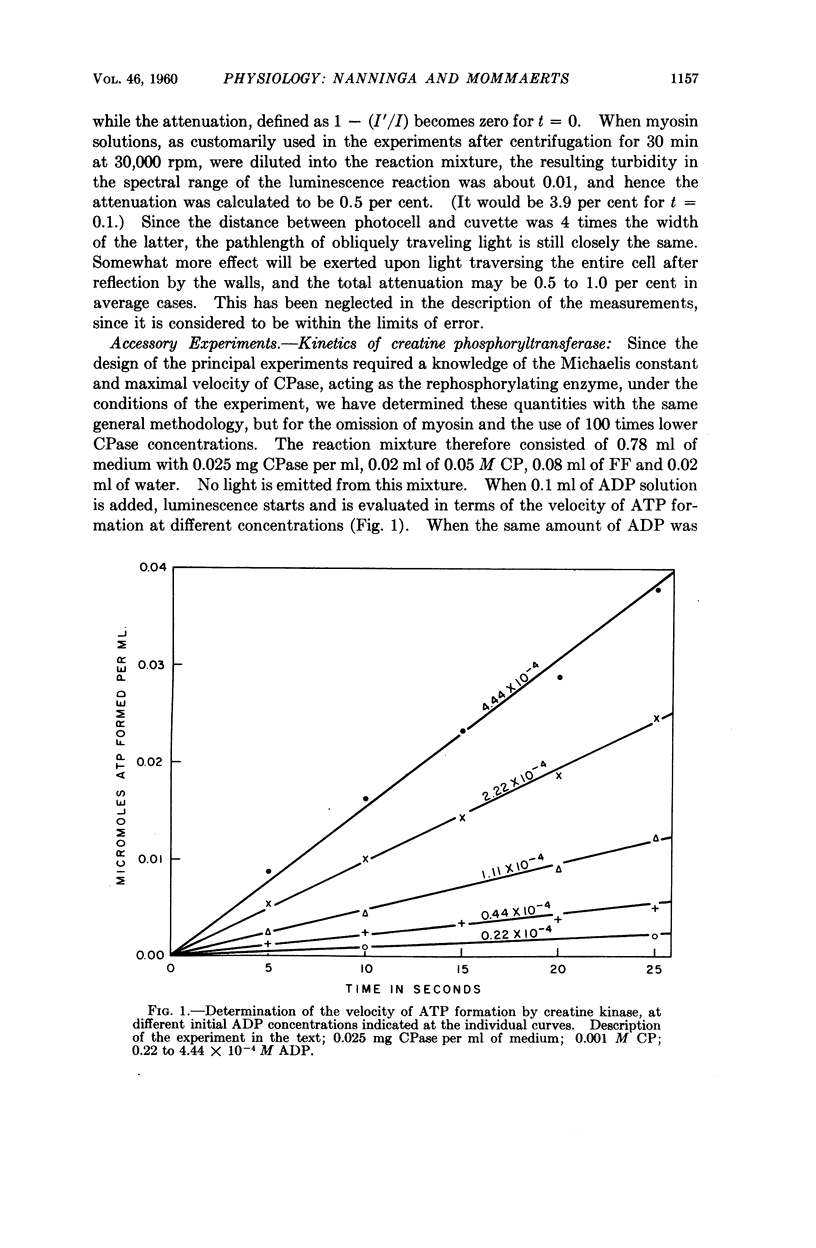
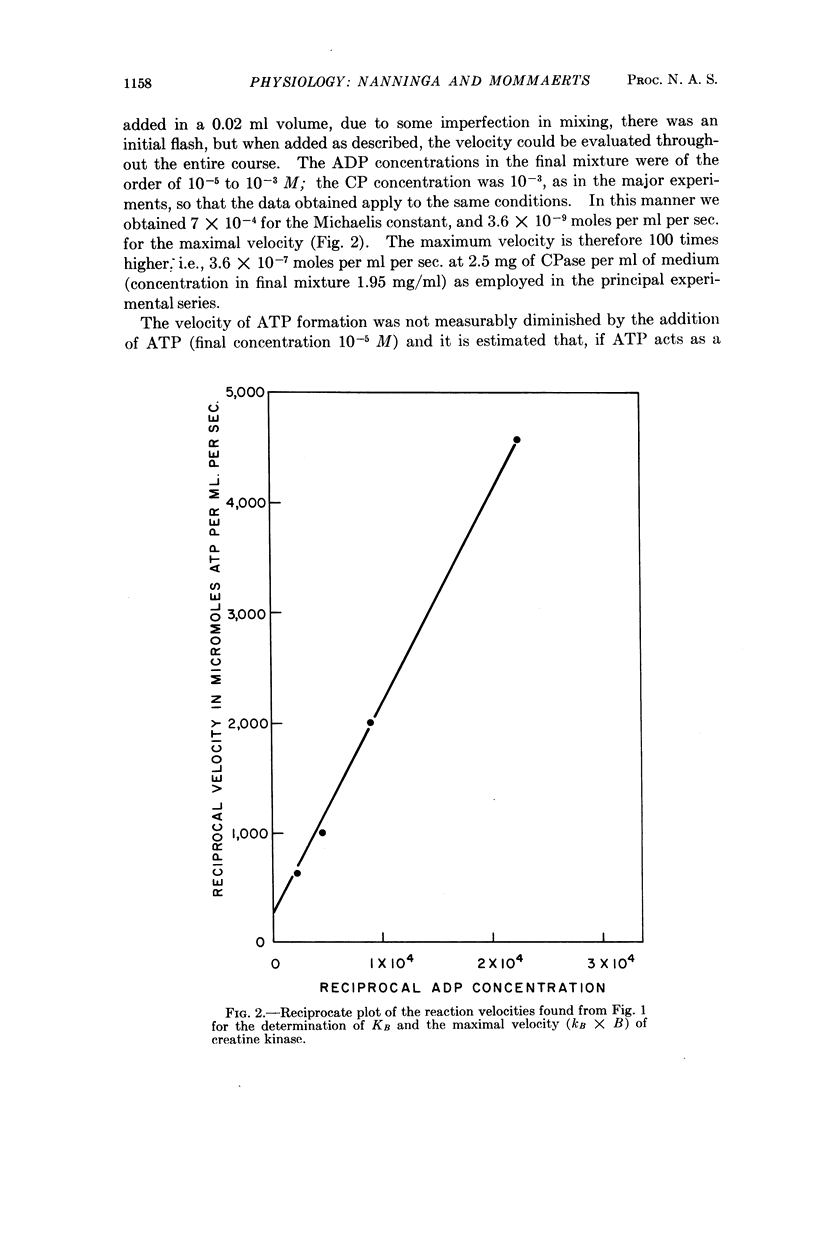
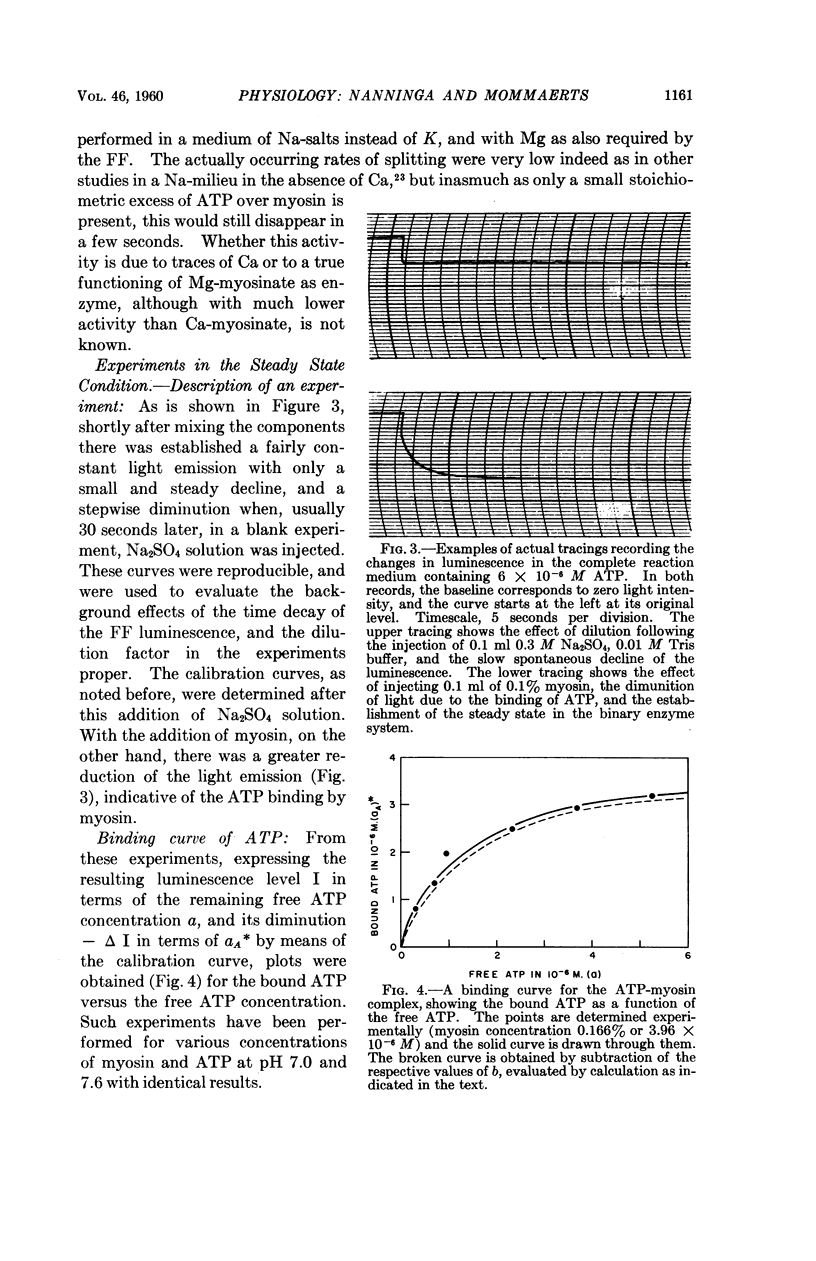
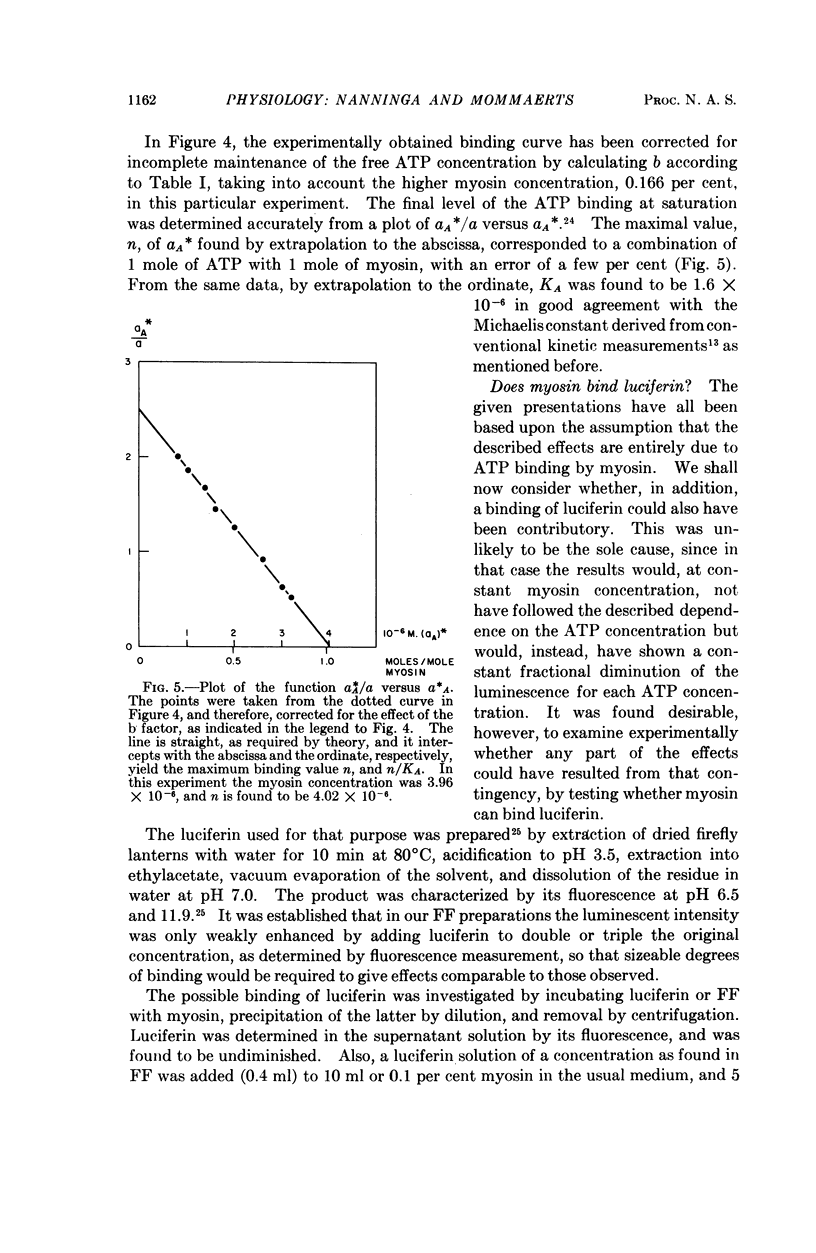
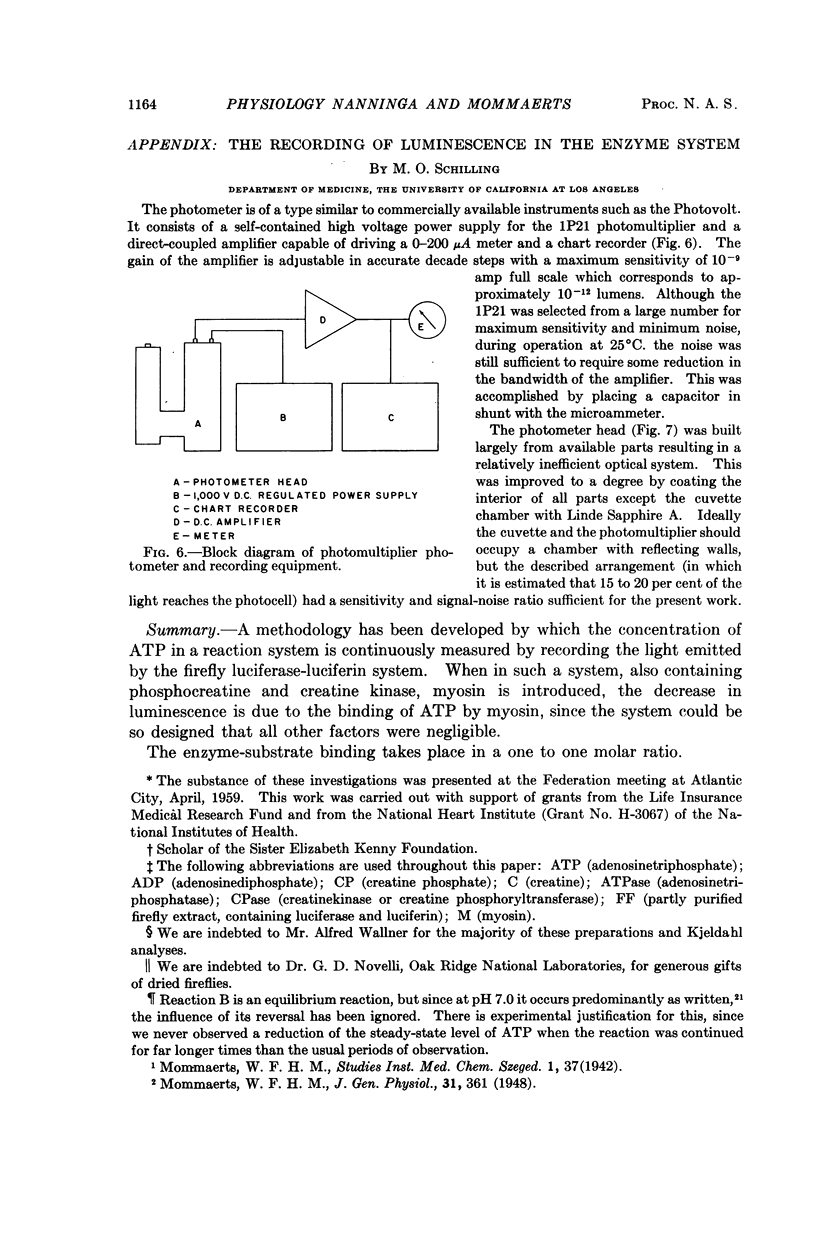
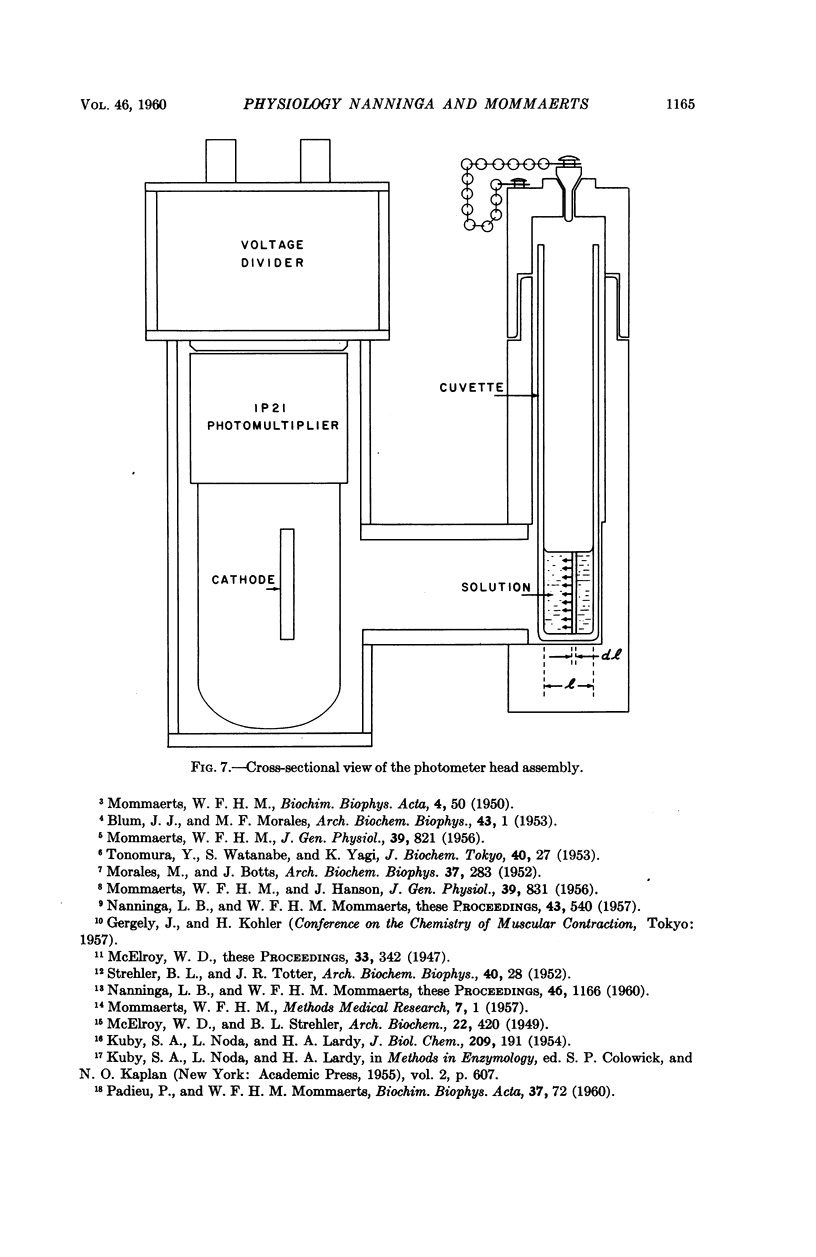
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLEN D., STREHLER B. L., STAPLETON G. E., BRIGHAM E. Postirradiation release of adenosine triphosphate from Escherichia coli B/r1. Arch Biochem Biophys. 1953 Mar;43(1):1–10. doi: 10.1016/0003-9861(53)90078-2. [DOI] [PubMed] [Google Scholar]
- BITLER B., McELROY W. D. The preparation and properties of crystalline firefly luciferin. Arch Biochem Biophys. 