Abstract
BACKGROUND: Resistance to thyroid hormone (RTH) is a syndrome characterized by refractoriness of the pituitary and/or peripheral tissues to the action of thyroid hormone. Mutations in the thyroid hormone receptor beta (TR beta) gene result in TR beta 1 mutants that mediate the clinical phenotype by interfering with transcription of thyroid hormone-regulated genes via a dominant negative effect. In this study, we developed transgenic mice harboring PV, a potent dominant negative human mutant TR beta 1 devoid of thyroid hormone binding and transcriptional activation, as an animal model to understand the molecular basis of this human disease. MATERIALS AND METHODS: Standard molecular biology approaches were used to obtain a cDNA fragment containing mutant PV which was injected into the pronucleus of fertilized egg. Founders were identified by Southern analysis and the expression of PV in tissues was determined by RNA and immunohistochemistry. Thyroid function was determined by radioimmunoassays of the hormones and the behavior of mice was observed using standard methods. RESULTS: The expression of mutant PV was directed by the beta-actin promoter. Mutant PV mRNA was detected in all tissues of transgenic mice, but the levels varied with tissues and with different lines of founders. Thyroid function tests in transgenic mice with high expression of mutant PV showed a significantly (approximately 1.5-fold) higher mean serum total of L-thyroxine levels (p < 0.01) than those of nontransgenic mice. Moreover, thyroid-stimulating hormone levels were not significantly different from those of nontransgenic mice. In addition, these mice displayed decreased weights and a behavioral phenotype characterized by hyperactivity. CONCLUSIONS: These mice have phenotypic features consistent with the commonly observed clinical features of RTH and could be used as a model system to better understand the action of mutant TR beta 1 in a physiological context, which could lead to better treatment for this disease.
Full text
PDF


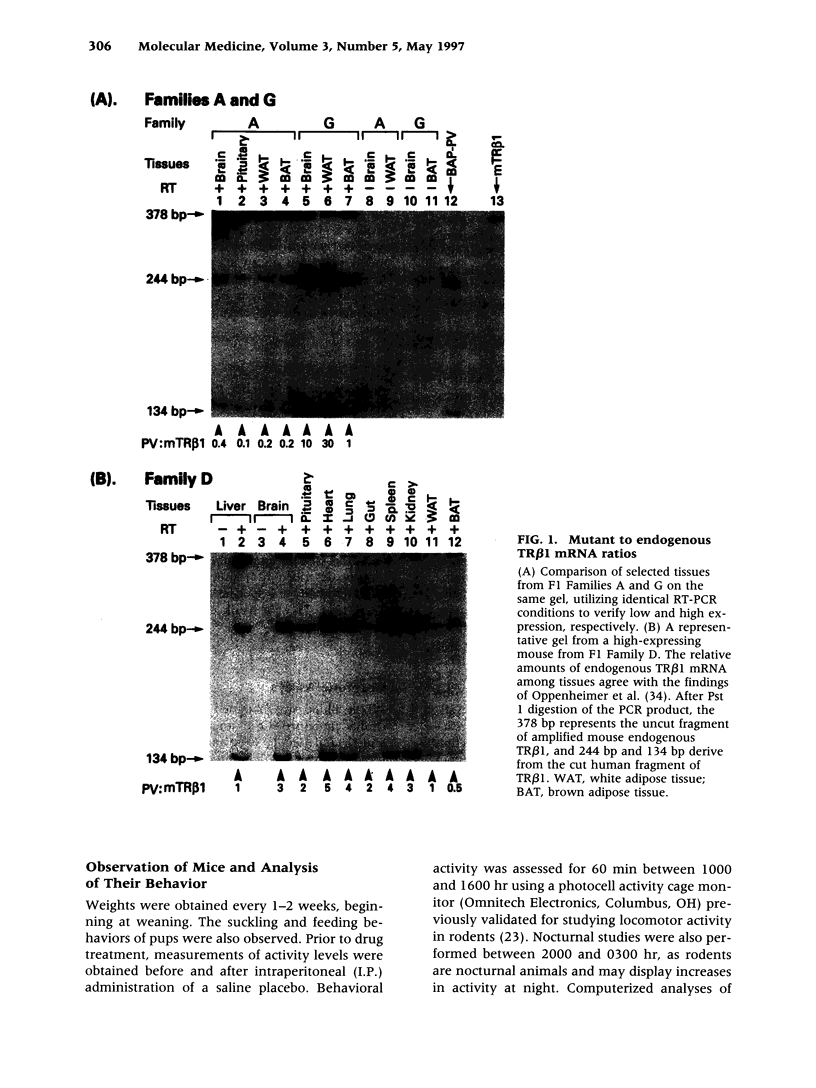


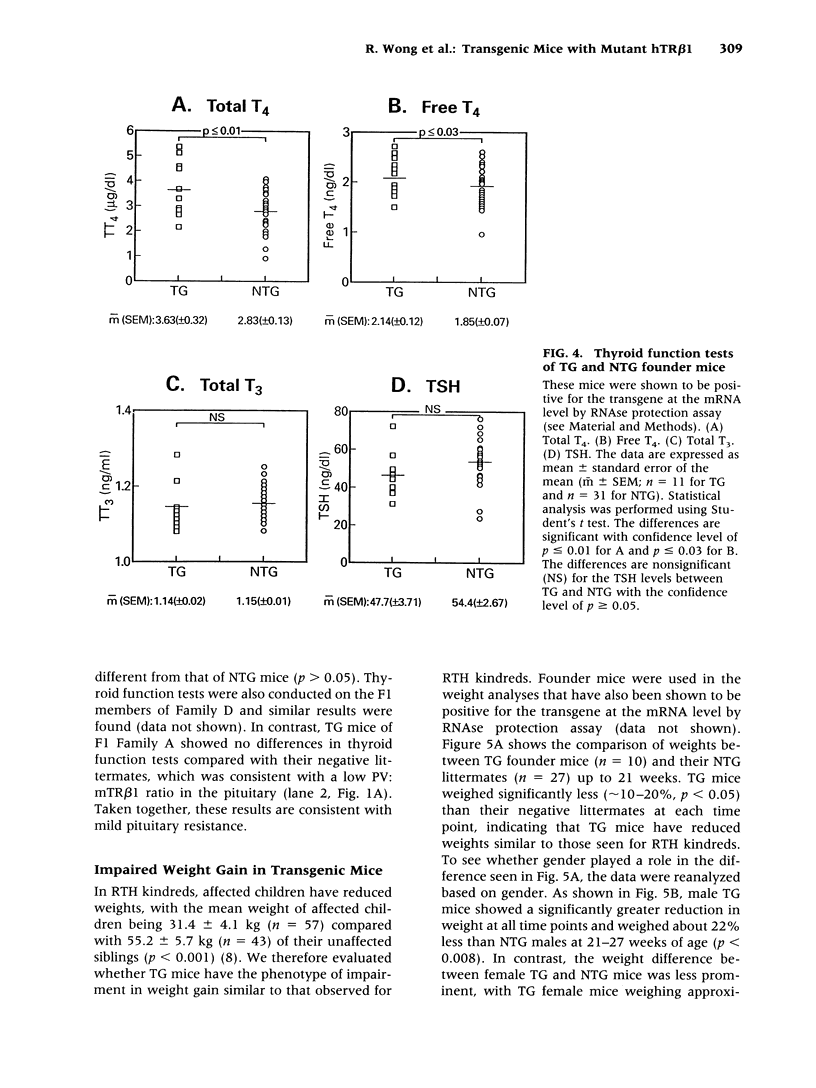
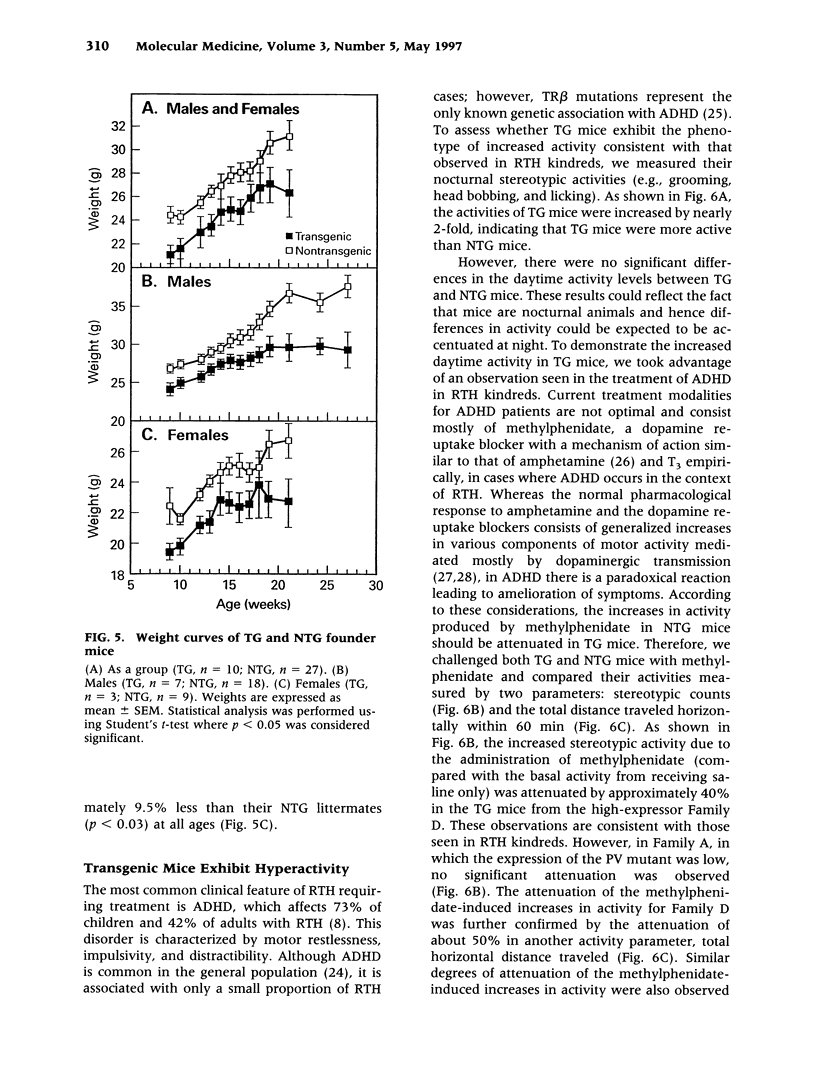




