Abstract
BACKGROUND: The human maspin gene encodes a protein in the serine proteinase inhibitor (serpin) family with tumor-suppressing functions in cell culture and in nude mice. In order to examine the role of maspin in an intact mammal, we cloned and sequenced the cDNA of mouse maspin. The recombinant protein was produced and its activity in cell culture was assessed. MATERIALS AND METHODS: Mouse maspin (mMaspin) was cloned by screening a mouse mammary gland cDNA library with the human maspin cDNA probe. Northern blot analysis was used to examine the expression patterns in mouse tissues, mammary epithelial cells, and carcinomas. Recombinant mMaspin protein was produced in E. coli. Invasion and motility assays were used to assess the biological function of mMaspin. RESULTS: mMaspin is 89% homologous with human maspin at the amino acid level. Like its human homolog, mMaspin is expressed in normal mouse mammary epithelial cells and down-regulated in mouse breast tumor cell lines. The expression is altered at different developmental stages in mammary gland. Addition of the recombinant mMaspin protein to mouse tumor cells was shown to inhibit invasion in a dose-dependent manner. As with the human protein, recombinant mMaspin protein also inhibited mouse mammary tumor motility. Deletion in the putative mMaspin reactive site loop (RSL) region resulted in the loss of its inhibitory functions. CONCLUSIONS: mMaspin is the mouse homolog of a human tumor suppressor gene. The expression of mMaspin is down-regulated in tumor cells and is altered at different developmental stages of mammary gland. mMaspin has inhibitory properties similar to those of human maspin in cell culture, suggesting that the homologous proteins play similar physiological roles in vivo.
Full text
PDF



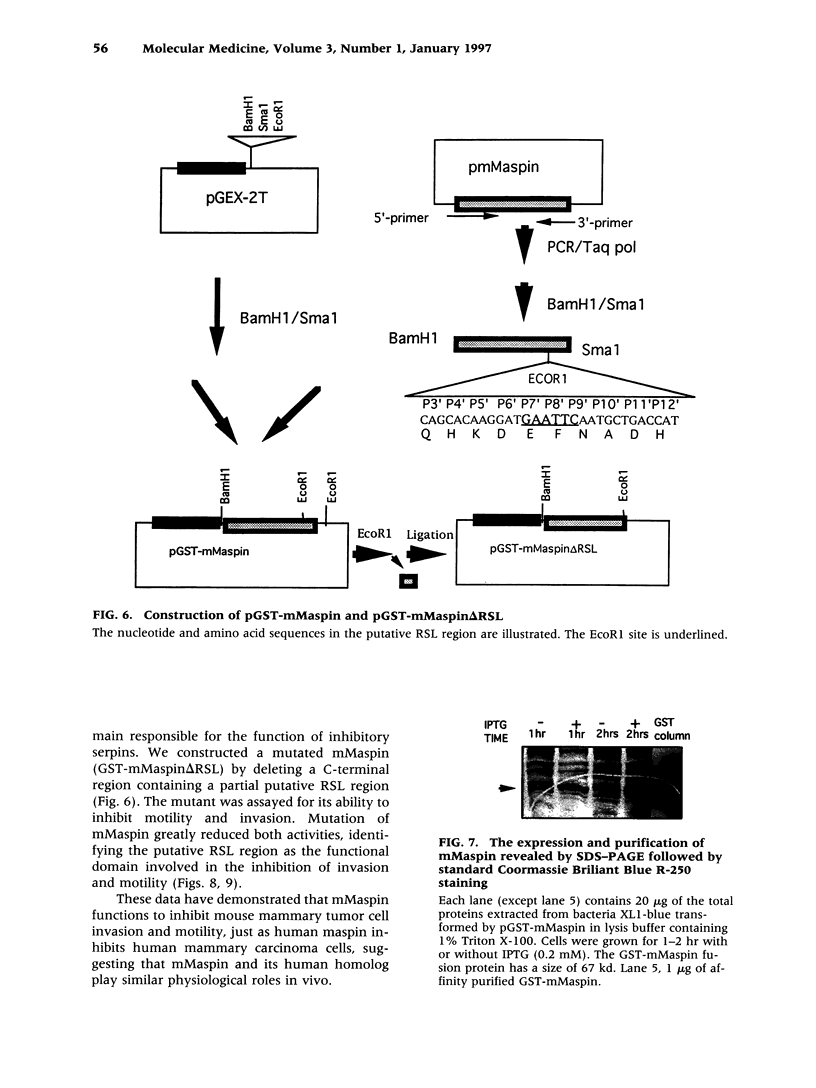


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chu Y. W., Runyan R. B., Oshima R. G., Hendrix M. J. Expression of complete keratin filaments in mouse L cells augments cell migration and invasion. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4261–4265. doi: 10.1073/pnas.90.9.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S., Silberstein G. B., Daniel C. W. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol. 1988 Jun;127(2):304–315. doi: 10.1016/0012-1606(88)90317-x. [DOI] [PubMed] [Google Scholar]
- Hendrix M. J., Seftor E. A., Seftor R. E., Fidler I. J. A simple quantitative assay for studying the invasive potential of high and low human metastatic variants. Cancer Lett. 1987 Dec;38(1-2):137–147. doi: 10.1016/0304-3835(87)90209-6. [DOI] [PubMed] [Google Scholar]
- Hopkins P. C., Whisstock J. Function of maspin. Science. 1994 Sep 23;265(5180):1893–1894. [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Imagawa W., Bandyopadhyay G. K., Nandi S. Regulation of mammary epithelial cell growth in mice and rats. Endocr Rev. 1990 Nov;11(4):494–523. doi: 10.1210/edrv-11-4-494. [DOI] [PubMed] [Google Scholar]
- Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991 Jul 25;19(14):3998–3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre O., Wolf C., Limacher J. M., Hutin P., Wendling C., LeMeur M., Basset P., Rio M. C. The breast cancer-associated stromelysin-3 gene is expressed during mouse mammary gland apoptosis. J Cell Biol. 1992 Nov;119(4):997–1002. doi: 10.1083/jcb.119.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton P. A., Wong D. T., Gibson H. L., Kiefer M. C., Fitzpatrick P. A., Sager R., Barr P. J. The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin. J Biol Chem. 1995 Jun 30;270(26):15832–15837. doi: 10.1074/jbc.270.26.15832. [DOI] [PubMed] [Google Scholar]
- Robinson S. D., Silberstein G. B., Roberts A. B., Flanders K. C., Daniel C. W. Regulated expression and growth inhibitory effects of transforming growth factor-beta isoforms in mouse mammary gland development. Development. 1991 Nov;113(3):867–878. doi: 10.1242/dev.113.3.867. [DOI] [PubMed] [Google Scholar]
- Sakakura T. New aspects of stroma-parenchyma relations in mammary gland differentiation. Int Rev Cytol. 1991;125:165–202. doi: 10.1016/s0074-7696(08)61219-x. [DOI] [PubMed] [Google Scholar]
- Sheng S., Carey J., Seftor E. A., Dias L., Hendrix M. J., Sager R. Maspin acts at the cell membrane to inhibit invasion and motility of mammary and prostatic cancer cells. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11669–11674. doi: 10.1073/pnas.93.21.11669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S., Pemberton P. A., Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem. 1994 Dec 9;269(49):30988–30993. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Snedeker S. M., Brown C. F., DiAugustine R. P. Expression and functional properties of transforming growth factor alpha and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):276–280. doi: 10.1073/pnas.88.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisshelm K., Ryan K., Lee X., Tsou H. C., Peacocke M., Sager R. Down-regulation of retinoic acid receptor beta in mammary carcinoma cell lines and its up-regulation in senescing normal mammary epithelial cells. Cell Growth Differ. 1994 Feb;5(2):133–141. [PubMed] [Google Scholar]
- Talhouk R. S., Bissell M. J., Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992 Sep;118(5):1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk R. S., Chin J. R., Unemori E. N., Werb Z., Bissell M. J. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991 Jun;112(2):439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis J., Guzdek A., Potempa J., Watorek W. Serpins: structure and mechanism of action. Biol Chem Hoppe Seyler. 1990 May;371 (Suppl):3–11. [PubMed] [Google Scholar]
- Tulchinsky E., Kramerov D., Ford H. L., Reshetnyak E., Lukanidin E., Zain S. Characterization of a positive regulatory element in the mts1 gene. Oncogene. 1993 Jan;8(1):79–86. [PubMed] [Google Scholar]
- Zou Z., Anisowicz A., Hendrix M. J., Thor A., Neveu M., Sheng S., Rafidi K., Seftor E., Sager R. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994 Jan 28;263(5146):526–529. doi: 10.1126/science.8290962. [DOI] [PubMed] [Google Scholar]