Abstract
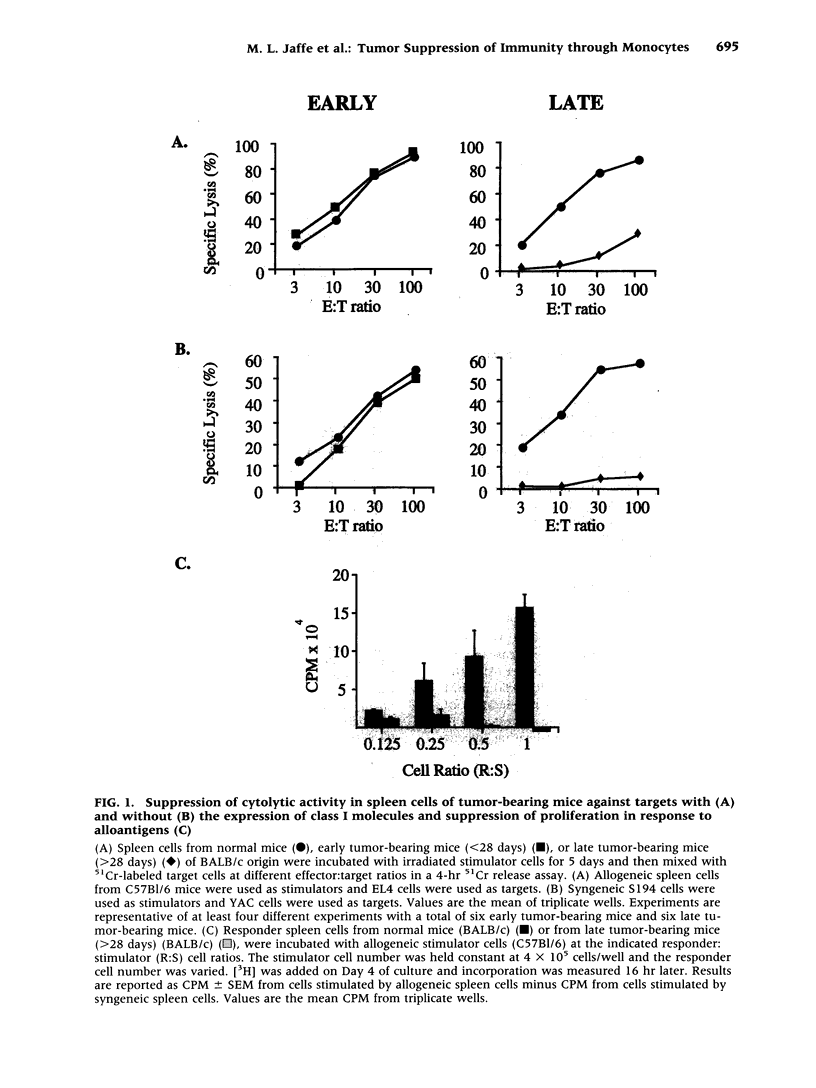
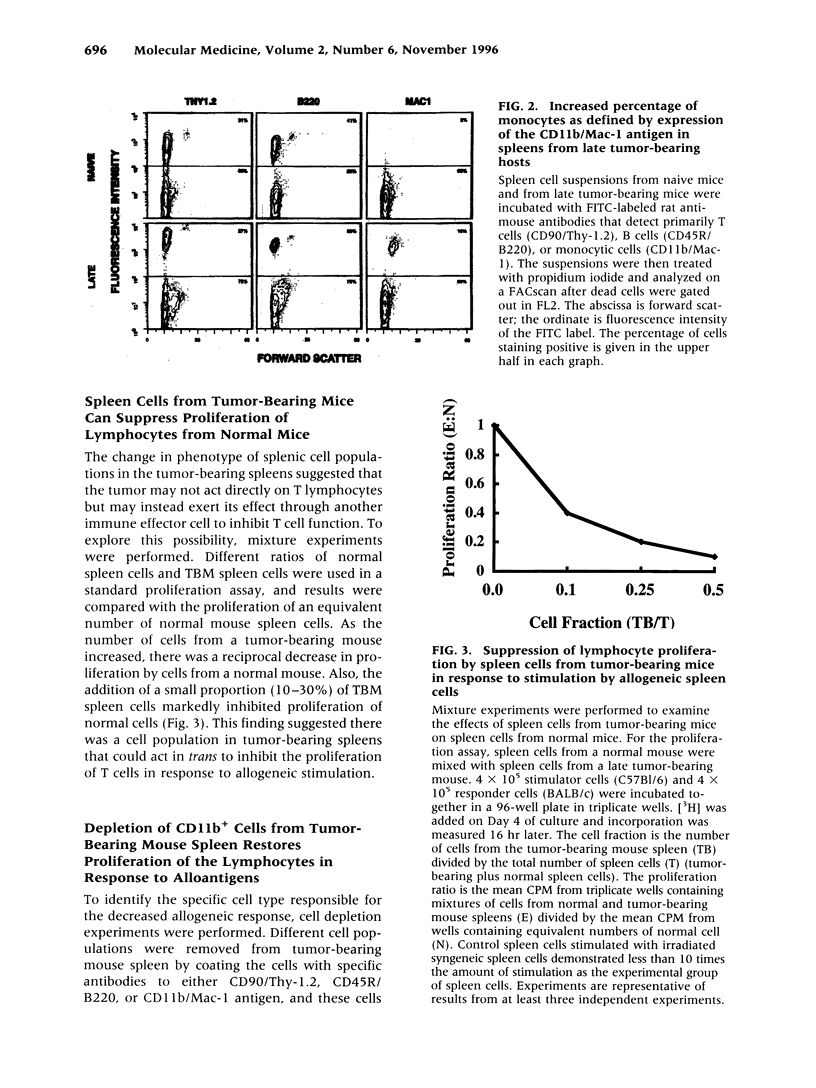
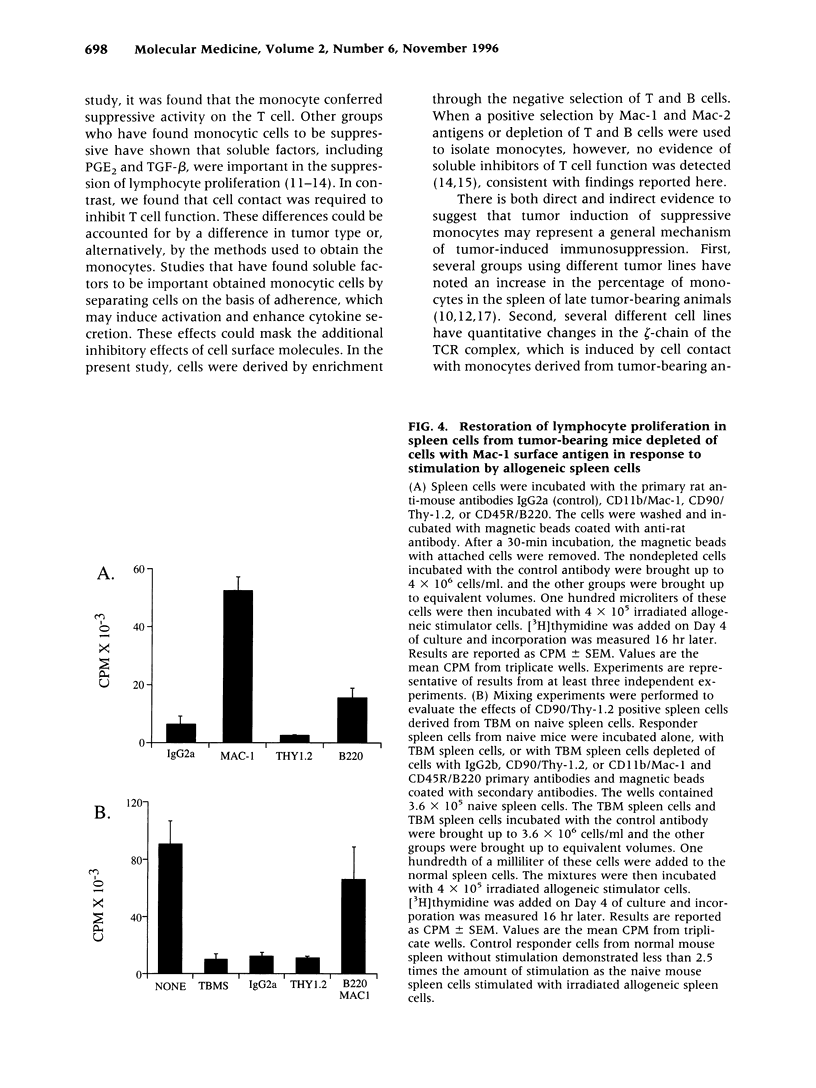
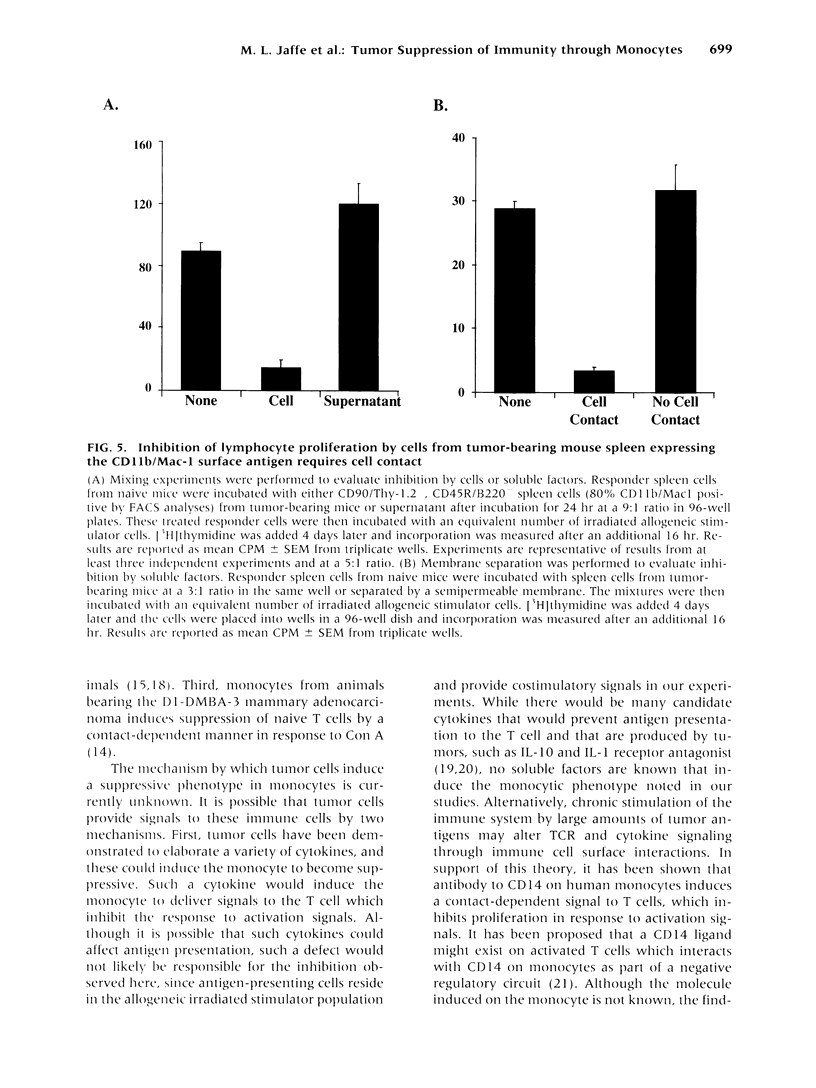
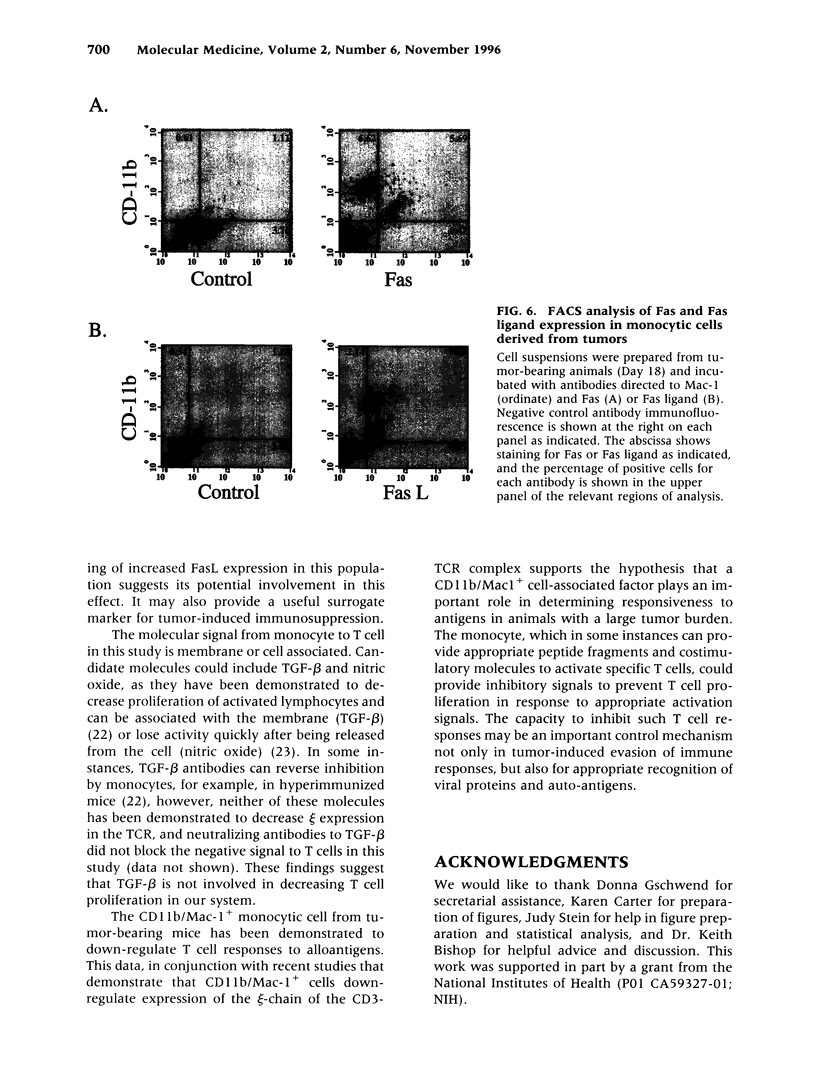
BACKGROUND: The progressive growth of tumors in mice is accompanied by down-regulation of specific T cell responses. The factors involved in this suppression are not completely understood. Here, we have developed a model to examine the role of host immune effector cells in the inhibition of T cell function. In this model, progressive growth of a colon carcinoma line, CT26, is accompanied by loss of T cell response to alloantigens in both cytolytic and proliferation assays. MATERIALS AND METHODS: The CT26 tumor was inoculated into BALB/c syngeneic mice. Tumor growth, cytolytic T cell responses, lymphocyte proliferation, and flow cytometric analysis was performed in tumor-bearing animals 7 or 28 days after tumor inoculation. RESULTS: Spleen cells from tumor-bearing mice were found to suppress the proliferative response of spleen cells from normal mice to alloantigens. Examination of the spleen cell population by FACS analysis revealed an increase in the percentage of monocytes as defined by expression of CD11b, the Mac-1 antigen. Removal of the Mac-1-positive cells from the tumor-bearing hosts spleen relieved suppression of the tumor-bearing mouse spleen