Abstract
BACKGROUND: CSF-1 expression precedes renal injury in autoimmune MRL-lpr mice and is responsible for macrophage (M phi) proliferation and survival in the kidney. By comparison, C3H-lpr mice do not express CSF-1 in the kidney, and despite the lpr mutation, kidneys remain normal. The purpose of this study was to test the capacity of local and systemic expression of M phi growth factor, CSF-1 to initiate renal injury in normal (C3H-(++), MRL-(++) and autoimmune (C3H-lpr, MRL-lpr) mice. MATERIALS AND METHODS: We designed a gene transfer system to deliver cytokines into the kidney by transducing renal tubular epithelial cells (TEC) using retroviral vectors expressing CSF-1 or another M phi growth factor, GM-CSF. We placed transduced syngeneic cytokine-TEC under the renal capsule of normal and autoimmune prone mice prior to renal injury and evaluated renal pathology at 3, 7, 14, 28, and 90 days postimplant. RESULTS: CSF-1-TEC and GM-CSF-TEC, but not uninfected TEC, caused extensive local renal injury in strains with the lpr mutation. At 3-7 days the infiltrating cells were mainly M phi, and by 28 days they were predominantly lymphocytes. By comparison, the kidneys of MRL-(++) and C3H-(++) mice remained normal. Implanted genetically modified TEC caused a sustained increase of CSF-1 or GM-CSF in the circulation which did not modify the contralateral kidney. CONCLUSIONS: Gene transfer of M phi growth factors into the kidney initiates severe local renal injury in autoimmune prone mice with the lpr mutation, but does not compromise the kidney in nonautoimmune hosts. Of note, introduction of M phi growth factors into the kidney of C3H-lpr mice which do not spontaneously develop renal injury incites renal damage. These studies offer a gene transfer approach to explore the impact of local and systemic cytokine production on renal injury.
