Abstract
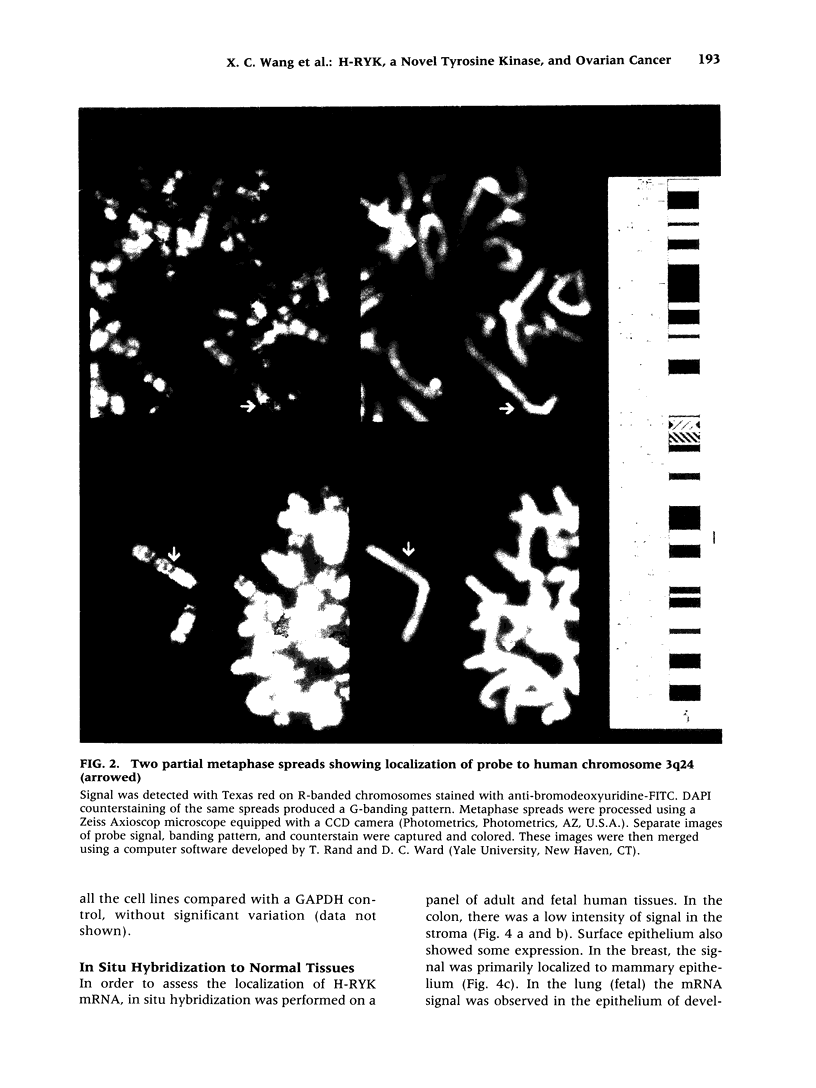
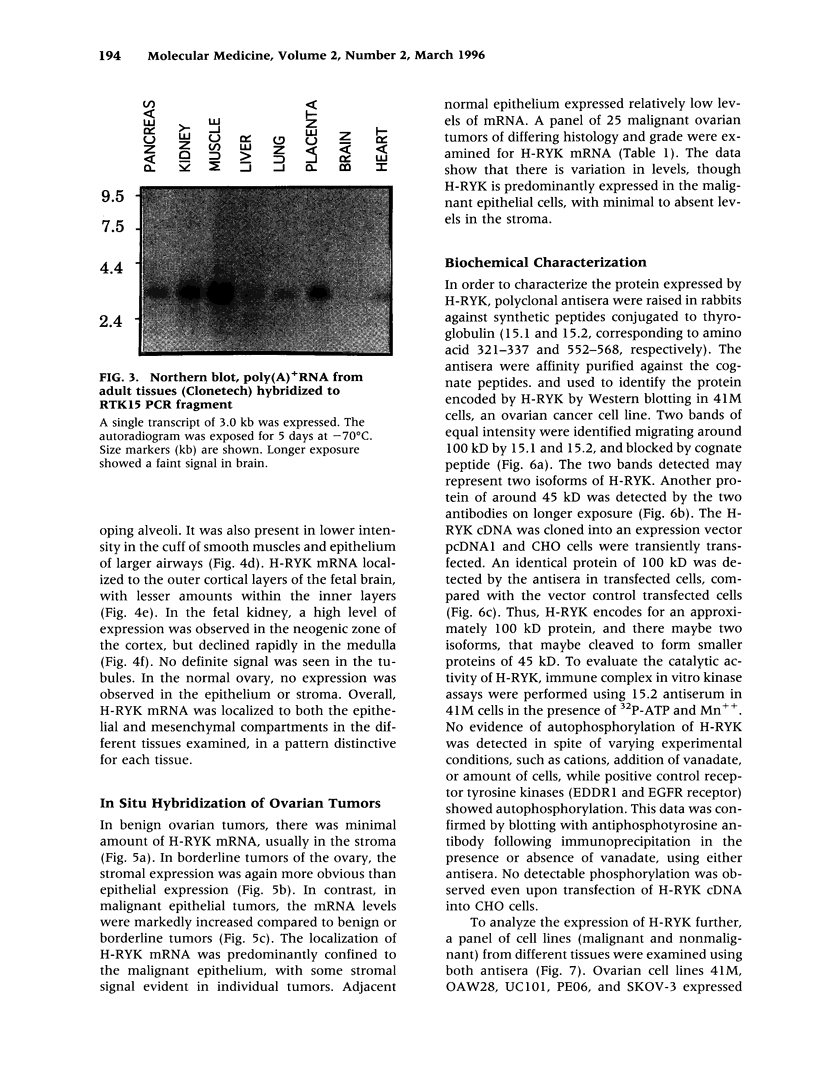
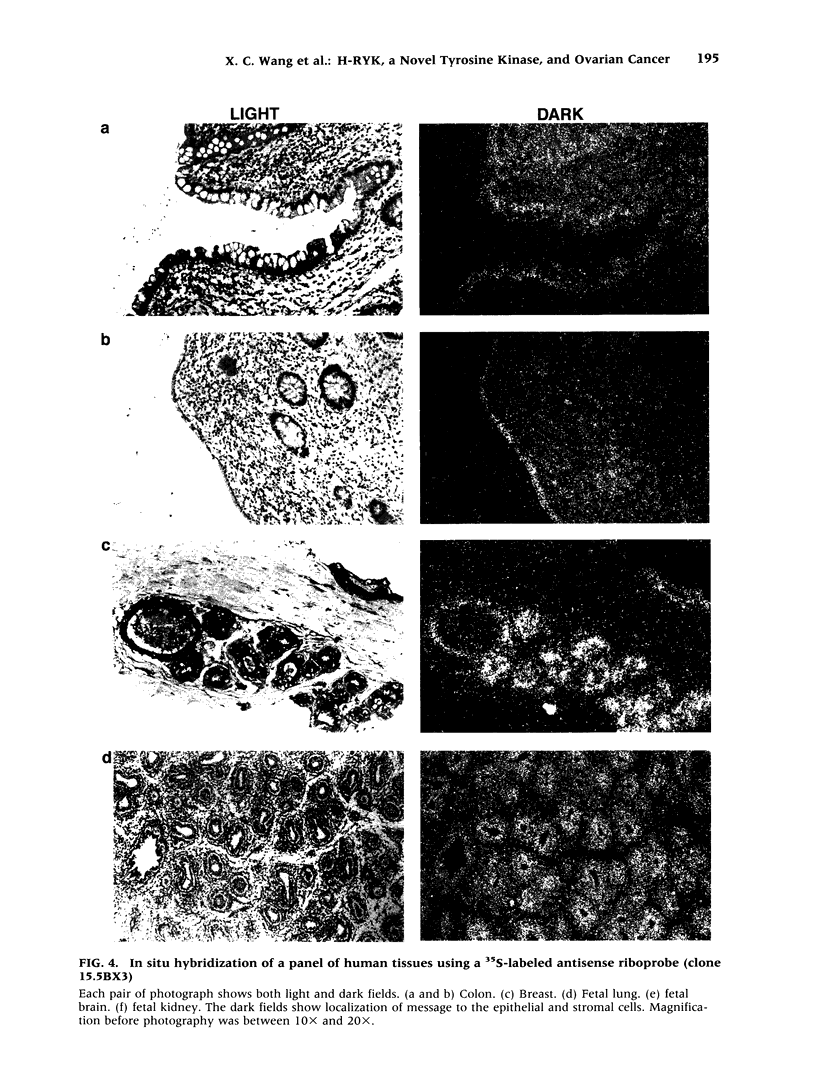
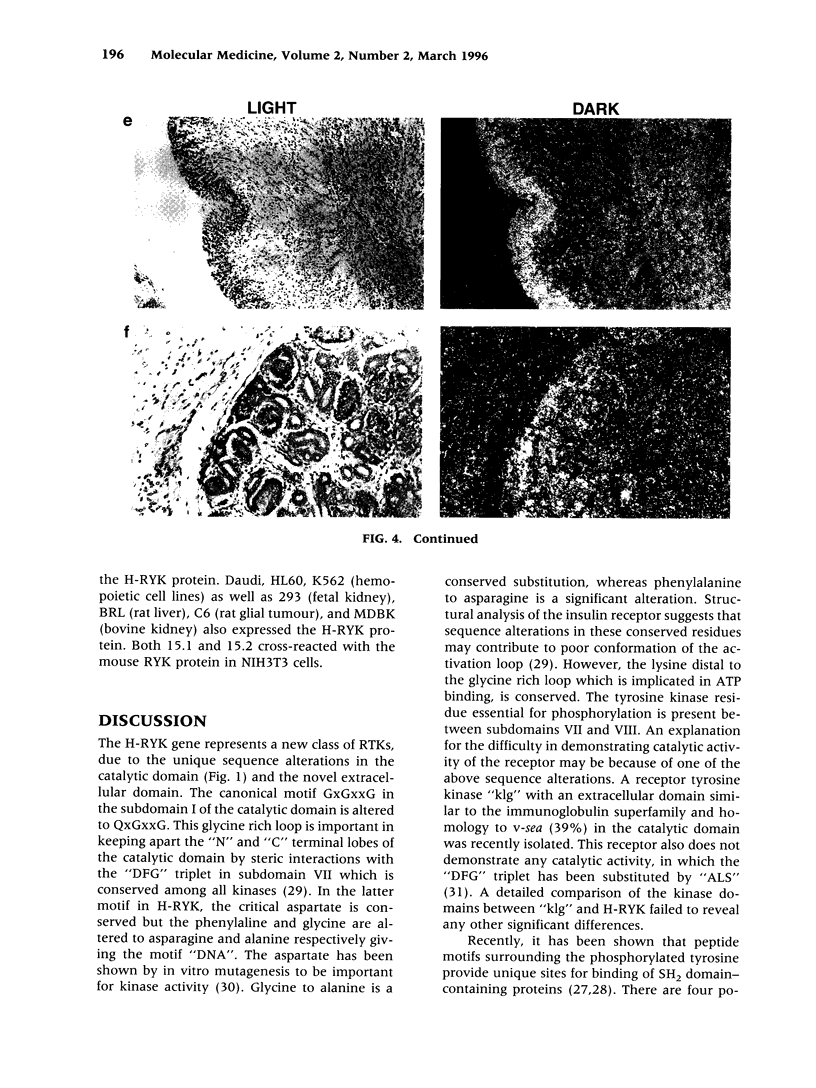
BACKGROUND: Protein tyrosine kinases play an important role in cellular metabolism as key components of signal transduction pathways. They are involved in cellular growth, differentiation, and development. Receptor tyrosine kinases (EGF receptor and c-erbB2) have been shown to be important in the pathogenesis of cancer. In ovarian cancer, overexpression of c-erbB2, a type I receptor, has been correlated with an adverse effect on survival of patients. MATERIAL AND METHODS: An unusual receptor tyrosine kinase, H-RYK, has been isolated from a complimentary DNA library of SKOV-3, an epithelial ovarian