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Fishburn C. S., Drago J., Steiner H., Lachowicz J. E., Park B. H., Gauda E. B., Lee E. J., Cool M. H., Sibley D. R. A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1945–1949. doi: 10.1073/pnas.93.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T., Danysz W., Fredriksson A., Jonsson G., Luthman J., Sundström E., Teiling A. Neonatal 6-hydroxydopamine-induced dopamine depletions: motor activity and performance in maze learning. Pharmacol Biochem Behav. 1988 Oct;31(2):357–364. doi: 10.1016/0091-3057(88)90358-9. [DOI] [PubMed] [Google Scholar]
- Barlow C., Meister B., Lardelli M., Lendahl U., Vennström B. Thyroid abnormalities and hepatocellular carcinoma in mice transgenic for v-erbA. EMBO J. 1994 Sep 15;13(18):4241–4250. doi: 10.1002/j.1460-2075.1994.tb06744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat M. K., McPhie P., Ting Y. T., Zhu X. G., Cheng S. Y. Structure of the carboxy-terminal region of thyroid hormone nuclear receptors and its possible role in hormone-dependent intermolecular interactions. Biochemistry. 1995 Aug 22;34(33):10591–10599. doi: 10.1021/bi00033a034. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F., Skarulis M. C., Grace M. B., Benichou J., Hauser P., Wiggs E., Weintraub B. D. Genetic and clinical features of 42 kindreds with resistance to thyroid hormone. The National Institutes of Health Prospective Study. Ann Intern Med. 1995 Oct 15;123(8):572–583. doi: 10.7326/0003-4819-123-8-199510150-00002. [DOI] [PubMed] [Google Scholar]
- Cheng Sy S.-y. New Insights into the Structure and Function of the Thyroid Hormone Receptor. J Biomed Sci. 1995 Apr;2(2):77–89. doi: 10.1007/BF02253060. [DOI] [PubMed] [Google Scholar]
- Crocker A. D., Overstreet D. H. Modification of the behavioural effects of haloperidol and of dopamine receptor regulation by altered thyroid status. Psychopharmacology (Berl) 1984;82(1-2):102–106. doi: 10.1007/BF00426390. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. E., Berres M., Schaeppi U. Validation of a photobeam system for assessment of motor activity in rats. Toxicology. 1988 May;49(2-3):433–439. doi: 10.1016/0300-483x(88)90029-7. [DOI] [PubMed] [Google Scholar]
- Forrest D., Hanebuth E., Smeyne R. J., Everds N., Stewart C. L., Wehner J. M., Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 1996 Jun 17;15(12):3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Hasumura S., Kitagawa S., Lovelace E., Willingham M. C., Pastan I., Cheng S. Characterization of a membrane-associated 3,3',5-triiodo-L-thyronine binding protein by use of monoclonal antibodies. Biochemistry. 1986 Dec 2;25(24):7881–7888. doi: 10.1021/bi00372a014. [DOI] [PubMed] [Google Scholar]