Full text
PDF



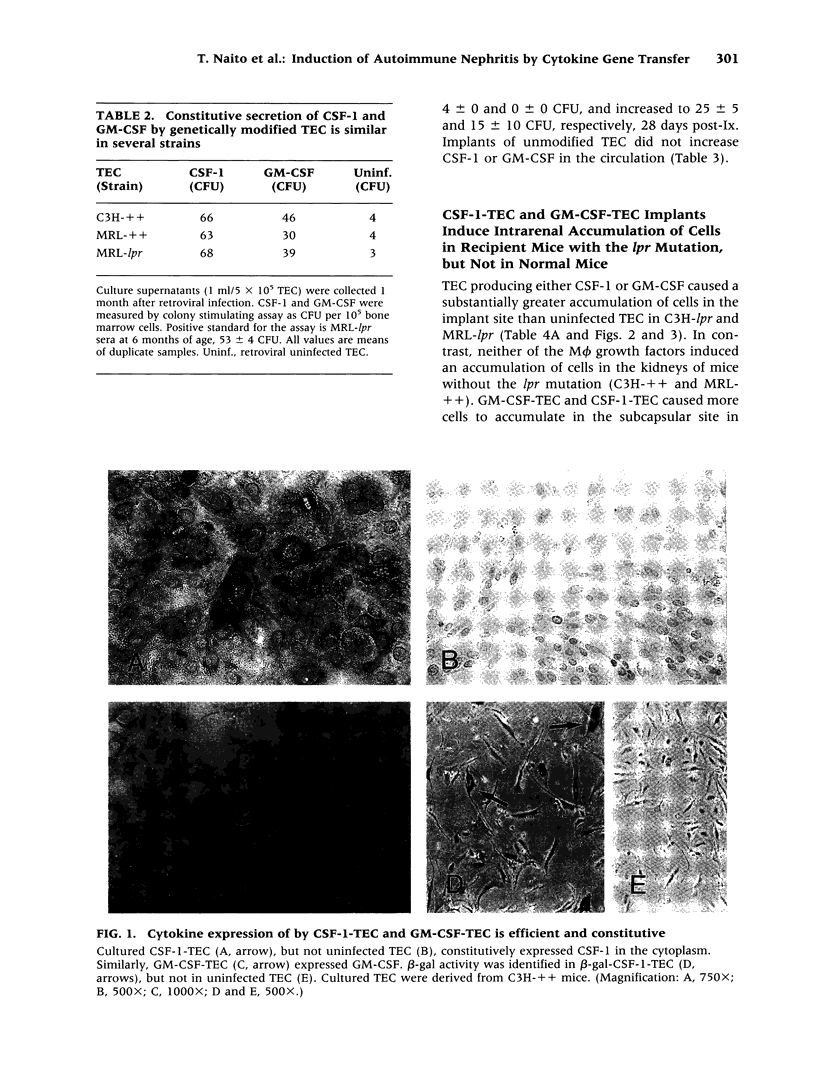



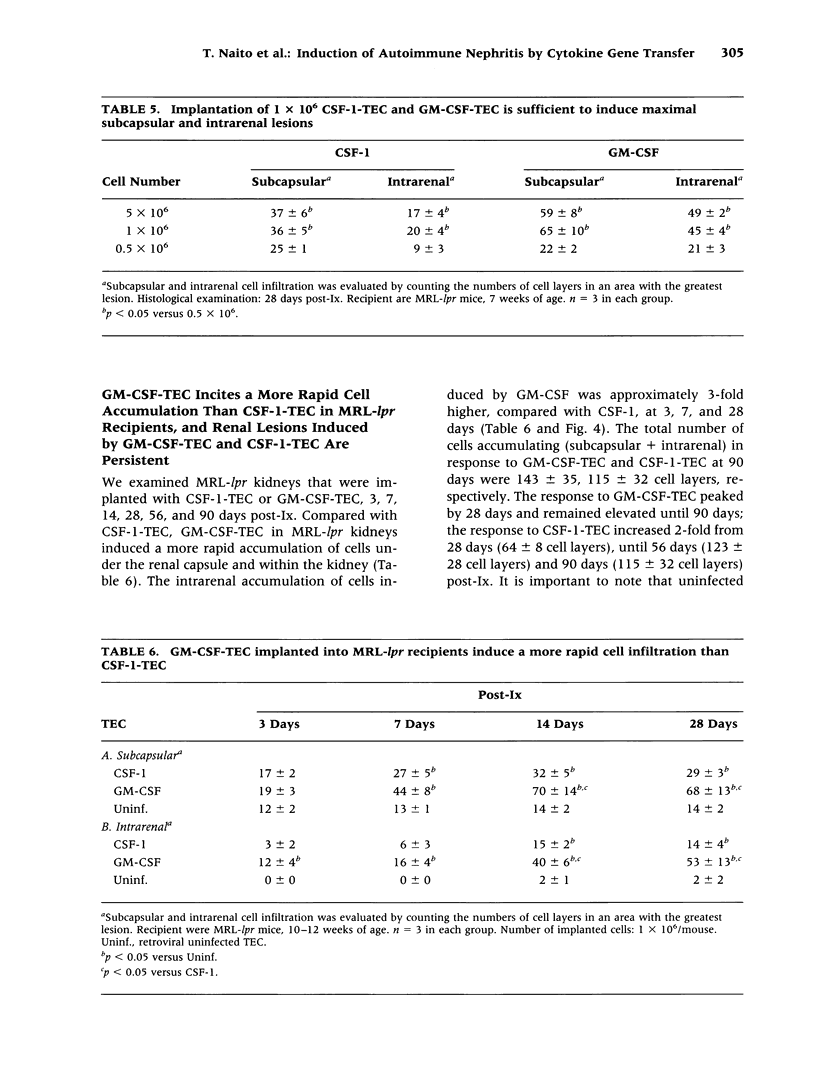

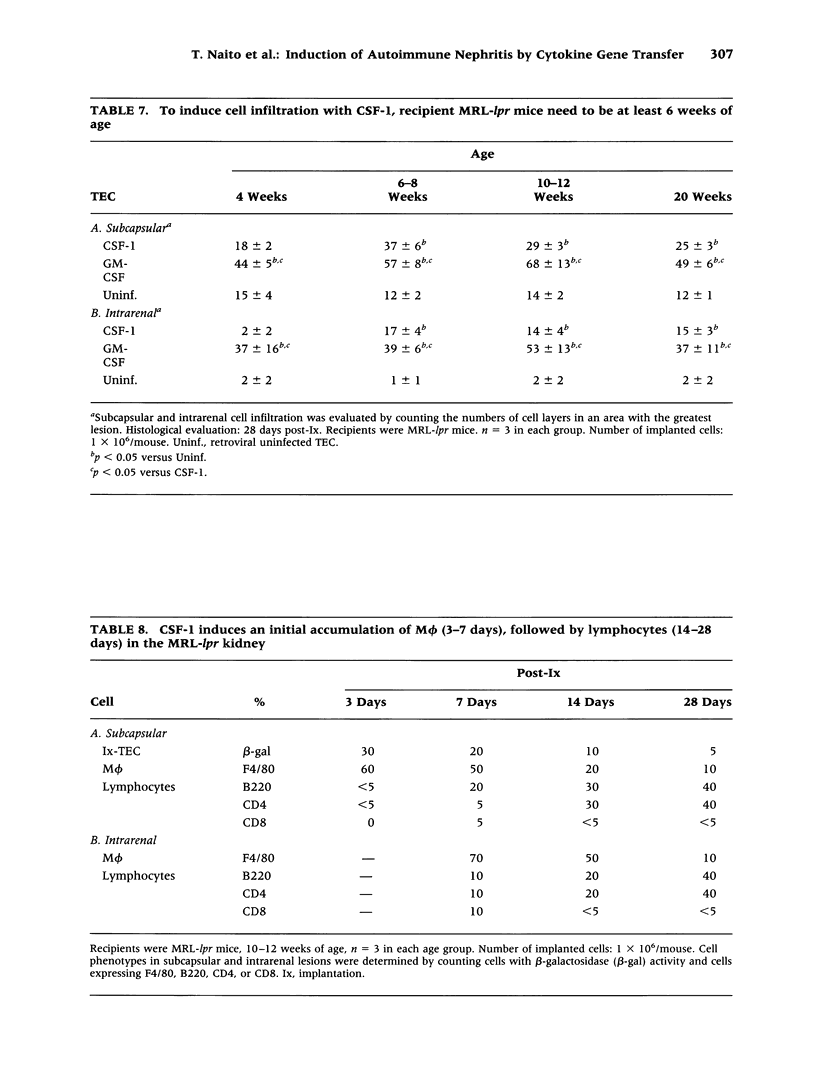





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- American Board of Internal Medicine, Inc. Middleton W. S. Subject: The American Board of Internal Medicine, Inc. Cal West Med. 1939 Apr;50(4):312–312. [PMC free article] [PubMed] [Google Scholar]
- Bloom R. D., Florquin S., Singer G. G., Brennan D. C., Kelley V. R. Colony stimulating factor-1 in the induction of lupus nephritis. Kidney Int. 1993 May;43(5):1000–1009. doi: 10.1038/ki.1993.141. [DOI] [PubMed] [Google Scholar]
- Bosch R. J., Woolf A. S., Fine L. G. Gene transfer into the mammalian kidney: direct retrovirus-transduction of regenerating tubular epithelial cells. Exp Nephrol. 1993 Jan-Feb;1(1):49–54. [PubMed] [Google Scholar]
- Boswell J. M., Yui M. A., Burt D. W., Kelley V. E. Increased tumor necrosis factor and IL-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988 Nov 1;141(9):3050–3054. [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Brennan D. C., Jevnikar A. M., Bloom R. D., Brissette W. H., Singer G. G., Kelley V. R. Cultured mesangial cells from autoimmune MRL-lpr mice have decreased secreted and surface M-CSF. Kidney Int. 1992 Aug;42(2):279–284. doi: 10.1038/ki.1992.287. [DOI] [PubMed] [Google Scholar]
- Danos O., Mulligan R. C. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6460–6464. doi: 10.1073/pnas.85.17.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz Gallo C., Jevnikar A. M., Brennan D. C., Florquin S., Pacheco-Silva A., Kelley V. R. Autoreactive kidney-infiltrating T-cell clones in murine lupus nephritis. Kidney Int. 1992 Oct;42(4):851–859. doi: 10.1038/ki.1992.360. [DOI] [PubMed] [Google Scholar]
