Abstract
Successful targeting methods represent a major hurdle to the use of retroviral vectors in cell-specific gene-delivery applications. We recently described an approach for retroviral targeting with a retroviral receptor-ligand bridge protein that was bound to the cognate cell-surface ligand receptors before viral challenge. We now report a significant improvement made to this viral targeting method by using a related bridge protein, designated TVB-EGF, comprised of the extracellular domain of the TVB receptor for subgroup B avian leukosis virus fused to epidermal growth factor (EGF). The most important activity of TVB-EGF was that it allowed specific viral entry when preloaded onto virions. Furthermore, virions preloaded with TVB-EGF were thermostable and could be produced directly from virus- packaging cells. These data suggest an approach for targeting retroviral vectors to specific cell types by using virions preloaded with a retroviral receptor-ligand bridge protein and indicate that these types of bridge proteins may be useful reagents for studying the normal mechanism of retroviral entry.
One of the major challenges facing retrovirus-based gene-delivery systems is in the development of approaches for efficiently targeting viral infection to only relevant cell types. A number of approaches have been tested in an effort to overcome this problem, including chemical modification of viral envelope (Env) proteins (1), the use of antibodies to bridge viral Env proteins with specific cell-surface molecules (2, 3), and the use of recombinant Env proteins containing cell-specific ligands or single-chain antibodies (4–17). Although these approaches have allowed for some degree of cell-type-specific viral entry, the level of infection observed is usually too low to be considered useful for most retrovirus-based gene-delivery applications (18, 19). Thus, there is clearly a need to develop new and improved methods for retroviral targeting.
We recently reported an approach for retroviral targeting with a retroviral receptor-ligand bridge protein (20). The bridge protein tested was comprised of the low density lipoprotein receptor-related extracellular domain of the TVA receptor for subgroup A avian leukosis virus (ALV-A) fused to human epidermal growth factor (EGF). When this bridge protein was added to the surfaces of cells that express EGF receptors (EGFR), these cells became highly susceptible to infection by ALV-A vectors (20). EGF was chosen as the prototype ligand to test this viral targeting approach, because its interaction with EGFR has been extensively characterized biochemically. Furthermore, EGFR is a relevant target for this method of retroviral vector delivery, because this receptor and other related receptors such as c-erbB-2 and c-erbB-3 are often overexpressed or mutated in solid human tumors (21).
Here, we show the functional activities of another type of bridge protein comprised of the tumor necrosis factor receptor-related extracellular domain of the TVB receptor for ALV-B fused to EGF. Most importantly, we show that it is possible to target retroviral vectors specifically to cells that express EGFR by preloading virions with the TVB-EGF bridge protein.
MATERIALS AND METHODS
Plasmids.
A DNA fragment encoding the extracellular domain of TVBS3 (amino acid residues 1–155) was amplified by PCR from pBK7.6-2 template DNA (22) by using primers OAB2 (5′-CATTGTTCTCGAGATGCGCTCAGCTGCGCTCCG-3′) and OAB5 (5′-CATTGTTCGGCCGTGAGTGGAGGAGCTGGAGGAG-3′). The PCR product was subcloned into the pCI expression vector (Promega) and ligated with a DNA fragment from plasmid pSS1 (20), encoding a glycine- and proline-rich linker region and human EGF. The resulting plasmid, pAB1, encodes TVB-EGF, and the sequence was validated by DNA sequencing (Department of Microbiology and Molecular Genetics DNA Sequencing Core Facility, Harvard Medical School).
A 2,208-bp KpnI–BamHI DNA fragment encoding a correctly spliced envA mRNA transcript (K. Zingler, L. Connolly, and J.A.T.Y., unpublished work) was subcloned into a modified version of the pCI expression plasmid to generate pAB6. Plasmid pAB7 encoding ALV-B Env was then generated by replacing the XhoI–ApaI env fragment of pAB6 with the corresponding 925-bp XhoI–ApaI fragment of the subgroup B RAV-2 env. Plasmid pMMP-nlslacZ, containing a murine leukemia virus (MLV) vector encoding β-galactosidase (lacZ); plasmid pMD.old.gag.pol, encoding the MLV proteins Gag and Pol (23); and plasmid pCMMP.GFP/neo, containing the gene encoding green fluorescent protein (GFP), were generous gifts from J.-S. Lee and R. C. Mulligan (Children’s Hospital, Boston).