- Hauser P., Zametkin A. J., Martinez P., Vitiello B., Matochik J. A., Mixson A. J., Weintraub B. D. Attention deficit-hyperactivity disorder in people with generalized resistance to thyroid hormone. N Engl J Med. 1993 Apr 8;328(14):997–1001. doi: 10.1056/NEJM199304083281403. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Mangoura D., Refetoff S. A mouse model of resistance to thyroid hormone produced by somatic gene transfer of a mutant thyroid hormone receptor. Mol Endocrinol. 1996 Jan;10(1):100–106. doi: 10.1210/mend.10.1.8838149. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Jackson T. A., Bain D. L., Richer J. K., Takimoto G. S., Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996 Oct;10(10):1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- Malone M. A., Kershner J. R., Swanson J. M. Hemispheric processing and methylphenidate effects in attention-deficit hyperactivity disorder. J Child Neurol. 1994 Apr;9(2):181–189. doi: 10.1177/088307389400900216. [DOI] [PubMed] [Google Scholar]
- Meier C. A., Dickstein B. M., Ashizawa K., McClaskey J. H., Muchmore P., Ransom S. C., Menke J. B., Hao E. H., Usala S. J., Bercu B. B. Variable transcriptional activity and ligand binding of mutant beta 1 3,5,3'-triiodothyronine receptors from four families with generalized resistance to thyroid hormone. Mol Endocrinol. 1992 Feb;6(2):248–258. doi: 10.1210/mend.6.2.1569968. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Strait K. A. Thyroid hormone action 1994: the plot thickens. Eur J Endocrinol. 1994 Jan;130(1):15–24. doi: 10.1530/eje.0.1300015. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Mixson A. J., McPherson J. A., McClaskey J. H., Weintraub B. D. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two "hot spot" regions of the ligand binding domain. J Clin Invest. 1991 Dec;88(6):2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R. F., Mani S. K., Codina J., Conneely O. M., O'Malley B. W. Dopaminergic and ligand-independent activation of steroid hormone receptors. Science. 1991 Dec 13;254(5038):1636–1639. doi: 10.1126/science.1749936. [DOI] [PubMed] [Google Scholar]
- Ray P., Higgins K. M., Tan J. C., Chu T. Y., Yee N. S., Nguyen H., Lacy E., Besmer P. Ectopic expression of a c-kitW42 minigene in transgenic mice: recapitulation of W phenotypes and evidence for c-kit function in melanoblast progenitors. Genes Dev. 1991 Dec;5(12A):2265–2273. doi: 10.1101/gad.5.12a.2265. [DOI] [PubMed] [Google Scholar]