1957 Dec;72(2):358–368. doi: 10.1016/0003-9861(57)90212-6. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- MALMSTROM B. G. The interaction of purified enolase with its activating metal-ions. Arch Biochem Biophys. 1953 Oct;46(2):345–363. doi: 10.1016/0003-9861(53)90207-0. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F. H. M. The molecular transformations of actin. I. Globular actin. J Biol Chem. 1952 Sep;198(1):445–457. [PubMed] [Google Scholar]
- MOMMAERTS W. F., ALDRICH B. B. Determination of the molecular weight of myosin; interference-optical measurements during the approach to ultracentrifugal sedimentation and diffusion equilibrium. Biochim Biophys Acta. 1958 Jun;28(3):627–636. doi: 10.1016/0006-3002(58)90530-4. [DOI] [PubMed] [Google Scholar]
- MOMMAERTS W. F. Chemical investigation of muscular tissues. Methods Med Res. 1958;7:1–59. [PubMed] [Google Scholar]
- MOMMAERTS W. F., HANSON J. The effect upon actomyosin of stoichiometric amounts of adenosinetriphosphate regenerated in a coupled enzyme system. J Gen Physiol. 1956 Jul 20;39(6):831–839. doi: 10.1085/jgp.39.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOMMAERTS W. F. The effect of adenosinetriphosphate upon actomyosin solutions, studied with a recording dual beam light-scattering photometer. J Gen Physiol. 