Abstract
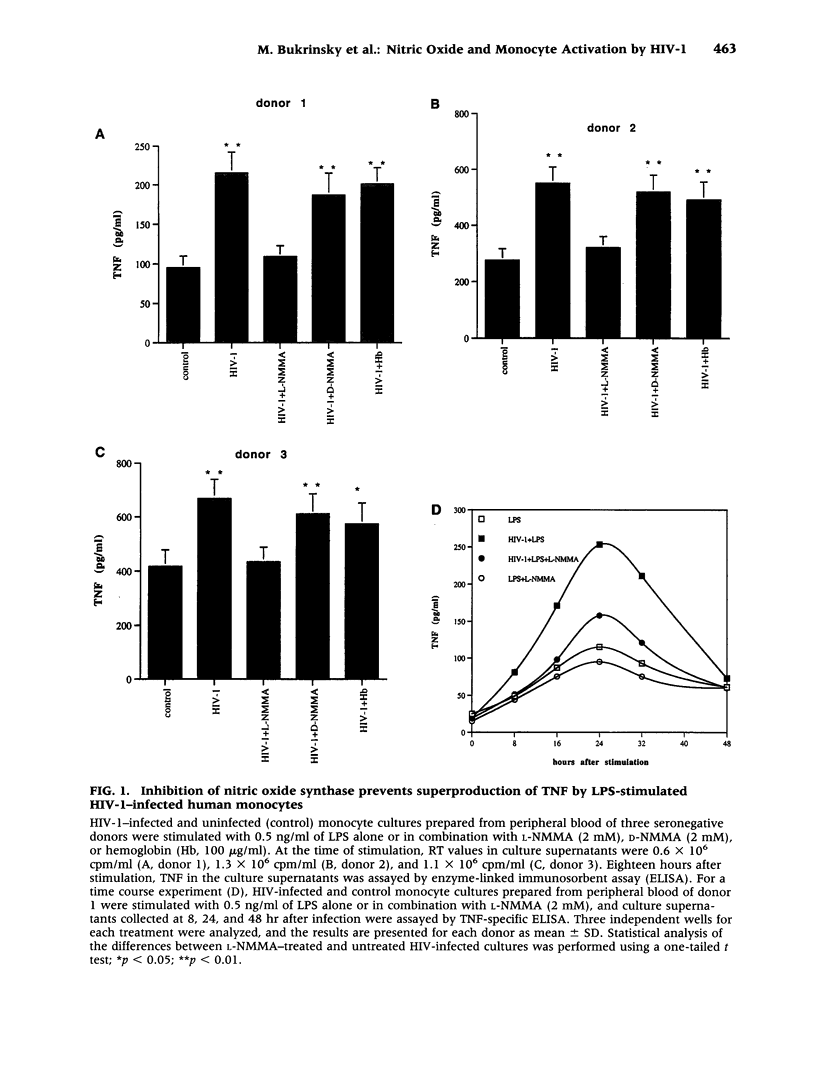
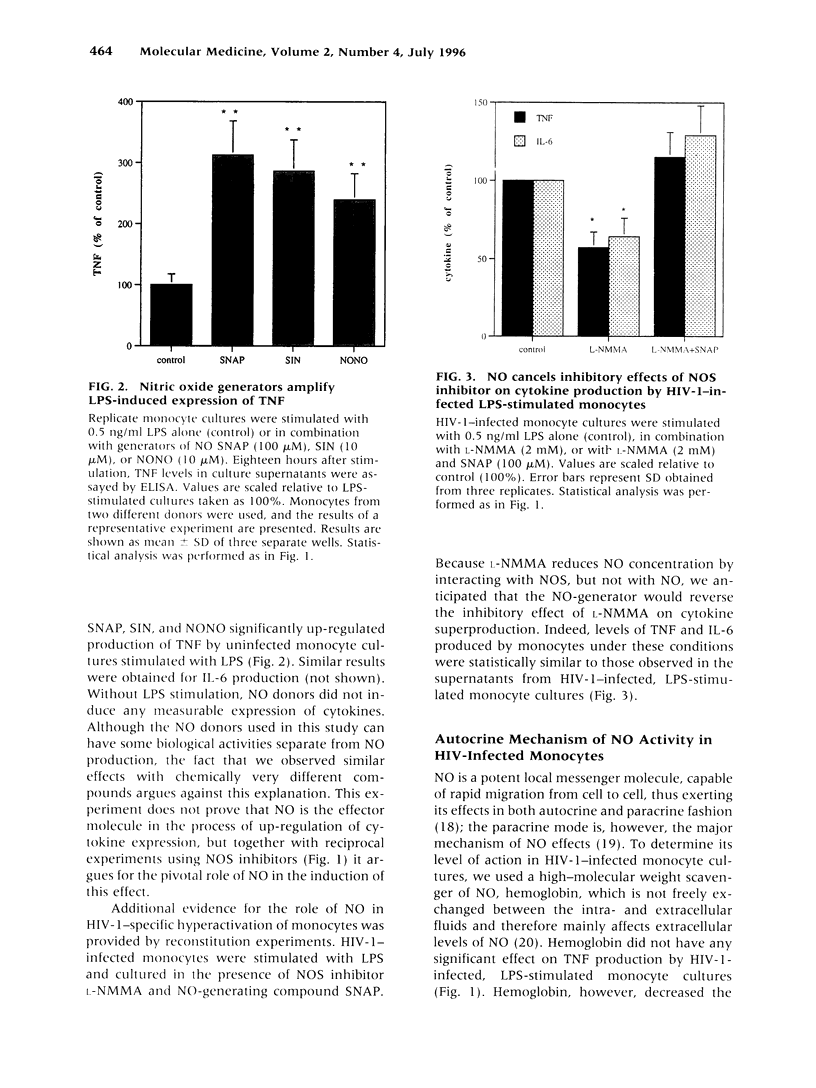
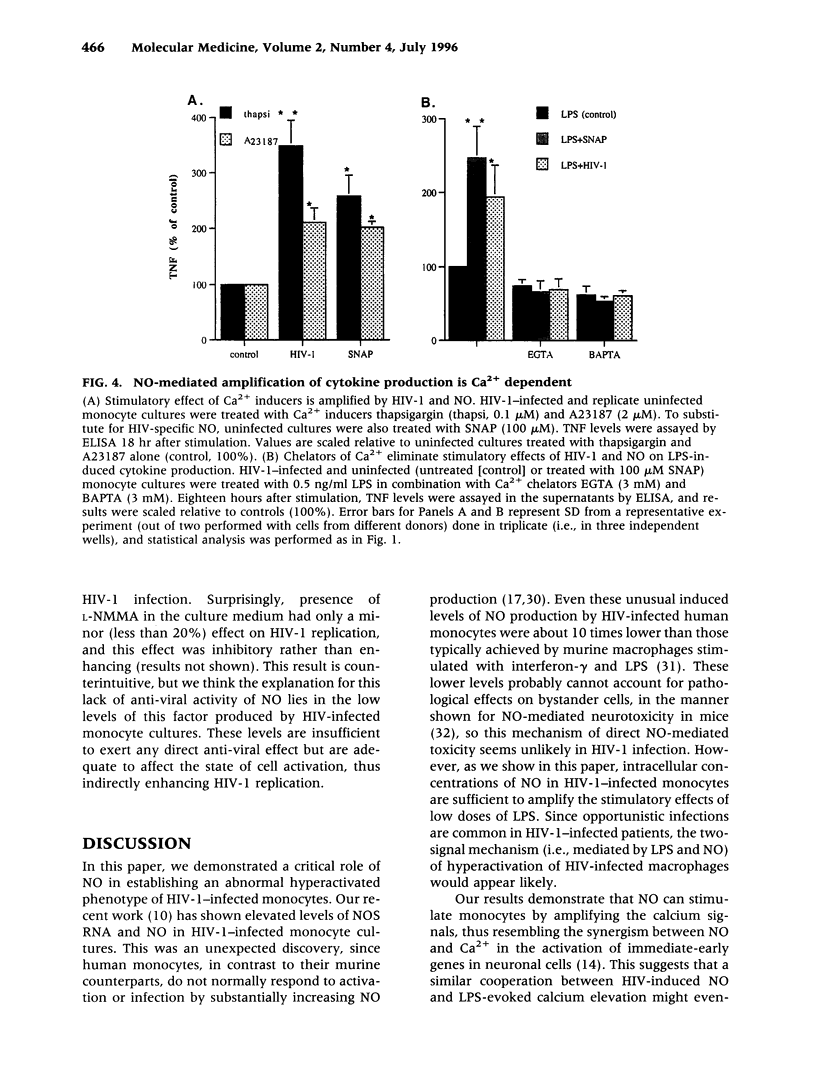
BACKGROUND: Human immunodeficiency virus type 1 (HIV-1) infection leads to a general exhaustion of the immune system. Prior to this widespread decline of immune functions, however, there is an evident hyperactivation of the monocyte/macrophage arm. Increased levels of cytokines and other biologically active molecules produced by activated monocytes may contribute to the pathogenesis of HIV disease both by activating expression of HIV-1 provirus and by direct effects on cytokine-sensitive tissues, such as lung or brain. In this article, we investigate mechanisms of hyperresponsiveness of HIV-infected monocytes. MATERIALS AND METHODS: The study was performed on monocyte cultures infected in vitro with a monocytetropic strain HIV-1ADA. Cytokine production was induced by stimulation of cultures with lipopolysaccharides (LPS) and measured by ELISA. To study involvement of nitric oxide (NO) in the regulation of cytokine expression, inhibitors of nitric oxide synthase (NOS) or chemical donors of NO were used. RESULTS: We demonstrate that infection with HIV-1 in vitro primes human monocytes for subsequent activation with LPS, resulting in increased production of pro-inflammatory cytokines tumor necrosis factor (TNF) and interleukin 6 (IL-6). This priming effect can be blocked by Ca(2+)-chelating agents and by the NOS inhibitor L-NMMA, but not by hemoglobin. It could be reproduced on uninfected monocyte cultures by using donors of NO, but not cGMP, together with LPS. CONCLUSIONS: NO, which is expressed in HIV-1-infected monocyte cultures, induces hyperresponsiveness of monocytes by synergizing with calcium signals activated in response to LPS stimulation. This activation is cGMP independent. Our findings demonstrate the critical role of NO in HIV-1-specific hyperactivation of monocytes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akarid K., Sinet M., Desforges B., Gougerot-Pocidalo M. A. Inhibitory effect of nitric oxide on the replication of a murine retrovirus in vitro and in vivo. J Virol. 