cancer cell line, using a polymerase chain reaction-mediated approach. RESULTS: The primary structure of the predicted amino acid sequence of the protein shows a novel NH2-terminal region. The catalytic region shows homology to other tyrosine kinases, the closest homology being with v-sea (39%). A significant alteration in the catalytic domain is that the highly conserved "DFG" triplet in subdomain VII is altered to "DNA." The gene was mapped to chromosome 3q22. A single transcript of 3.0 kb is expressed in heart, brain, lung, placenta, liver, muscle, kidney, and pancreas by Northern analysis with maximal expression in skeletal muscle. In situ hybridization analysis on human tissues demonstrated localization of message in the epithelial and stromal compartment of tissues such as brain, lung, colon, kidney, and breast. There was minimal to absent expression of H-RYK on surface epithelium of ovaries. In benign (3) and borderline tumors of the ovary (5), there was expression in the stromal compartment. However, in malignant tumors (24) there was increased expression predominantly confined to the epithelium. Polyclonal antisera raised against synthetic peptides recognize a 100-kD protein in ovarian cancer cells and other cell lines. In contrast to other receptor tyrosine kinases, the receptor did not phosphorylate in an in vitro kinase assay. CONCLUSIONS: The expression of this unusual receptor tyrosine kinase in epithelial ovarian cancer suggests that it may be involved in tumor progression, which needs further investigation.