1956 Jul 20;39(6):821–830. doi: 10.1085/jgp.39.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORALES M. F., BOTTS J., BLUM J. J., HILL T. L. Elementary processes in muscle action: an examination of current concepts. Physiol Rev. 1955 Jul;35(3):475–505. doi: 10.1152/physrev.1955.35.3.475. [DOI] [PubMed] [Google Scholar]
- MORALES M., BOTTS J. A model for the elementary process in muscle action. Arch Biochem Biophys. 1952 Jun;37(2):283–300. doi: 10.1016/0003-9861(52)90193-8. [DOI] [PubMed] [Google Scholar]
- McElroy W. D. The Energy Source for Bioluminescence in an Isolated System. Proc Natl Acad Sci U S A. 1947 Nov;33(11):342–345. doi: 10.1073/pnas.33.11.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NANNINGA L. B. Investigation on the effect of calcium ions on the splitting of adenosinetriphosphate by myosin. Biochim Biophys Acta. 1959 Nov;36:191–202. doi: 10.1016/0006-3002(59)90084-8. [DOI] [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. IV. Equilibrium studies. J Biol Chem. 1954 Sep;210(1):83–95. [PubMed] [Google Scholar]
- Nanninga L. B., Mommaerts W. F. ON THE BINDING OF ADENOSINE TRIPHOSPHATE BY ACTOMYOSIN. Proc Natl Acad Sci U S A. 1957 Jun 15;43(6):540–542. doi: 10.1073/pnas.43.6.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PADIEU P., MOMMAERTS W. F. Creatine phosphoryl transferase and phosphoglyceraldehyde dehydrogenase in iodoacetate poisoned muscle. Biochim Biophys Acta. 1960 Jan 1;37:72–77. doi: 10.1016/0006-3002(60)90080-9. [DOI] [PubMed] [Google Scholar]
- STREHLER B. L., TOTTER J. R. Firefly luminescence in the study of energy transfer mechanisms. I. Substrate and enzyme determination. Arch Biochem Biophys. 1952 Sep;40(1):28–41. doi: 10.1016/0003-9861(52)90070-2. [DOI] [PubMed] [Google Scholar]
- VON HIPPEL P. H., SCHACHMAN H. K., APPEL P., MORALES M. F. On the molecular weight of myosin. Biochim Biophys Acta. 1958 Jun;28(3):504–507. doi: 10.1016/0006-3002(58)90511-0. [DOI] [PubMed] [Google Scholar]