1995 Nov;69(11):7001–7005. doi: 10.1128/jvi.69.11.7001-7005.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Nitric oxide: a physiologic messenger molecule. Annu Rev Biochem. 1994;63:175–195. doi: 10.1146/annurev.bi.63.070194.001135. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Nottet H. S., Schmidtmayerova H., Dubrovsky L., Flanagan C. R., Mullins M. E., Lipton S. A., Gendelman H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995 Feb 1;181(2):735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouse K. A., Powell D., Washington I., Poli G., Strebel K., Farrar W., Barstad P., Kovacs J., Fauci A. S., Folks T. M. Monokine regulation of human immunodeficiency virus-1 expression in a chronically infected human T cell clone. J Immunol. 1989 Jan 15;142(2):431–438. [PubMed] [Google Scholar]
- Croen K. D. Evidence for antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J Clin Invest. 1993 Jun;91(6):2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emilie D., Fior R., Jarrousse B., Marfaing-Koka A., Merrien D., Devergne O., Crevon M. C., Maillot M. C., Galanaud P. Cytokines in HIV infection. Int J Immunopharmacol. 1994 May-Jun;16(5-6):391–396. doi: 10.1016/0192-0561(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Gurram M., Chirmule N., Wang X. P., Ponugoti N., Pahwa S. Increased spontaneous secretion of interleukin 6 and tumor necrosis factor alpha by peripheral blood lymphocytes of human immunodeficiency virus-infected children. Pediatr Infect Dis J. 1994 Jun;13(6):496–501. doi: 10.1097/00006454-199406000-00006. [DOI] [PubMed] [Google Scholar]
- Hurme M., Viherluoto J., Nordström T. The effect of calcium mobilization on LPS-induced IL-1 beta production depends on the differentiation stage of the monocytes/macrophages. Scand J Immunol. 1992 Sep;36(3):507–511. doi: 10.1111/j.1365-3083.1992.tb02966.x. [DOI] [PubMed] [Google Scholar]