cell proliferative response to alloantigens, and addition of the Mac-1-positive enriched cells suppressed proliferation of normal T cells in response to alloantigens. Cell contact was required for this inhibition. CONCLUSIONS: Tumor induction of suppressive monocytes plays an important role in the general immunosuppression noted in animals bearing CT26 tumors. Identification of the mechanisms responsible for this effect and reversal of tumor-induced macrophage suppression may facilitate efforts to develop effective immunotherapy for malignancy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoe T., Okamoto Y., Saito T. Activated macrophages induce structural abnormalities of the T cell receptor-CD3 complex. J Exp Med. 1995 May 1;181(5):1881–1886. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broder S., Waldmann T. A. The suppressor-cell network in cancer (first of two parts). N Engl J Med. 1978 Dec 7;299(23):1281–1284. doi: 10.1056/NEJM197812072992305. [DOI] [PubMed] [Google Scholar]
- Bursuker I., North R. J. Generation and decay of the immune response to a progressive fibrosarcoma. II. Failure to demonstrate postexcision immunity after the onset of T cell-mediated suppression of immunity. J Exp Med. 1984 May 1;159(5):1312–1321. doi: 10.1084/jem.159.5.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham S., Rowland I. J. Inhibition of the reactive proliferation of lymphocytes by activated macrophages: the role of nitric oxide. Clin Exp Immunol. 