Abstract
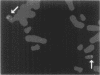
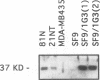
BACKGROUND: Using differential display (DD), we discovered a new member of the serine protease family of protein-cleaving enzymes, named protease M. The gene is most closely related by sequence to the kallikreins, to prostate-specific antigen (PSA), and to trypsin. The diagnostic use of PSA in prostate cancer suggested that a related molecule might be a predictor for breast or ovarian cancer. This, in turn, led to studies designed to characterize the protein and to screen for its expression in cancer. MATERIALS AND METHODS: The isolation of protease M by DD, the cloning and sequencing of the cDNA, and the comparison of the predicted protein structure with related proteins are described, as are methods to produce recombinant proteins and polyclonal antibody preparations. Protease M expression was examined in mammary, prostate, and ovarian cancer, as well as normal, cells and tissues. Stable transfectants expressing the protease M gene were produced in mammary carcinoma cells. RESULTS: Protease M was localized by fluorescent in situ hybridization analysis to chromosome 19q13.3, in a region to which other kallikreins and PSA also map. The gene is expressed in the primary mammary carcinoma lines tested but not in the corresponding cell lines of metastatic origin. It is strongly expressed in ovarian cancer tissues and cell lines. The enzyme activity could not be established, because of difficulties in producing sufficient recombinant protein, a common problem with proteases. Transfectants were selected that overexpress the mRNA, but the protein levels remained very low. CONCLUSIONS: Protease M expression (mRNA) may be a useful marker in the detection of primary mammary carcinomas, as well as primary ovarian cancers. Other medical applications are also likely, based on sequence relatedness to trypsin and PSA.
Full text
PDF





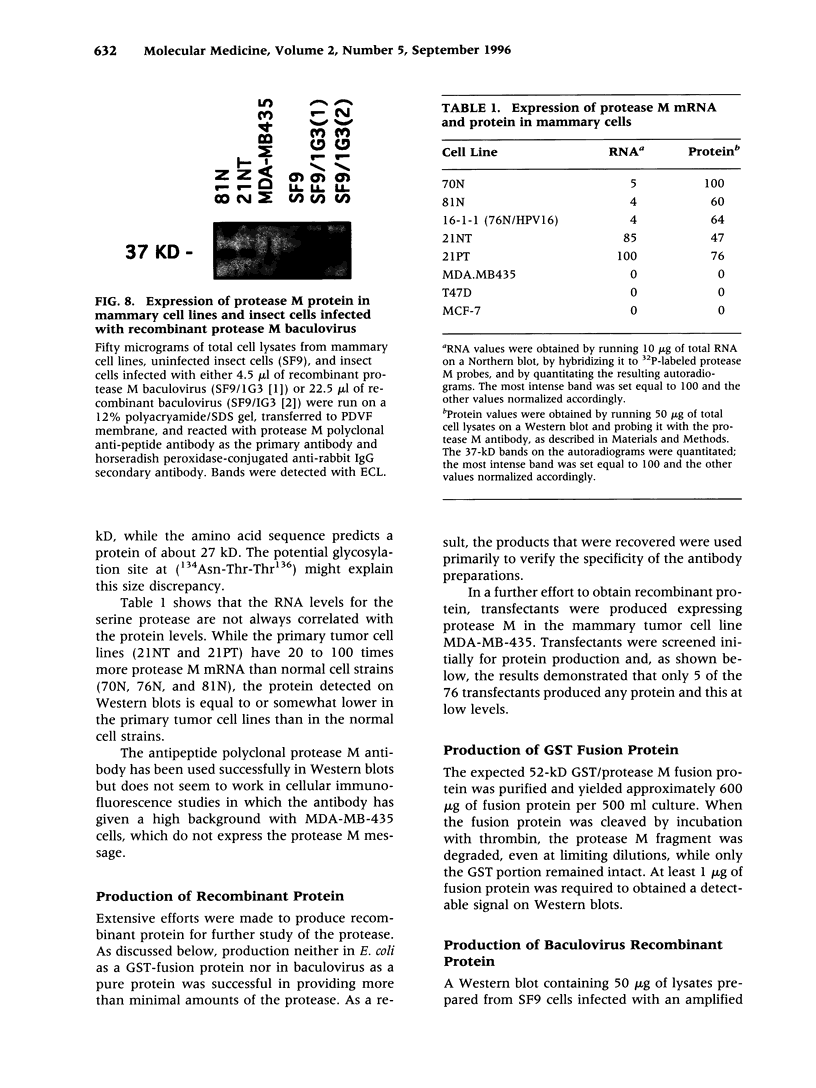
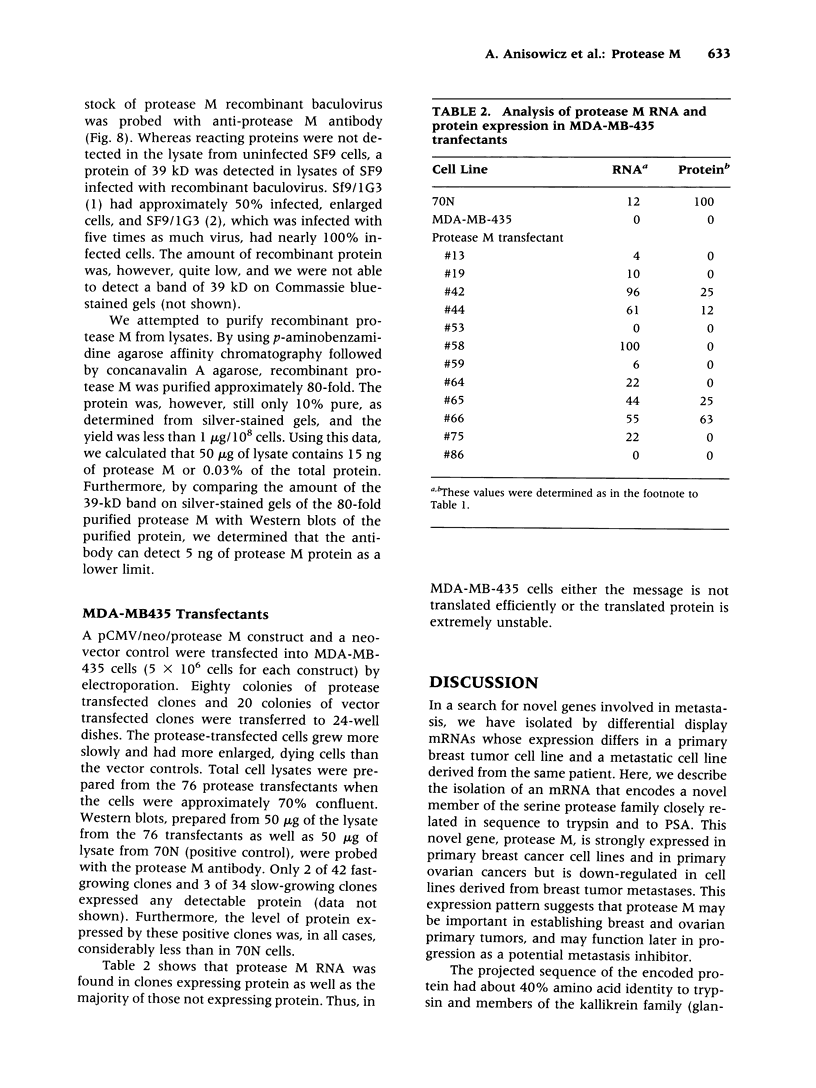