- Karupiah G., Xie Q. W., Buller R. M., Nathan C., Duarte C., MacMicking J. D. Inhibition of viral replication by interferon-gamma-induced nitric oxide synthase. Science. 1993 Sep 10;261(5127):1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P. K., Novogrodsky A. Nitric oxide signaling: a possible role for G proteins. J Immunol. 1993 Dec 15;151(12):7182–7187. [PubMed] [Google Scholar]
- Lander H. M., Sehajpal P., Levine D. M., Novogrodsky A. Activation of human peripheral blood mononuclear cells by nitric oxide-generating compounds. J Immunol. 1993 Feb 15;150(4):1509–1516. [PubMed] [Google Scholar]
- Lee C. G., Demarquoy J., Jackson M. J., O'Brien W. E. Molecular cloning and characterization of a murine LPS-inducible cDNA. J Immunol. 1994 Jun 15;152(12):5758–5767. [PubMed] [Google Scholar]
- Letari O., Nicosia S., Chiavaroli C., Vacher P., Schlegel W. Activation by bacterial lipopolysaccharide causes changes in the cytosolic free calcium concentration in single peritoneal macrophages. J Immunol. 1991 Aug 1;147(3):980–983. [PubMed] [Google Scholar]
- Lorsbach R. B., Murphy W. J., Lowenstein C. J., Snyder S. H., Russell S. W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J Biol Chem. 1993 Jan 25;268(3):1908–1913. [PubMed] [Google Scholar]
- Mannick J. B., Asano K., Izumi K., Kieff E., Stamler J. S. Nitric oxide produced by human B lymphocytes inhibits apoptosis and Epstein-Barr virus reactivation. Cell. 1994 Dec 30;79(7):1137–1146. doi: 10.1016/0092-8674(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Mülsch A., Lückhoff A., Pohl U., Busse R., Bassenge E. LY 83583 (6-anilino-5,8-quinolinedione) blocks nitrovasodilator-induced cyclic GMP increases and inhibition of platelet activation. Naunyn Schmiedebergs Arch Pharmacol. 1989 Jul;340(1):119–125. doi: 10.1007/BF00169217. [DOI] [PubMed] [Google Scholar]
- Nottet H. S., Jett M., Flanagan C. R., Zhai Q. H., Persidsky Y., Rizzino A., Bernton E. W., Genis P., Baldwin T., Schwartz J. A regulatory role for astrocytes in HIV-1 encephalitis. An overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-alpha by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995 Apr 1;154(7):3567–3581. [PubMed] [Google Scholar]