1992 Jan;87(1):157–162. doi: 10.1111/j.1365-2249.1992.tb06430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cruz E., Gilman S. C., Feldman J. D. Altered levels of mononuclear leukocytes in tumor-bearing rats: decrease of helper T lymphocytes and increase of suppressor cells. J Immunol. 1982 Sep;129(3):1324–1328. [PubMed] [Google Scholar]
- Fu Y. X., Watson G., Jimenez J. J., Wang Y., Lopez D. M. Expansion of immunoregulatory macrophages by granulocyte-macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res. 1990 Jan 15;50(2):227–234. [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Golstein P. Lymphocyte activation and effector functions. Editorial overview. The role of cell surface molecules. Curr Opin Immunol. 1993 Jun;5(3):313–323. doi: 10.1016/0952-7915(93)90048-w. [DOI] [PubMed] [Google Scholar]
- Lue K. H., Lauener R. P., Winchester R. J., Geha R. S., Vercelli D. Engagement of CD14 on human monocytes terminates T cell proliferation by delivering a negative signal to T cells. J Immunol. 1991 Aug 15;147(4):1134–1138. [PubMed] [Google Scholar]
- Maccubbin D. L., Mace K. F., Ehrke M. J., Mihich E. Modification of host antitumor defense mechanisms in mice by progressively growing tumor. Cancer Res. 1989 Aug 1;49(15):4216–4224. [PubMed] [Google Scholar]