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Angermann A., Bergmann C., Appelhans H. Cloning and expression of human salivary-gland kallikrein in Escherichia coli. Biochem J. 1989 Sep 15;262(3):787–793. doi: 10.1042/bj2620787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley P. L., MacDonald R. J. Kallikrein-related mRNAs of the rat submaxillary gland: nucleotide sequences of four distinct types including tonin. Biochemistry. 1985 Aug 13;24(17):4512–4520. doi: 10.1021/bi00338a005. [DOI] [PubMed] [Google Scholar]
- Baker A. R., Shine J. Human kidney kallikrein: cDNA cloning and sequence analysis. DNA. 1985 Dec;4(6):445–450. doi: 10.1089/dna.1985.4.445. [DOI] [PubMed] [Google Scholar]
- Band V., Sager R. Distinctive traits of normal and tumor-derived human mammary epithelial cells expressed in a medium that supports long-term growth of both cell types. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1249–1253. doi: 10.1073/pnas.86.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band V., Zajchowski D., Swisshelm K., Trask D., Kulesa V., Cohen C., Connolly J., Sager R. Tumor progression in four mammary epithelial cell lines derived from the same patient. Cancer Res. 1990 Nov 15;50(22):7351–7357. [PubMed] [Google Scholar]
- Berg T., Bradshaw R. A., Carretero O. A., Chao J., Chao L., Clements J. A., Fahnestock M., Fritz H., Gauthier F., MacDonald R. J. A common nomenclature for members of the tissue (glandular) kallikrein gene families. Agents Actions Suppl. 1992;38(Pt 1):19–25. doi: 10.1007/978-3-0348-7321-5_3. [DOI] [PubMed] [Google Scholar]
- Emi M., Nakamura Y., Ogawa M., Yamamoto T., Nishide T., Mori T., Matsubara K. Cloning, characterization and nucleotide sequences of two cDNAs encoding human pancreatic trypsinogens. Gene. 1986;41(2-3):305–310. doi: 10.1016/0378-1119(86)90111-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fukushima D., Kitamura N., Nakanishi S. Nucleotide sequence of cloned cDNA for human pancreatic kallikrein. Biochemistry. 1985 Dec 31;24(27):8037–8043. doi: 10.1021/bi00348a030. [DOI] [PubMed] [Google Scholar]
- Henttu P., Vihko P. cDNA coding for the entire human prostate specific antigen shows high homologies to the human tissue kallikrein genes. Biochem Biophys Res Commun. 1989 Apr 28;160(2):903–910. doi: 10.1016/0006-291x(89)92520-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Lilja H. A kallikrein-like serine protease in prostatic fluid cleaves the predominant seminal vesicle protein. J Clin Invest. 