- Padgett E. L., Pruett S. B. Evaluation of nitrite production by human monocyte-derived macrophages. Biochem Biophys Res Commun. 1992 Jul 31;186(2):775–781. doi: 10.1016/0006-291x(92)90813-z. [DOI] [PubMed] [Google Scholar]
- Peunova N., Enikolopov G. Amplification of calcium-induced gene transcription by nitric oxide in neuronal cells. Nature. 1993 Jul 29;364(6436):450–453. doi: 10.1038/364450a0. [DOI] [PubMed] [Google Scholar]
- Poli G., Fauci A. S. Cytokine modulation of HIV expression. Semin Immunol. 1993 Jun;5(3):165–173. doi: 10.1006/smim.1993.1020. [DOI] [PubMed] [Google Scholar]
- Schmidtmayerova H., Nottet H. S., Nuovo G., Raabe T., Flanagan C. R., Dubrovsky L., Gendelman H. E., Cerami A., Bukrinsky M., Sherry B. Human immunodeficiency virus type 1 infection alters chemokine beta peptide expression in human monocytes: implications for recruitment of leukocytes into brain and lymph nodes. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):700–704. doi: 10.1073/pnas.93.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann M., Schoedon G., Hofer S., Blau N., Guerrero L., Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993 Jun;167(6):1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- Stamler J. S. Redox signaling: nitrosylation and related target interactions of nitric oxide. Cell. 1994 Sep 23;78(6):931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor W. R., Glass J. D., Griffin J. W., Becker P. S., McArthur J. C., Bezman L., Griffin D. E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992 Apr;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Wesselingh S. L., Power C., Glass J. D., Tyor W. R., McArthur J. C., Farber J. M., Griffin J. W., Griffin D. E. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993 Jun;33(6):576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Zinetti M., Fantuzzi G., Delgado R., Di Santo E., Ghezzi P., Fratelli M. Endogenous nitric oxide production by human monocytic cells regulates LPS-induced TNF production. Eur Cytokine Netw. 1995 Jan-Feb;6(1):45–48. [PubMed] [Google Scholar]