- Mizoguchi H., O'Shea J. J., Longo D. L., Loeffler C. M., McVicar D. W., Ochoa A. C. Alterations in signal transduction molecules in T lymphocytes from tumor-bearing mice. Science. 1992 Dec 11;258(5089):1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- Parhar R. S., Lala P. K. Prostaglandin E2-mediated inactivation of various killer lineage cells by tumor-bearing host macrophages. J Leukoc Biol. 1988 Dec;44(6):474–484. doi: 10.1002/jlb.44.6.474. [DOI] [PubMed] [Google Scholar]
- Plautz G. E., Yang Z. Y., Wu B. Y., Gao X., Huang L., Nabel G. J. Immunotherapy of malignancy by in vivo gene transfer into tumors. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4645–4649. doi: 10.1073/pnas.90.10.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. R., Kunkel S. L., Burdick M. D., Wilke C. A., Orringer M. B., Whyte R. I., Strieter R. M. Production of interleukin-10 by human bronchogenic carcinoma. Am J Pathol. 1994 Jul;145(1):18–25. [PMC free article] [PubMed] [Google Scholar]
- Stach R. M., Rowley D. A. A first or dominant immunization. II. Induced immunoglobulin carries transforming growth factor beta and suppresses cytolytic T cell responses to unrelated alloantigens. J Exp Med. 1993 Sep 1;178(3):841–852. doi: 10.1084/jem.178.