Abstract
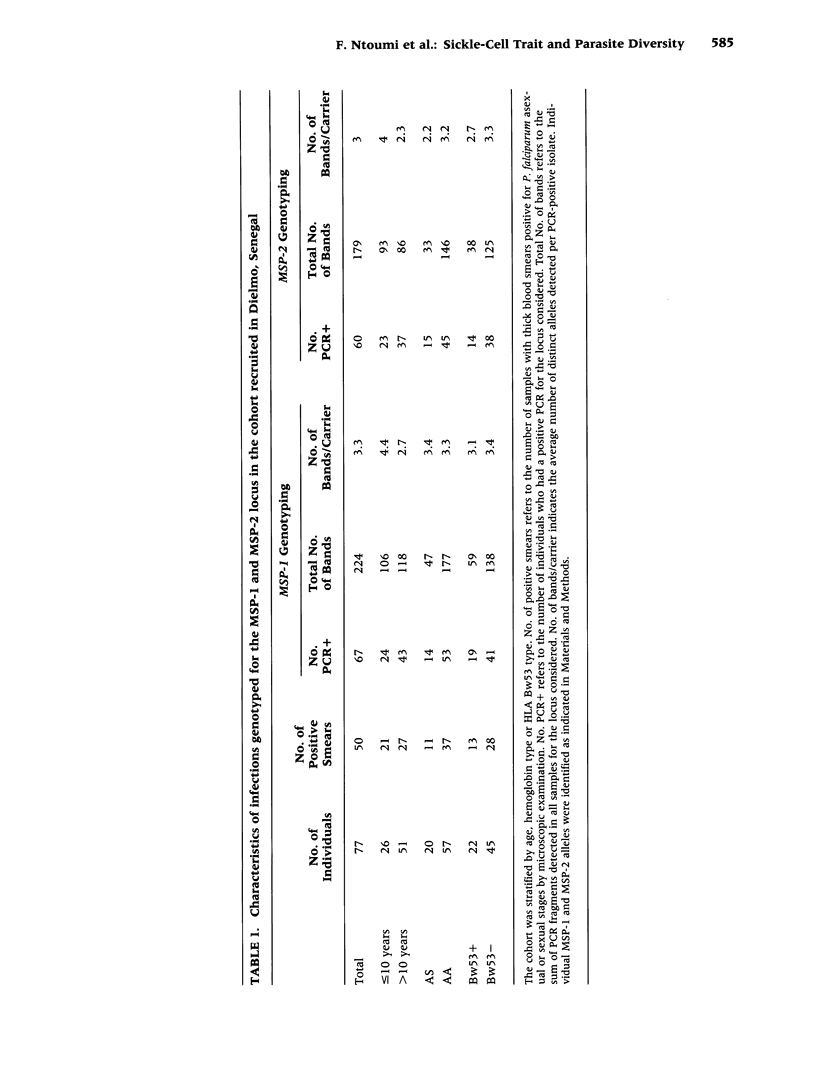
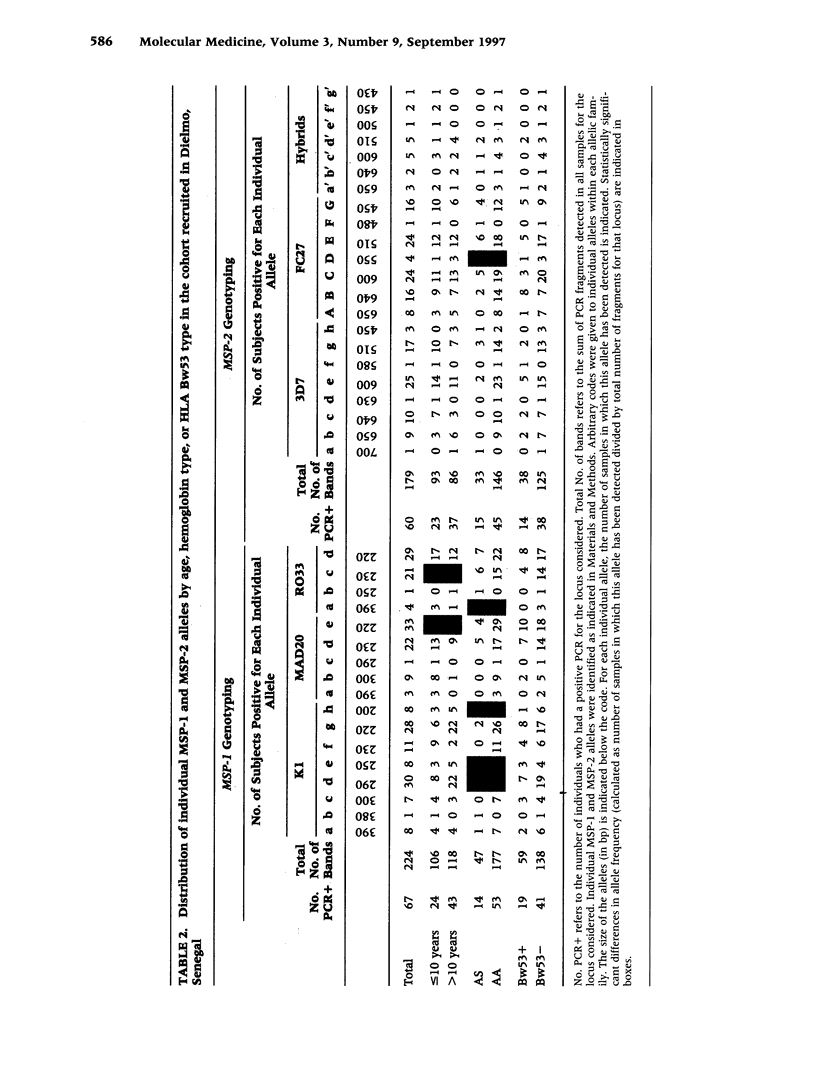
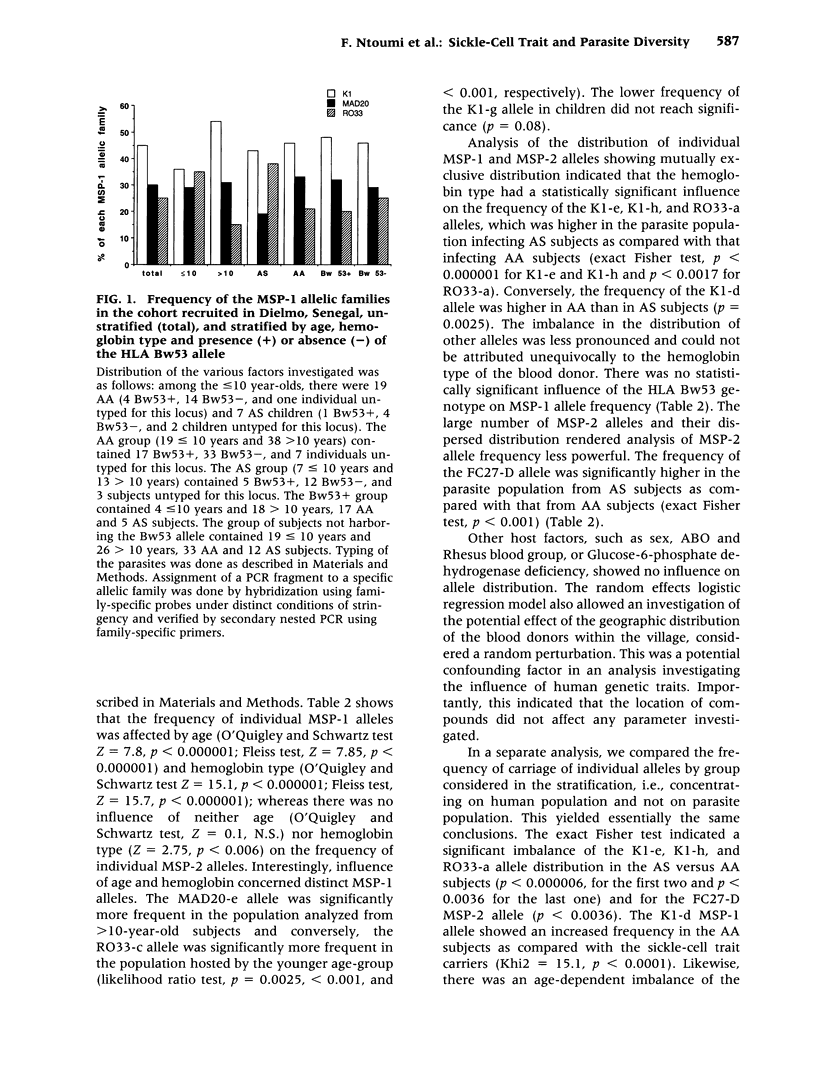
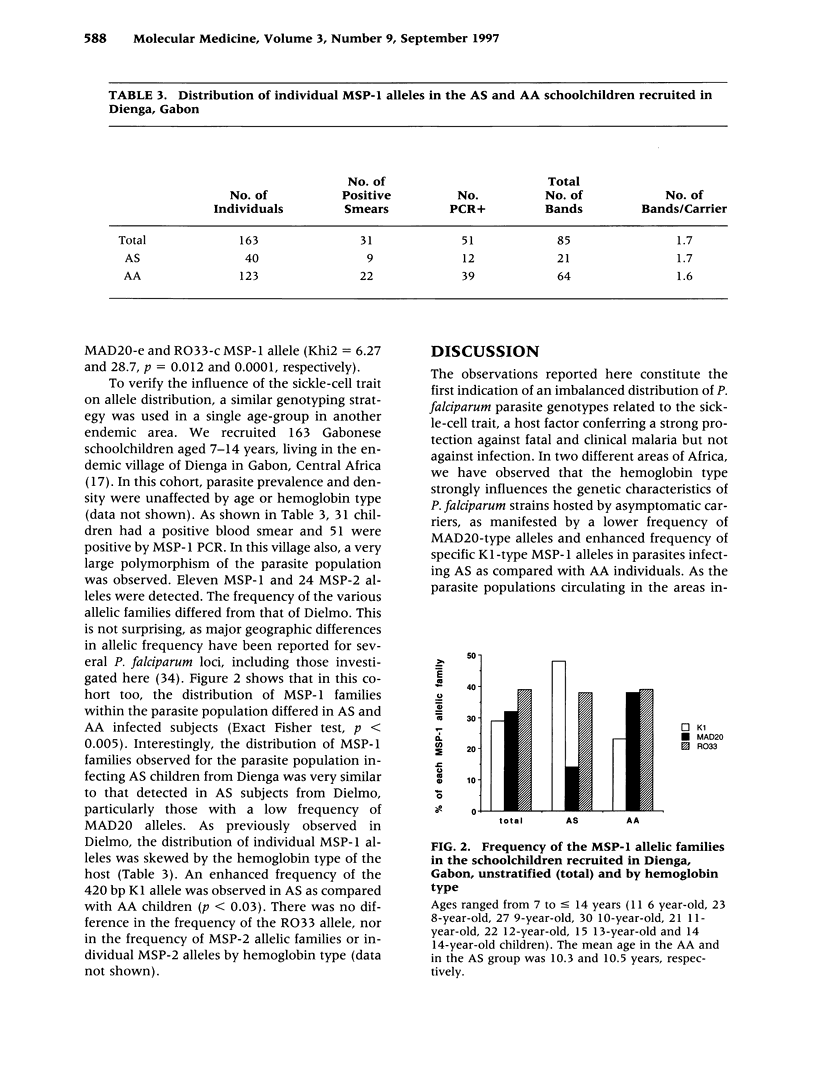
BACKGROUND: The sickle-cell trait protects against severe Plasmodium falciparum malaria and reduces susceptibility to mild malaria but does not prevent infection. The exact mechanism of this protection remains unclear. We have hypothesized that AS individuals are protected by virtue of being less susceptible to a subset of parasite strains; thus we compared some genetic characteristics of parasites infecting AS and AA subjects. MATERIALS AND METHODS: Blood was collected from asymptomatic individuals living in two different regions of Africa. The polymorphic MSP-1 and MSP-2 loci were genotyped using a PCR-based methodology. Individual alleles were identified by size polymorphism, amplification using family-specific primers, and hybridization using family-specific probes. Multivariate logistic regression was used to analyze allele distribution. RESULTS: In Senegalese carriers, age and hemoglobin type influenced differently the distribution of the three MSP-1 families and had an impact on distinct individual alleles, whereas the distribution of MSP-2 alleles was marginally affected. There was no influence of other genetic traits, including the HLA Bw53 genotype, or factors such as place of residence within the village. In a cohort of Gabonese schoolchildren in which the influence of age was abrogated, a similar imbalance in the MSP-1 allelic distribution but not of MSP-2 allelic distribution by hemoglobin type was observed. CONCLUSIONS: The influence of the host's hemoglobin type on P. falciparum genotypes suggests that parasite fitness for a specific host is strain-dependent, which is consistent with our hypothesis that innate resistance might result from reduced fitness of some parasite strains for individuals with sickle-cell traits.