Cell Lines.
Human 293 cells were obtained from the American Type Culture Collection. B82 cells are a clonal line of mouse L cells, which lack EGFR. As described in refs. 20, 24, and 25, these cells were transfected with plasmids encoding either a kinase-deficient EGFR or wild-type EGFR to generate clonal derivatives designated M5 and T23 cells, respectively. The M5:S3/T cell line was obtained after stable transfection of M5 cells with plasmids encoding FLAG-epitope tagged TVBS3 and TVBT, formerly SEAR, a subgroup E-specific ALV receptor (ref. 26 and H. Adkins and J.A.T.Y., unpublished work). Expression of TVBS3 in this cell line was confirmed by flow cytometry by using an ALV-B surface envelope protein (SU)-Ig and a FITC-conjugated secondary antibody as described (26).
Production of TVB-EGF.
TVB-EGF was produced in the extracellular supernatants of transiently transfected 293 cells as described (20), and 50-μl aliquots of extracellular supernatants from transfected and nontransfected 293 cells were subjected to electrophoresis on a 12% polyacrylamide gel containing SDS and transferred to a nylon membrane. The samples were subjected to immunoblotting by using an EGF-specific antibody (PeproTech, Rocky Hill, NJ) diluted 1:500 in TBST (25 mM Tris, pH 7.4/130 mM NaCl/0.1% Triton X-100) followed by a horseradish peroxidase-coupled antibody specific for rabbit Igs (Amersham Pharmacia) diluted 1:3,000 in TBST. The bound antibodies were detected by enhanced chemiluminescence.
Flow Cytometry.
Flow cytometric analysis to detect TVB-EGF binding to cells that express EGFR was performed on ice essentially as described (20). B82, M5, and T23 cells were incubated with different amounts of TVB-EGF-containing supernatants, and M5 cells were incubated with a saturating amount of TVB-EGF (100 μl of crude extracellular supernatant in 500 μl of medium) in the presence or absence of 4 μg of recombinant EGF (Upstate Biotechnology, Lake Placid, NY). The bound TVB-EGF proteins were detected by adding 5 μg of ALV-B SU-Ig and 5 μg of an FITC-coupled anti-rabbit secondary antibody (Dako).
Production of Viral Pseudotypes.
MLV-lacZ (ALV-B) virions were generated by using a tripartite transfection system (27). Typically, a 60% confluent plate of 293 cells was transfected with 15 μg of plasmid pMD.old.gag.pol, 20 μg of plasmid pMMP-nlslacZ, and 5 μg of plasmid pAB7 encoding ALV-B Env. Virions contained in extracellular supernatants were harvested at approximately 48 and 60 h after transfection and pooled. These virions were used for infection studies either before or after being concentrated by ultracentrifugation as described (28). To generate preloaded virions, MLV-lacZ(ALV-B) was incubated at either a 1:1 or a 1:2 ratio with extracellular supernatants containing TVB-EGF, and the virion/bridge-protein complexes were purified by ultracentrifugation at 109,000 × g for 1.5 h at 4°C. The MLV-lacZ(ALV-B)/TVB-EGF complexes were resuspended in TNE buffer (described in ref. 28; at 1/100, 1/50, or 1/33 of the original volume to generate 100×, 50×, or 33× stocks of preloaded virions).
To generate preloaded virions directly from virus packaging cells, 293 cells were transiently transfected essentially as described above, except that variable amounts of pAB1 plasmid DNA were cotransfected. To monitor expression levels of TVB-EGF, 40-μl aliquots of extracellular supernatants prepared from these cells were subjected to electrophoresis on a 10% polyacrylamide gel containing SDS and transferred to a nylon membrane. These samples were immunoblotted with 3 ml of extracellular supernatants containing a subgroup B ALV SU-Ig fusion protein (22) diluted in 10 ml of TBST followed by an horseradish peroxidase-coupled antibody specific for rabbit Igs (Amersham Pharmacia) diluted 1:3,000 in TBST. Virions produced from cells transfected with 0.01 μg, 0.1 μg, or 1 μg of pAB1 plasmid DNA were collected 48 h after transfection and either left unconcentrated or concentrated 100-fold before infection. Human 293 cells transiently producing MLV-GFP/TVB-EGF complexes were generated as described above except that plasmid pCMMP.GFP/neo (J.-S. Lee and R. C. Mulligan, unpublished work) was used instead of pMMP-nlslacZ.