- Hayek A., Culler F. L., Beattie G. M., Lopez A. D., Cuevas P., Baird A. An in vivo model for study of the angiogenic effects of basic fibroblast growth factor. Biochem Biophys Res Commun. 1987 Sep 15;147(2):876–880. doi: 10.1016/0006-291x(87)91011-4. [DOI] [PubMed] [Google Scholar]
- Isaka Y., Fujiwara Y., Ueda N., Kaneda Y., Kamada T., Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993 Dec;92(6):2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura M., Taylor S., Unwin R., Burton S., Shimizu F., Fine L. G. Gene transfer into the rat renal glomerulus via a mesangial cell vector: site-specific delivery, in situ amplification, and sustained expression of an exogenous gene in vivo. J Clin Invest. 1994 Aug;94(2):497–505. doi: 10.1172/JCI117361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen C. P., Ritchie S. C., Hendrix R., Linsley P. S., Hathcock K. S., Hodes R. J., Lowry R. P., Pearson T. C. Regulation of immunostimulatory function and costimulatory molecule (B7-1 and B7-2) expression on murine dendritic cells. J Immunol. 1994 Jun 1;152(11):5208–5219. [PubMed] [Google Scholar]
- Mendola J. F., Goity C., Fernández-Alvarez J., Saenz A., Benarroch G., Fernández-Cruz L., Gomis R. Immunocytochemical study of pancreatic islet revascularization in islet isograft. Effect of hyperglycemia of the recipient and of in vitro culture of islets. Transplantation. 1994 Mar 15;57(5):725–730. doi: 10.1097/00007890-199403150-00015. [DOI] [PubMed] [Google Scholar]
- Metcalf D. The granulocyte-macrophage colony-stimulating factors. Science. 1985 Jul 5;229(4708):16–22. doi: 10.1126/science.2990035. [DOI] [PubMed] [Google Scholar]
- Moullier P., Friedlander G., Calise D., Ronco P., Perricaudet M., Ferry N. Adenoviral-mediated gene transfer to renal tubular cells in vivo. Kidney Int. 1994 Apr;45(4):1220–1225. doi: 10.1038/ki.1994.162. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F. The role of the macrophage in glomerular injury. Semin Nephrol. 