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C. Protection afforded by sickle-cell trait against subtertian malareal infection. Br Med J. 1954 Feb 6;1(4857):290–294. doi: 10.1136/bmj.1.4857.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C. The distribution of the sickle-cell trait in East Africa and elsewhere, and its apparent relationship to the incidence of subtertian malaria. Trans R Soc Trop Med Hyg. 1954 Jul;48(4):312–318. doi: 10.1016/0035-9203(54)90101-7. [DOI] [PubMed] [Google Scholar]
- Abu-Zeid Y. A., Abdulhadi N. H., Hviid L., Theander T. G., Saeed B. O., Jepsen S., Jensen J. B., Bayoumi R. A. Lymphoproliferative responses to Plasmodium falciparum antigens in children with and without the sickle cell trait. Scand J Immunol. 1991 Aug;34(2):237–242. doi: 10.1111/j.1365-3083.1991.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Bayoumi R. A., Abu-Zeid Y. A., Abdulhadi N. H., Saeed B. O., Theander T. G., Hviid L., Ghalib H. W., Nugud A. H., Jepsen S., Jensen J. B. Cell-mediated immune responses to Plasmodium falciparum purified soluble antigens in sickle-cell trait subjects. Immunol Lett. 1990 Aug;25(1-3):243–249. doi: 10.1016/0165-2478(90)90122-7. [DOI] [PubMed] [Google Scholar]
- Chippaux J. P., Massougbodji A., Boulard J. C., Akogbeto M. Etude de la morbidité palustre et de la gravité des accès pernicieux chez les porteurs du trait drépanocytaire. Rev Epidemiol Sante Publique. 1992;40(4):240–245. [PubMed] [Google Scholar]