Infection of Cells Preloaded with TVB-EGF.
M5 cells plated at 20% confluence in six-well tissue culture plates were placed on ice and incubated for 1 h at 4°C with 1 ml of ice-cold extracellular supernatants that either contained or lacked TVB-EGF. The cells were then washed with ice-cold PBS and incubated for 1 h at 4°C with 1.5 ml of ice-cold medium containing 5 μg/ml polybrene and 0.75 μl of (100×) MLV-lacZ (ALV-B). The M5:S3/T cells were also incubated with the same amount of virus under identical conditions. The cells were transferred to a 37°C incubator and, 52 h later, were washed with PBS and fixed for 15 min at room temperature with 1% formaldehyde/0.2% glutaraldehyde in PBS. The cells were then washed twice with PBS and incubated with 2 mM MgCl2/5 mM potassium ferrocyanide/5 mM potassium ferricyanide/0.01 g of 5-bromo-4-chloro-3-indolyl β-d-galactoside (GIBCO)/1% N,N-dimethylformamide (Mallinckrodt) in PBS to detect the viral-encoded lacZ protein.
Infection of Cells with Virions Preloaded with TVB-EGF.
Cells were plated at approximately 20% confluence on 12-well tissue culture plates before viral challenge. M5, B82, and T23 cells were incubated with 190 μl of (33×) MLV-lacZ (ALV-B), and M5:S3/T cells were incubated with 0.1 μl of these concentrated virions. M5, T23, and B82 cells were incubated separately with 0.5 μl, 1 μl, and 200 μl of (33×) MLV-lacZ (ALV-B)/TVB-EGF complexes, respectively. The cells were immediately placed at 37°C and, approximately 60 h later, were analyzed for infection by staining for lacZ activity as before.
To analyze virion thermostability, 0.5 μl of (50×) MLV-lacZ (ALV-B) or 2 μl of (50×) MLV-lacZ (ALV-B)/TVB-EGF complexes were incubated either on ice for 9 h (preincubation time = 0) or at 37°C for the time periods indicated and then returned to ice before addition to M5:S3/T cells or to M5 cells, respectively.
In the cocultivation experiment, human 293 cells producing MLV-GFP (ALV-B)/TVB-EGF complexes were placed, 16 h after transfection, in transwell inserts with a 0.4-μm pore size (CoStar). These transwell inserts were placed in six-well tissue culture plates that contained either M5 or B82 cells plated at approximately 20% confluence and incubated at 37°C. The transwell inserts were removed 56 h after the start of cocultivation, and the numbers of infected cells were determined by using flow cytometry to detect expression of GFP.
RESULTS
TVB-EGF Is a Bifunctional Reagent That Can Bind Both to Cell Surface EGF Receptors and to ALV-B SU.