Full text
PDF


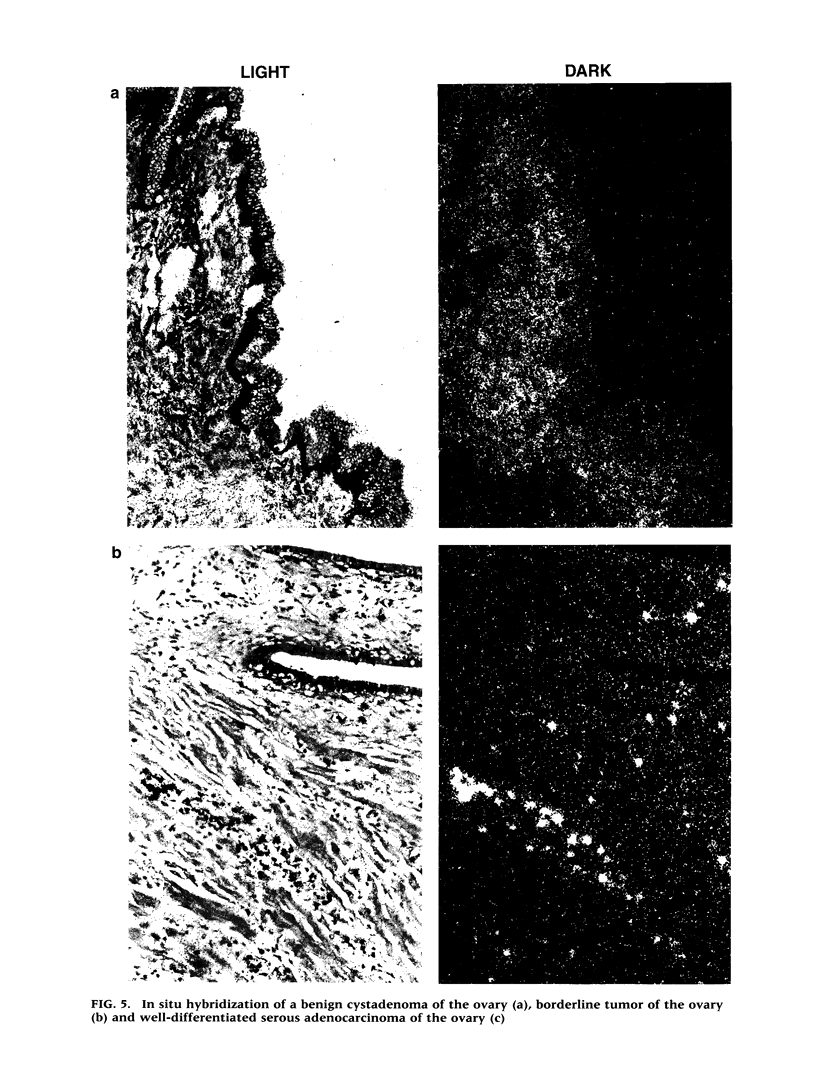

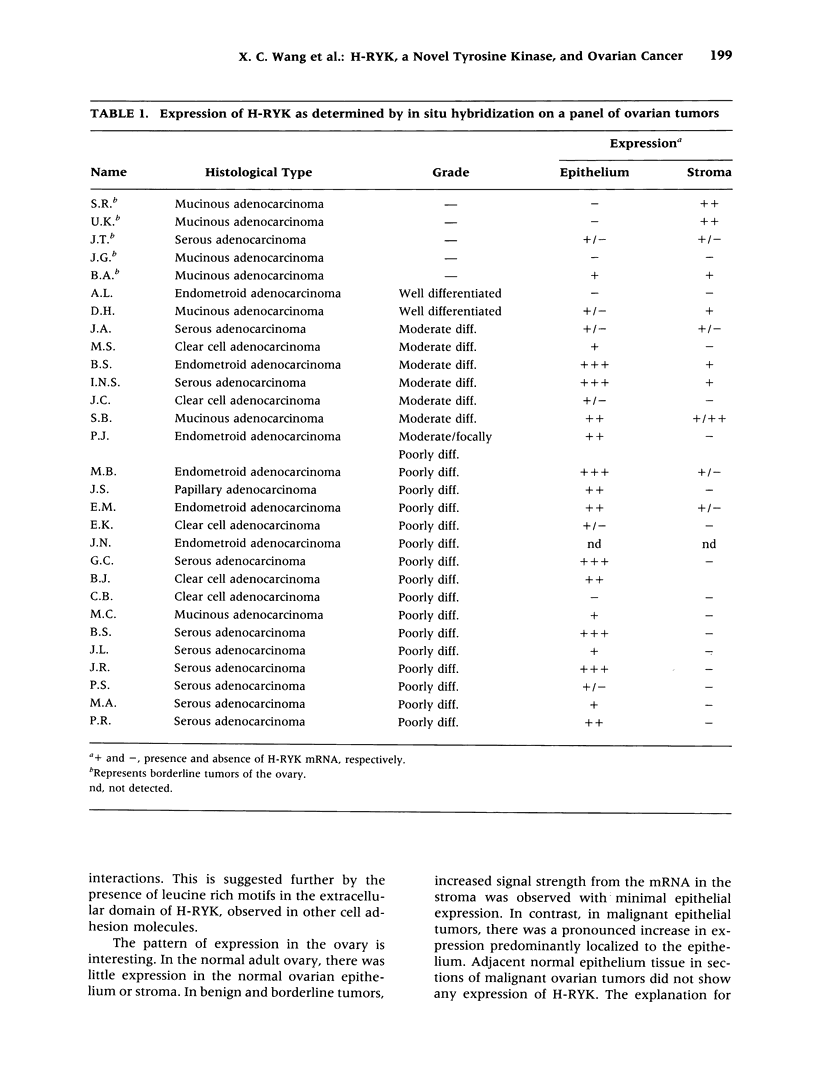




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bansal A., Gierasch L. M. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991 Dec 20;67(6):1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995 Jan 15;55(2):249–252. [PubMed] [Google Scholar]
- Callahan C. A., Muralidhar M. G., Lundgren S. E., Scully A. L., Thomas J. B. Control of neuronal pathway selection by a Drosophila receptor protein-tyrosine kinase family member. Nature. 1995 Jul 13;376(6536):171–174. doi: 10.1038/376171a0. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chen W. J., Goldstein J. L., Brown M. S. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990 Feb 25;265(6):3116–3123. [PubMed] [Google Scholar]
- Chou Y. H., Hayman M. J. Characterization of a member of the immunoglobulin gene superfamily that possibly represents an additional class of growth factor receptor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4897–4901. doi: 10.1073/pnas.88.11.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Eng C., Smith D. P., Mulligan L. M., Nagai M. A., Healey C. S., Ponder M. A., Gardner E., Scheumann G. F., Jackson C. E., Tunnacliffe A. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994 Feb;3(2):237–241. doi: 10.1093/hmg/3.2.237. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Hovens C. M., Stacker S. A., Andres A. C., Harpur A. G., Ziemiecki A., Wilks A. F. RYK, a receptor tyrosine kinase-related molecule with unusual kinase domain motifs. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11818–11822. doi: 10.1073/pnas.89.24.11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard S. R., Wei L., Ellis L., Hendrickson W. A. Crystal structure of the tyrosine kinase domain of the human insulin receptor. Nature. 1994 Dec 22;372(6508):746–754. doi: 10.1038/372746a0. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Kavanaugh W. M., Turck C. W., Williams L. T. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science. 1995 May 26;268(5214):1177–1179. doi: 10.1126/science.7539155. [DOI] [PubMed] [Google Scholar]