- Chippaux J. P., Massougbodji A., Castel J., Akogbeto M., Zohoun I., Zohoun T. Parasitémies à Plasmodium falciparum ou P. malariae chez les porteurs du trait drépanocytaire dans différents biotopes du Bénin. Rev Epidemiol Sante Publique. 1992;40(4):246–251. [PubMed] [Google Scholar]
- Contamin H., Fandeur T., Bonnefoy S., Skouri F., Ntoumi F., Mercereau-Puijalon O. PCR typing of field isolates of Plasmodium falciparum. J Clin Microbiol. 1995 Apr;33(4):944–951. doi: 10.1128/jcm.33.4.944-951.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A. Merozoite surface antigen-I of plasmodium. Parasitol Today. 1993 Feb;9(2):50–54. doi: 10.1016/0169-4758(93)90031-a. [DOI] [PubMed] [Google Scholar]
- Creasey A., Fenton B., Walker A., Thaithong S., Oliveira S., Mutambu S., Walliker D. Genetic diversity of Plasmodium falciparum shows geographical variation. Am J Trop Med Hyg. 1990 May;42(5):403–413. doi: 10.4269/ajtmh.1990.42.403. [DOI] [PubMed] [Google Scholar]
- Dieye A., Diaw M. L., Rogier C., Trape J. F., Sarthou J. L. HLA-A, -B, -C, -DR, -DQ typing in a population group of Senegal: distribution of HLA antigens and HLA-DRB1*13 and DRB1*11 subtyping by PCR using sequence-specific primers (PCR-SSP). Tissue Antigens. 1996 Mar;47(3):194–199. doi: 10.1111/j.1399-0039.1996.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Fandeur T., Mercereau-Puijalon O., Bonnemains B. Plasmodium falciparum: genetic diversity of several strains infectious for the squirrel monkey (Saimiri sciureus). Exp Parasitol. 1996 Oct;84(1):1–15. doi: 10.1006/expr.1996.0085. [DOI] [PubMed] [Google Scholar]
- Fenton B., Clark J. T., Khan C. M., Robinson J. V., Walliker D., Ridley R., Scaife J. G., McBride J. S. Structural and antigenic polymorphism of the 35- to 48-kilodalton merozoite surface antigen (MSA-2) of the malaria parasite Plasmodium falciparum. Mol Cell Biol. 1991 Feb;11(2):963–971. doi: 10.1128/mcb.11.