1985 Nov;76(5):1899–1903. doi: 10.1172/JCI112185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilja H., Oldbring J., Rannevik G., Laurell C. B. Seminal vesicle-secreted proteins and their reactions during gelation and liquefaction of human semen. J Clin Invest. 1987 Aug;80(2):281–285. doi: 10.1172/JCI113070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. S., Lin F. K., Chao L., Chao J. Human urinary kallikrein. Complete amino acid sequence and sites of glycosylation. Int J Pept Protein Res. 1989 Apr;33(4):237–249. doi: 10.1111/j.1399-3011.1989.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Lundwall A., Lilja H. Molecular cloning of human prostate specific antigen cDNA. FEBS Lett. 1987 Apr 20;214(2):317–322. doi: 10.1016/0014-5793(87)80078-9. [DOI] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack R. T., Rittenhouse H. G., Finlay J. A., Sokoloff R. L., Wang T. J., Wolfert R. L., Lilja H., Oesterling J. E. Molecular forms of prostate-specific antigen and the human kallikrein gene family: a new era. Urology. 1995 May;45(5):729–744. doi: 10.1016/s0090-4295(99)80076-4. [DOI] [PubMed] [Google Scholar]
- Mok C. H., Tsao S. W., Knapp R. C., Fishbaugh P. M., Lau C. C. Unifocal origin of advanced human epithelial ovarian cancers. Cancer Res. 1992 Sep 15;52(18):5119–5122. [PubMed] [Google Scholar]
- Riegman P. H., Klaassen P., van der Korput J. A., Romijn J. C., Trapman J. Molecular cloning and characterization of novel prostate antigen cDNA's. Biochem Biophys Res Commun. 1988 Aug 30;155(1):181–188. doi: 10.1016/s0006-291x(88)81066-0. [DOI] [PubMed] [Google Scholar]
- Riegman P. H., Vlietstra R. J., Suurmeijer L., Cleutjens C. B., Trapman J. Characterization of the human kallikrein locus. Genomics. 1992 Sep;14(1):6–11. doi: 10.1016/s0888-7543(05)80275-7. [DOI] [PubMed] [Google Scholar]
- Riegman P. H., Vlietstra R. J., van der Korput H. A., Romijn J. C., Trapman J. Identification and androgen-regulated expression of two major human glandular kallikrein-1 (hGK-1) mRNA species. Mol Cell Endocrinol. 1991 Apr;76(1-3):181–190. doi: 10.1016/0303-7207(91)90272-t. [DOI] [PubMed] [Google Scholar]
- Sager R., Anisowicz A., Neveu M., Liang P., Sotiropoulou G. Identification by differential display of alpha 6 integrin as a candidate tumor suppressor gene. FASEB J. 1993 Jul;7(10):964–970. doi: 10.1096/fasebj.7.10.8344495. [DOI] [PubMed] [Google Scholar]