- Kelman Z., Simon-Chazottes D., Guénet J. L., Yarden Y. The murine vik gene (chromosome 9) encodes a putative receptor with unique protein kinase motifs. Oncogene. 1993 Jan;8(1):37–44. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laval S., Butler R., Shelling A. N., Hanby A. M., Poulsom R., Ganesan T. S. Isolation and characterization of an epithelial-specific receptor tyrosine kinase from an ovarian cancer cell line. Cell Growth Differ. 1994 Nov;5(11):1173–1183. [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Sadowski I., Pawson T. Mutational analysis of a phosphotransfer motif essential for v-fps tyrosine kinase activity. Oncogene. 1988 Dec;3(6):665–672. [PubMed] [Google Scholar]
- Mulligan L. M., Kwok J. B., Healey C. S., Elsdon M. J., Eng C., Gardner E., Love D. R., Mole S. E., Moore J. K., Papi L. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993 Jun 3;363(6428):458–460. doi: 10.1038/363458a0. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein modules and signalling networks. Nature. 1995 Feb 16;373(6515):573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- Pawson T. Protein-tyrosine kinases. Getting down to specifics. Nature. 1995 Feb 9;373(6514):477–478. doi: 10.1038/373477a0. [DOI] [PubMed] [Google Scholar]
- Prigent S. A., Gullick W. J. Identification of c-erbB-3 binding sites for phosphatidylinositol 3'-kinase and SHC using an EGF receptor/c-erbB-3 chimera. EMBO J. 1994 Jun 15;13(12):2831–2841. doi: 10.1002/j.1460-2075.1994.tb06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Ullrich A. Growth factor signaling by receptor tyrosine kinases. Neuron. 1992 Sep;9(3):383–391. doi: 10.1016/0896-6273(92)90177-f. [DOI] [PubMed] [Google Scholar]
- Senior P. V., Critchley D. R., Beck F., Walker R. A., Varley J. M. The localization of laminin mRNA and protein in the postimplantation embryo and placenta of the mouse: an in situ hybridization and immunocytochemical study. Development. 1988 Nov;104(3):431–446. doi: 10.1242/dev.104.3.431. [DOI] [PubMed] [Google Scholar]
- Shelling A. N., Butler R., Jones T., Laval S., Boyle J. M., Ganesan T. S. Localization of an epithelial-specific receptor kinase (EDDR1) to chromosome 6q16. Genomics. 1995 Jan 20;25(2):584–587. doi: 10.1016/0888-7543(95)80065-t. [DOI] [PubMed] [Google Scholar]
- Shiang R., Thompson L. M., Zhu Y. Z., Church D. M., Fielder T. J., Bocian M., Winokur S. T., Wasmuth J. J. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994 Jul 29;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., Stuart S. G., Udove J., Ullrich A. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989 May 12;244(4905):707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Vogt P. K., Hayman M. J. The v-sea oncogene of avian erythroblastosis retrovirus S13: another member of the protein-tyrosine kinase gene family. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5291–5295. doi: 10.1073/pnas.86.14.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songyang Z., Carraway K. L., 3rd, Eck M. J., Harrison S. C., Feldman R. A., Mohammadi M., Schlessinger J., Hubbard S. R., Smith D. P., Eng C. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995 Feb 9;373(6514):536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., Chaudhuri M., Gish G., Pawson T., Haser W. G., King F., Roberts T., Ratnofsky S., Lechleider R. J. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993 Mar 12;72(5):767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- Songyang Z., Shoelson S. E., McGlade J., Olivier P., Pawson T., Bustelo X. R., Barbacid M., Sabe H., Hanafusa H., Yi T. Specific motifs recognized by the SH2 domains of Csk, 3BP2, fps/fes, GRB-2, HCP, SHC, Syk, and Vav. Mol Cell Biol. 1994 Apr;14(4):2777–2785. doi: 10.1128/mcb.14.4.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacker S. A., Hovens C. M., Vitali A., Pritchard M. A., Baker E., Sutherland G. R., Wilks A. F. Molecular cloning and chromosomal localisation of the human homologue of a receptor related to tyrosine kinases (RYK). Oncogene. 1993 May;8(5):1347–1356. [PubMed] [Google Scholar]
- Tamagnone L., Partanen J., Armstrong E., Lasota J., Ohgami K., Tazunoki T., LaForgia S., Huebner K., Alitalo K. The human ryk cDNA sequence predicts a protein containing two putative transmembrane segments and a tyrosine kinase catalytic domain. Oncogene. 1993 Jul;8(7):2009–2014. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Ganesan T. S., Dhut S., Gibbons B., Lister T. A., Rothbard J., Young B. D. Novel chimaeric protein expressed in Philadelphia positive acute lymphoblastic leukaemia. 1987 Oct 29-Nov 4Nature. 329(6142):851–853. doi: 10.1038/329851a0. [DOI] [PubMed] [Google Scholar]
- Wallasch C., Weiss F. U., Niederfellner G., Jallal B., Issing W., Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO J. 1995 Sep 1;14(17):4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. F. The IRS-1 signaling system. Curr Opin Genet Dev. 1994 Feb;4(1):47–54. doi: 10.1016/0959-437x(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Wilks A. F. Two putative protein-tyrosine kinases identified by application of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1603–1607. doi: 10.1073/pnas.86.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]