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. F., Storey J., Molineaux L., Iroko E. A., Attai E. D. Abnormal haemoglobins in the Sudan savanna of Nigeria. I. Prevalence of haemoglobins and relationships between sickle cell trait, malaria and survival. Ann Trop Med Parasitol. 1979 Apr;73(2):161–172. doi: 10.1080/00034983.1979.11687243. [DOI] [PubMed] [Google Scholar]
- Friedman M. J. Erythrocytic mechanism of sickle cell resistance to malaria. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1994–1997. doi: 10.1073/pnas.75.4.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V., Allsopp C. E., Kwiatkowski D., Anstey N. M., Twumasi P., Rowe P. A., Bennett S., Brewster D., McMichael A. J., Greenwood B. M. Common west African HLA antigens are associated with protection from severe malaria. Nature. 1991 Aug 15;352(6336):595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- Luty A. J., Mayombo J., Lekoulou F., Mshana R. Immunologic responses to soluble exoantigens of Plasmodium falciparum in Gabonese children exposed to continuous intense infection. Am J Trop Med Hyg. 1994 Dec;51(6):720–729. doi: 10.4269/ajtmh.1994.51.720. [DOI] [PubMed] [Google Scholar]
- Luzzatto L. Genetics of red cells and susceptibility to malaria. Blood. 1979 Nov;54(5):961–976. [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R. J., Carson D. C., Greenwood B. M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989 May-Jun;83(3):293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- Miller L. H. Impact of malaria on genetic polymorphism and genetic diseases in Africans and African Americans. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2415–2419. doi: 10.1073/pnas.91.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. H., Roberts T., Shahabuddin M., McCutchan T. F. Analysis of sequence diversity in the Plasmodium falciparum merozoite surface protein-1 (MSP-1). Mol Biochem Parasitol. 1993 May;59(1):1–14. doi: 10.1016/0166-6851(93)90002-f. [DOI] [PubMed] [Google Scholar]