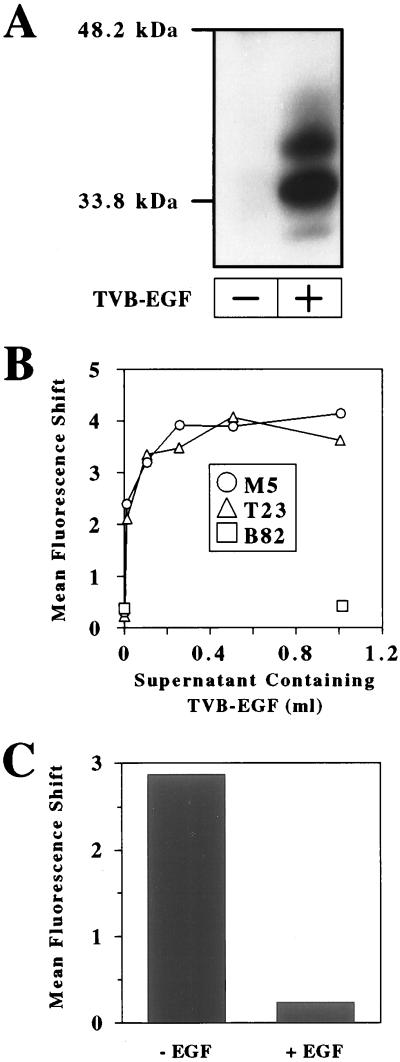
The TVB-EGF protein is comprised of the extracellular domain of the TVBS3 receptor for subgroups B and D ALV (ALV-B and ALV-D; refs. 22 and 29), fused via a glycine- and proline-rich linker region to the mature form of human EGF (30). TVB-EGF was produced in the extracellular supernatants of transiently transfected human 293 cells, and its production was confirmed by immunoblotting by using an EGF-specific antibody (Fig. 1A). Three predominant forms of TVB-EGF were detected (Fig. 1A), presumably resulting from differential glycosylation of the TVB domain as noted previously (22); the expected molecular mass of TVB-EGF without any N-linked carbohydrate residues is approximately 22 kDa.
Figure 1.
TVB-EGF binds specifically to cell surface EGFR. (A) Aliquots of extracellular supernatants from nontransfected 293 cells (−) and transfected 293 cells expressing TVB-EGF (+) were subjected to SDS/PAGE and to immunoblotting by using an EGF-specific antibody. (B) Mouse M5 cells expressing kinase-deficient EGFR, T23 cells expressing wild-type EGFR, and B82 cells, which lack EGFR, were incubated with increasing amounts of TVB-EGF. (C) M5 cells were incubated with TVB-EGF in the presence or absence of a recombinant EGF protein. (B–C) After each of these incubations, the cells were incubated with ALV-B SU-Ig and a FITC-conjugated secondary antibody and analyzed by flow cytometry as described (20).
Flow cytometric analysis was used to assess whether TVB-EGF is a bifunctional reagent that can bind simultaneously to cell surface EGF receptors and to the ALV-B SU subunit that is responsible for TVB binding. Cells were incubated with increasing amounts of supernatants that contained TVB-EGF and then incubated with a subgroup B-specific ALV SU-Ig fusion protein (22) followed by an FITC-conjugated secondary antibody. TVB-EGF bound in a saturable manner to transfected mouse L cells expressing kinase-deficient human EGFR (M5 cells) and to transfected mouse L cells expressing wild-type human EGFR (T23 cells; Fig. 1B). By contrast, the bridge protein did not bind to the parental mouse L cells, which lack EGF receptors (B82 cells), even at the highest amount of TVB-EGF added (Fig. 1B). In addition, TVB-EGF binding to M5 cells was specifically competed in the presence of recombinant EGF (Fig. 1C). Taken together, these data show that TVB-EGF binds specifically to ALV-B SU and to the ligand-binding regions of EGFR expressed on the surfaces of M5 and T23 cells.
TVB-EGF Mediates Viral Infection when Attached to Cell-Surface EGFR.
To determine whether TVB-EGF can facilitate viral entry when attached to cell surface EGFR, we asked whether this bridge protein could mediate infection by an MLV vector encoding lacZ and bearing ALV-B Env [designated MLV-lacZ (ALV-B)]. An MLV vector was chosen for this study, because, to date, these are the best characterized retroviral vectors used for infecting dividing cell types such as cancer cells (31). As expected, the MLV-lacZ (ALV-B) pseudotyped virus required cell surface TVB receptors for entry; in contrast to parental M5 cells, which were resistant to viral infection, transfected M5 cells (M5:S3/T) expressing a transmembrane form of TVBS3 were susceptible to infection by MLV-lacZ (ALV-B) (Table 1).
Table 1.
TVB-EGF mediates efficient viral entry when attached to M5 cells
| Cell line | Viral titer per microliter |
|---|---|
| M5:S3/T | (1.3 ± 0.2) × 103 |
| M5 (+TVB-EGF) | (7.1 ± 2.4) × 102 |
| M5 (−TVB-EGF) | 0 |
M5 cells were incubated with extracellular supernatants that contained (+) or lacked (−) TVB-EGF. These cells, and for control purposes M5:S3/T cells, were then challenged with 0.75-μl aliquots of (100×) concentrated MLV-lacZ (ALV-B), and the infected cells were identified by analyzing for lacZ expression.