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Ohzeki S., Utsumi K., Takiuchi H., Muramatsu M., Li X. F., Shimizu J., Fujiwara H., Hamaoka T. Transforming growth factor-beta-induced inhibition of T cell function. Susceptibility difference in T cells of various phenotypes and functions and its relevance to immunosuppression in the tumor-bearing state. J Immunol. 1991 Feb 1;146(3):1077–1082. [PubMed] [Google Scholar]
- Walker T. M., Burger C. J., Elgert K. D. Tumor growth alters T cell and macrophage production of and responsiveness to granulocyte-macrophage colony-stimulating factor: partial dysregulation through interleukin-10. Cell Immunol. 1994 Apr 1;154(1):342–357. doi: 10.1006/cimm.1994.1082. [DOI] [PubMed] [Google Scholar]
- Watson G. A., Fu Y. X., Lopez D. M. Splenic macrophages from tumor-bearing mice co-expressing MAC-1 and MAC-2 antigens exert immunoregulatory functions via two distinct mechanisms. J Leukoc Biol. 1991 Feb;49(2):126–138. doi: 10.1002/jlb.49.2.126. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Weiss A. T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell. 1993 Apr 23;73(2):209–212. doi: 10.1016/0092-8674(93)90221-b. [DOI] [PubMed] [Google Scholar]
- Young M. R., Wright M. A., Coogan M., Young M. E., Bagash J. Tumor-derived cytokines induce bone marrow suppressor cells that mediate immunosuppression through transforming growth factor beta. Cancer Immunol Immunother. 1992;35(1):14–18. doi: 10.1007/BF01741049. [DOI] [PMC free article] [PubMed] [Google Scholar]