- Mozzarelli A., Hofrichter J., Eaton W. A. Delay time of hemoglobin S polymerization prevents most cells from sickling in vivo. Science. 1987 Jul 31;237(4814):500–506. doi: 10.1126/science.3603036. [DOI] [PubMed] [Google Scholar]
- Ntoumi F., Contamin H., Rogier C., Bonnefoy S., Trape J. F., Mercereau-Puijalon O. Age-dependent carriage of multiple Plasmodium falciparum merozoite surface antigen-2 alleles in asymptomatic malaria infections. Am J Trop Med Hyg. 1995 Jan;52(1):81–88. doi: 10.4269/ajtmh.1995.52.81. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Weatherall D. J., Wilson R. J. Cellular mechanism for the protective effect of haemoglobin S against P. falciparum malaria. Nature. 1978 Aug 17;274(5672):701–703. doi: 10.1038/274701a0. [DOI] [PubMed] [Google Scholar]
- Prescott N., Stowers A. W., Cheng Q., Bobogare A., Rzepczyk C. M., Saul A. Plasmodium falciparum genetic diversity can be characterised using the polymorphic merozoite surface antigen 2 (MSA-2) gene as a single locus marker. Mol Biochem Parasitol. 1994 Feb;63(2):203–212. doi: 10.1016/0166-6851(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Robert F., Ntoumi F., Angel G., Candito D., Rogier C., Fandeur T., Sarthou J. L., Mercereau-Puijalon O. Extensive genetic diversity of Plasmodium falciparum isolates collected from patients with severe malaria in Dakar, Senegal. Trans R Soc Trop Med Hyg. 1996 Nov-Dec;90(6):704–711. doi: 10.1016/s0035-9203(96)90446-0. [DOI] [PubMed] [Google Scholar]