To assess whether TVB-EGF could mediate viral entry when bound to M5 cells, these cells were incubated with the bridge protein before challenge with MLV-lacZ (ALV-B). TVB-EGF rendered these cells highly susceptible to viral infection; the level of infection mediated by TVB-EGF was approximately 55% of that seen when control M5:S3/T cells were challenged with the same number of native virions (Table 1). M5 cells that were incubated with TVB-EGF were also very susceptible to infection by subgroup B-specific ALV-based vectors (data not shown). These results establish that TVB-EGF is a highly efficient mediator of targeted retroviral entry when attached to cell surface EGFR before viral challenge.
Virions Preloaded with TVB-EGF Specifically Infect Cells That Express EGFR.
For most retroviral delivery applications, it would be desirable to preload the bridge protein onto virions, as opposed to cell surfaces, provided that such viral complexes retain their cell-type specificity. To determine whether retroviruses preloaded with TVB-EGF have this property, MLV-lacZ (ALV-B) virions were incubated with the bridge protein, and the resultant complexes were purified and used to infect M5, T23, and B82 cells.
The level of preloaded virus infection observed with M5 cells was extremely high and, in fact, exceeded the level obtained when the same number of native virions were used to infect the control M5:S3/T cells (Table 2). The level of preloaded virus infection of T23 cells was also high, representing approximately 29% of the level seen with native virus infection of M5:S3/T cells (Table 2). By contrast, the level of preloaded virus infection of B82 cells was low (Table 2). Indeed, similar low levels of infection were seen with B82, M5, and T23 cells that were challenged with native virions (Table 2), showing that these events are caused by an intrinsic ability of MLV-lacZ (ALV-B) to enter these cell types with low efficiency. Based on a comparison of the numbers of infection events obtained with cells that express or lack EGFR (Table 2), it seems that 99.97% of the preloaded virus infection events seen with M5 cells and 99.89% of those seen with T23 cells require the specific interaction of the virus-bound TVB-EGF and cell-surface EGFR.
Table 2.
MLV-lacZ (ALV-B) virions preloaded with TVB-EGF specifically infect M5 and T23 cells with a high efficiency
| Cell line | TVB-EGF | Viral titer per milliliter |
|---|---|---|
| B82 | − | (6.6 ± 0.9) × 102 |
| + | (5.0 ± 0.2) × 102 | |
| M5 | − | (8.8 ± 1.2) × 102 |
| + | (2.2 ± 0.2) × 106 | |
| T23 | − | (2.1 ± 0.2) × 102 |
| + | (4.5 ± 0.4) × 105 | |
| M5:S3/T | − | (1.6 ± 0.04) × 106 |
B82, M5, and T23 cells were challenged with MLV-lacZ (ALV-B) and MLV-lacZ (ALV-B)/TVB-EGF complexes. M5:S3/T cells were also challenged with MLV-lacZ (ALV-B). Infected cells were identified by staining for lacZ activity.
Preloaded Virions Can Be Generated Directly from Virus Packaging Cells.