1991 May;11(3):268–275. [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Steward W. P., Scarffe J. H., Austin R., Bonnem E., Thatcher N., Morgenstern G., Crowther D. Recombinant human granulocyte macrophage colony stimulating factor (rhGM-CSF) given as daily short infusions--a phase I dose-toxicity study. Br J Cancer. 1989 Jan;59(1):142–145. doi: 10.1038/bjc.1989.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang B., Matsuda T., Akira S., Nagata N., Ikehara S., Hirano T., Kishimoto T. Age-associated increase in interleukin 6 in MRL/lpr mice. Int Immunol. 1991 Mar;3(3):273–278. doi: 10.1093/intimm/3.3.273. [DOI] [PubMed] [Google Scholar]
- Wang J. M., Griffin J. D., Rambaldi A., Chen Z. G., Mantovani A. Induction of monocyte migration by recombinant macrophage colony-stimulating factor. J Immunol. 1988 Jul 15;141(2):575–579. [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Jefferson D. M., Chowdhury J. R., Novikoff P. M., Johnston D. E., Mulligan R. C. Retrovirus-mediated transduction of adult hepatocytes. Proc Natl Acad Sci U S A. 1988 May;85(9):3014–3018. doi: 10.1073/pnas.85.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich R. P., Glimcher L. H., Yui M. A., Jevnikar A. M., Dumas S. E., Kelley V. E. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney Int. 1990 Feb;37(2):783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Kreft B., Kelley V. R. Biphasic increase in circulating and renal TNF-alpha in MRL-lpr mice with differing regulatory mechanisms. Kidney Int. 1995 Jan;47(1):122–130. doi: 10.1038/ki.1995.14. [DOI] [PubMed] [Google Scholar]
- Yui M. A., Brissette W. H., Brennan D. C., Wuthrich R. P., Rubin-Kelley V. E. Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am J Pathol. 1991 Aug;139(2):255–261. [PMC free article] [PubMed] [Google Scholar]