- Refetoff S., DeWind L. T., DeGroot L. J. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J Clin Endocrinol Metab. 1967 Feb;27(2):279–294. doi: 10.1210/jcem-27-2-279. [DOI] [PubMed] [Google Scholar]
- Refetoff S., Weiss R. E., Usala S. J. The syndromes of resistance to thyroid hormone. Endocr Rev. 1993 Jun;14(3):348–399. doi: 10.1210/edrv-14-3-348. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Shaywitz B. A., Teicher M. H., Cohen D. J., Anderson G. M., Young J. G., Levitt P. Dopaminergic but not noradrenergic mediation of hyperactivity and performance deficits in the developing rat pup. Psychopharmacology (Berl) 1984;82(1-2):73–77. doi: 10.1007/BF00426384. [DOI] [PubMed] [Google Scholar]
- Shenker A. The mechanism of action of drugs used to treat attention-deficit hyperactivity disorder: focus on catecholamine receptor pharmacology. Adv Pediatr. 1992;39:337–382. [PubMed] [Google Scholar]
- Strait K. A., Schwartz H. L., Perez-Castillo A., Oppenheimer J. H. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem. 1990 Jun 25;265(18):10514–10521. [PubMed] [Google Scholar]
- Takeda K., Sakurai A., DeGroot L. J., Refetoff S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor-beta gene. J Clin Endocrinol Metab. 1992 Jan;74(1):49–55. doi: 10.1210/jcem.74.1.1727829. [DOI] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Xu M., Hu X. T., Cooper D. C., Moratalla R., Graybiel A. M., White F. J., Tonegawa S. Elimination of cocaine-induced hyperactivity and dopamine-mediated neurophysiological effects in dopamine D1 receptor mutant mice. Cell. 1994 Dec 16;79(6):945–955. doi: 10.1016/0092-8674(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Yap N., Yu C. L., Cheng S. Y. Modulation of the transcriptional activity of thyroid hormone receptors by the tumor suppressor p53. Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4273–4277. doi: 10.1073/pnas.93.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen P. M., Chin W. W. Molecular mechanisms of dominant negative activity by nuclear hormone receptors. Mol Endocrinol. 1994 Nov;8(11):1450–1454. doi: 10.1210/mend.8.11.7877614. [DOI] [PubMed] [Google Scholar]
- Zametkin A. J., Rapoport J. L. Neurobiology of attention deficit disorder with hyperactivity: where have we come in 50 years? J Am Acad Child Adolesc Psychiatry. 1987 Sep;26(5):676–686. doi: 10.1097/00004583-198709000-00011. [DOI] [PubMed] [Google Scholar]