- Schachter M. Kallikreins (kininogenases)--a group of serine proteases with bioregulatory actions. Pharmacol Rev. 1979 Mar;31(1):1–17. [PubMed] [Google Scholar]
- Schaller J., Akiyama K., Tsuda R., Hara M., Marti T., Rickli E. E. Isolation, characterization and amino-acid sequence of gamma-seminoprotein, a glycoprotein from human seminal plasma. Eur J Biochem. 1987 Dec 30;170(1-2):111–120. doi: 10.1111/j.1432-1033.1987.tb13674.x. [DOI] [PubMed] [Google Scholar]
- Schedlich L. J., Bennetts B. H., Morris B. J. Primary structure of a human glandular kallikrein gene. DNA. 1987 Oct;6(5):429–437. doi: 10.1089/dna.1987.6.429. [DOI] [PubMed] [Google Scholar]
- Sheng S., Pemberton P. A., Sager R. Production, purification, and characterization of recombinant maspin proteins. J Biol Chem. 1994 Dec 9;269(49):30988–30993. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Irie A., Miyake Y. Primary structure of human urinary prokallikrein. J Biochem. 1988 Jul;104(1):22–29. doi: 10.1093/oxfordjournals.jbchem.a122415. [DOI] [PubMed] [Google Scholar]
- Tomasetto C., Neveu M. J., Daley J., Horan P. K., Sager R. Specificity of gap junction communication among human mammary cells and connexin transfectants in culture. J Cell Biol. 1993 Jul;122(1):157–167. doi: 10.1083/jcb.122.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S. W., Mok S. C., Fey E. G., Fletcher J. A., Wan T. S., Chew E. C., Muto M. G., Knapp R. C., Berkowitz R. S. Characterization of human ovarian surface epithelial cells immortalized by human papilloma viral oncogenes (HPV-E6E7 ORFs). Exp Cell Res. 1995 Jun;218(2):499–507. doi: 10.1006/excr.1995.1184. [DOI] [PubMed] [Google Scholar]
- Watt K. W., Lee P. J., M'Timkulu T., Chan W. P., Loor R. Human prostate-specific antigen: structural and functional similarity with serine proteases. Proc Natl Acad Sci U S A. 1986 May;83(10):3166–3170. doi: 10.1073/pnas.83.10.3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Diamandis E. P., Sutherland D. J. Immunoreactive prostate-specific antigen levels in female and male breast tumors and its association with steroid hormone receptors and patient age. Clin Biochem. 1994 Apr;27(2):75–79. doi: 10.1016/0009-9120(94)90015-9. [DOI] [PubMed] [Google Scholar]