- Robert V., Tchuinkam T., Mulder B., Bodo J. M., Verhave J. P., Carnevale P., Nagel R. L. Effect of the sickle cell trait status of gametocyte carriers of Plasmodium falciparum on infectivity to anophelines. Am J Trop Med Hyg. 1996 Feb;54(2):111–113. doi: 10.4269/ajtmh.1996.54.111. [DOI] [PubMed] [Google Scholar]
- Rogier C., Commenges D., Trape J. F. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am J Trop Med Hyg. 1996 Jun;54(6):613–619. doi: 10.4269/ajtmh.1996.54.613. [DOI] [PubMed] [Google Scholar]
- Roth E. F., Jr, Friedman M., Ueda Y., Tellez I., Trager W., Nagel R. L. Sickling rates of human AS red cells infected in vitro with Plasmodium falciparum malaria. Science. 1978 Nov 10;202(4368):650–652. doi: 10.1126/science.360396. [DOI] [PubMed] [Google Scholar]
- Snewin V. A., Herrera M., Sanchez G., Scherf A., Langsley G., Herrera S. Polymorphism of the alleles of the merozoite surface antigens MSA1 and MSA2 in Plasmodium falciparum wild isolates from Colombia. Mol Biochem Parasitol. 1991 Dec;49(2):265–275. doi: 10.1016/0166-6851(91)90070-m. [DOI] [PubMed] [Google Scholar]
- Stiratelli R., Laird N., Ware J. H. Random-effects models for serial observations with binary response. Biometrics. 1984 Dec;40(4):961–971. [PubMed] [Google Scholar]
- Trape J. F., Rogier C. Combating malaria morbidity and mortality by reducing transmission. Parasitol Today. 1996 Jun;12(6):236–240. doi: 10.1016/0169-4758(96)10015-6. [DOI] [PubMed] [Google Scholar]
- Viriyakosol S., Siripoon N., Petcharapirat C., Petcharapirat P., Jarra W., Thaithong S., Brown K. N., Snounou G. Genotyping of Plasmodium falciparum isolates by the polymerase chain reaction and potential uses in epidemiological studies. Bull World Health Organ. 1995;73(1):85–95. [PMC free article] [PubMed] [Google Scholar]




