kitagawa S., Azuma T., Kokudo S., Hamaoka T., Fujiwara H. Tolerance induction of allo-class II H-2 antigen-reactive L3T4+ helper T cells and prolonged survival of the corresponding class II H-2-disparate skin graft. J Immunol. 1989 Sep 1;143(5):1447–1452. [PubMed] [Google Scholar]
- Ildstad S. T., Sachs D. H. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984 Jan 12;307(5947):168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- Kang J., Wither J., Hozumi N. Long-term expression of a T-cell receptor beta-chain gene in mice reconstituted with retrovirus-infected hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9803–9807. doi: 10.1073/pnas.87.24.9803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Lai M. Z., Ross D. T., Guillet J. G., Briner T. J., Gefter M. L., Smith J. A. T lymphocyte response to bacteriophage lambda repressor cI protein. Recognition of the same peptide presented by Ia molecules of different haplotypes. J Immunol. 1987 Dec 15;139(12):3973–3980. [PubMed] [Google Scholar]
- Madsen J. C., Superina R. A., Wood K. J., Morris P. J. Immunological unresponsiveness induced by recipient cells transfected with donor MHC genes. Nature. 1988 Mar 10;332(6160):161–164. doi: 10.1038/332161a0. [DOI] [PubMed] [Google Scholar]
- Nemazee D., Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen S. U., Ohashi P. S., Bürki K., Hengartner H., Zinkernagel R. M., Aichele P. Escape of thymocytes and mature T cells from clonal deletion due to limiting tolerogen expression levels. Cell Immunol. 1994 Oct 15;158(2):342–352. doi: 10.1006/cimm.1994.1281. [DOI] [PubMed] [Google Scholar]
- Ramsdell F., Lantz T., Fowlkes B. J. A nondeletional mechanism of thymic self tolerance. Science. 1989 Nov 24;246(4933):1038–1041. doi: 10.1126/science.2511629. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Sharrow S. O., Singer A. Clonal deletion and clonal anergy in the thymus induced by cellular elements with different radiation sensitivities. J Exp Med. 1990 Mar 1;171(3):935–940. doi: 10.1084/jem.171.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. J., Gress R. E., Hathcock K. S., Hodes R. J. Recognition and response to alloantigens in vivo. II. Priming with accessory cell-depleted donor allogeneic splenocytes: induction of specific unresponsiveness to foreign major histocompatibility complex determinants. J Immunol. 1984 Nov;133(5):2343–2350. [PubMed] [Google Scholar]
- Sachs D. H., Bodine D. M., Moulton A. D., Pearson D. A., Nienhuis A. W., Sykes M. Tolerance induction using autologous bone marrow modified with an allogeneic class I MHC gene. Transplant Proc. 1993 Feb;25(1 Pt 1):348–349. [PubMed] [Google Scholar]
- Soloway P., Fish S., Passmore H., Gefter M., Coffee R., Manser T. Regulation of the immune response to peptide antigens: differential induction of immediate-type hypersensitivity and T cell proliferation due to changes in either peptide structure or major histocompatibility complex haplotype. J Exp Med. 1991 Oct 1;174(4):847–858. doi: 10.1084/jem.174.4.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent J., Schaefer M., Hurd M., Surh C. D., Ron Y. Mature murine B and T cells transferred to SCID mice can survive indefinitely and many maintain a virgin phenotype. J Exp Med. 1991 Sep 1;174(3):717–728. doi: 10.1084/jem.174.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutkowski N., Kuo M. L., Varela-Echavarria A., Dougherty J. P., Ron Y. A murine model for B-lymphocyte somatic cell gene therapy. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8875–8879. doi: 10.1073/pnas.91.19.8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisch R., Yang X. D., Singer S. M., Liblau R. S., Fugger L., McDevitt H. O. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature. 1993 Nov 4;366(6450):72–75. doi: 10.1038/366072a0. [DOI] [PubMed] [Google Scholar]
- Tripathy S. K., Black H. B., Goldwasser E., Leiden J. M. Immune responses to transgene-encoded proteins limit the stability of gene expression after injection of replication-defective adenovirus vectors. Nat Med. 1996 May;2(5):545–550. doi: 10.1038/nm0596-545. [DOI] [PubMed] [Google Scholar]
- Walunas T. L., Lenschow D. J., Bakker C. Y., Linsley P. S., Freeman G. J., Green J. M., Thompson C. B., Bluestone J. A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994 Aug;1(5):405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- Weiss S., Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991 Feb 22;64(4):767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- Yang Y., Li Q., Ertl H. C., Wilson J. M. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol. 1995 Apr;69(4):2004–2015. doi: 10.1128/jvi.69.4.2004-2015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurin V. L., Rudensky A. Y., Mazel S. M., Blechman J. M. Immunoglobulin-specific T-B cell interaction. II. T cell clones recognize the processed form of B cells' own surface immunoglobulin in the context of the major histocompatibility complex class II molecule. Eur J Immunol. 1989 Sep;19(9):1685–1691. doi: 10.1002/eji.1830190924. [DOI] [PubMed] [Google Scholar]
- Zaghouani H., Steinman R., Nonacs R., Shah H., Gerhard W., Bona C. Presentation of a viral T cell epitope expressed in the CDR3 region of a self immunoglobulin molecule. Science. 1993 Jan 8;259(5092):224–227. doi: 10.1126/science.7678469. [DOI] [PubMed] [Google Scholar]
- Zambidis E. T., Barth R. K., Scott D. W. Both resting and activated B lymphocytes expressing engineered peptide-Ig molecules serve as highly efficient tolerogenic vehicles in immunocompetent adult recipients. J Immunol. 1997 Mar 1;158(5):2174–2182. [PubMed] [Google Scholar]
- Zambidis E. T., Scott D. W. Epitope-specific tolerance induction with an engineered immunoglobulin. Proc Natl Acad Sci U S A. 1996 May 14;93(10):5019–5024. doi: 10.1073/pnas.93.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]