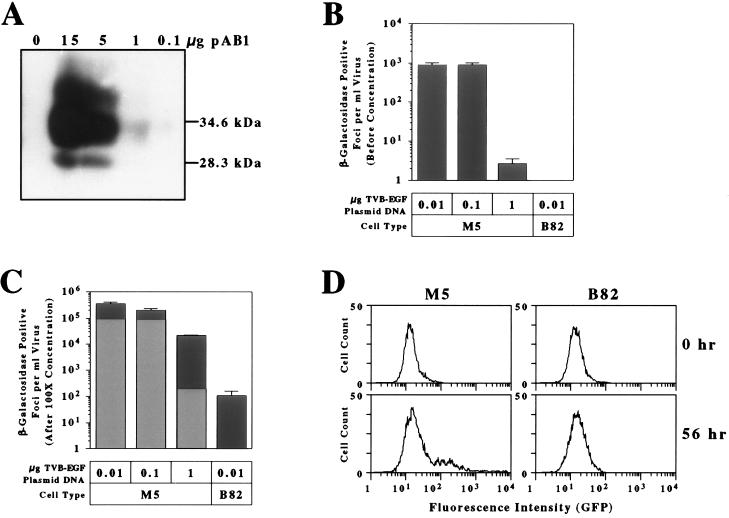
To determine whether virions preloaded with TVB-EGF could be produced from virus packaging cells, human 293 cells were cotransfected with plasmids encoding MLV-lacZ (ALV-B) and with different amounts of the pAB1 plasmid encoding TVB-EGF. The addition of increasing amounts of the pAB1 plasmid led to an increase in the production of TVB-EGF from the virus packaging cells (Fig. 2A). Virion/TVB-EGF complexes contained in the extracellular supernatants from virus-producing cells that were transfected with 0.01 μg, 0.1 μg, or 1 μg of pAB1 plasmid DNA were able to infect M5 cells but not B82 cells (Fig. 2B). However, the level of infection of M5 cells was substantially reduced when 1 μg of pAB1 plasmid DNA was transfected (Fig. 2B). Given the relationship between the amount of pAB1 plasmid DNA transfected and the amount of TVB-EGF produced (Fig. 2A), we reasoned that this inhibitory effect might be caused by the production of excess levels of the bridge protein, which might act as a competitive inhibitor of preloaded virus entry. To test this possibility, virion/TVB-EGF complexes were purified from unbound bridge protein in the extracellular supernatants by ultracentrifugation and were concentrated 100-fold before infection of M5 and B82 cells. With M5 cells, the infectious titers obtained with these complexes purified from cells transfected with 0.01 μg and 0.1 μg of pAB1 plasmid DNA were similar to those expected from the 100-fold concentration effect (Fig. 2C). Indeed, it is noteworthy that, by using 0.01 μg of pAB1 plasmid DNA, the purified preloaded virions infected M5 cells 3,300-fold more efficiently than B82 cells, which lack EGFR (Fig. 2C). This level is consistent with the specificity of infection obtained when TVB-EGF was preloaded onto native virions (Table 2). By contrast, the titer obtained with virions purified from cells transfected with 1 μg of pAB1 plasmid DNA was approximately 45-fold higher than that expected from the concentration effect alone (Fig. 2C), consistent with the idea that excess amounts of the bridge protein must be removed for efficient infection by these preloaded viruses.
Figure 2.
TVB-EGF-loaded virions produced directly from virus packaging cells infect M5 cells specifically. Human 293 cells were transfected with fixed amounts of plasmids encoding MLV-lacZ (ALV-B) and with variable amounts of plasmid pAB1 encoding TVB-EGF. (A) Aliquots of extracellular supernatants taken from these cells were monitored for TVB-EGF production by immunoblotting analysis by using a subgroup B ALV SU-Ig fusion protein (22) and a horseradish peroxidase-coupled secondary antibody. (B) M5 and B82 cells were challenged with MLV-lacZ (ALV-B) virions produced from cells that were cotransfected with the amounts of pAB1 plasmid DNA indicated, and the resultant numbers of infected cells were measured by lacZ staining. (C) MLV-lacZ (ALV-B)/TVB-EGF complexes shown in B were purified from unbound TVB-EGF and concentrated 100-fold before infection. Gray bars represent the expected infectious viral titer based on the 100-fold concentration effect (as compared with those numbers obtained in B). Black bars represent the actual infectious titers observed with the purified virion/TVB-EGF complexes. (D) M5 cells or B82 cells were cocultivated with human 293 cells that produce MLV-GFP (ALV-B)/TVB-EGF complexes. Flow cytometric profiles for M5 (Left) and B82 (Right) are shown after 0 or 56 h of cocultivation.
As an independent test of the specificity of the preloaded virions, virus packaging cells were generated that produced TVB-EGF and MLV (ALV-B) particles encoding GFP. These cells were cocultivated with either M5 or B82 cells, and the target cells were analyzed for infection by flow cytometry. After 56 h of cocultivation, approximately 20% of the M5 cells were infected by virus (Fig. 2D Left). In contrast to M5 cells, there was no discernable infection of B82 cells under the same conditions (Fig. 2D Right). These results underscore the strict cell-type-specific infection of the preloaded virions.
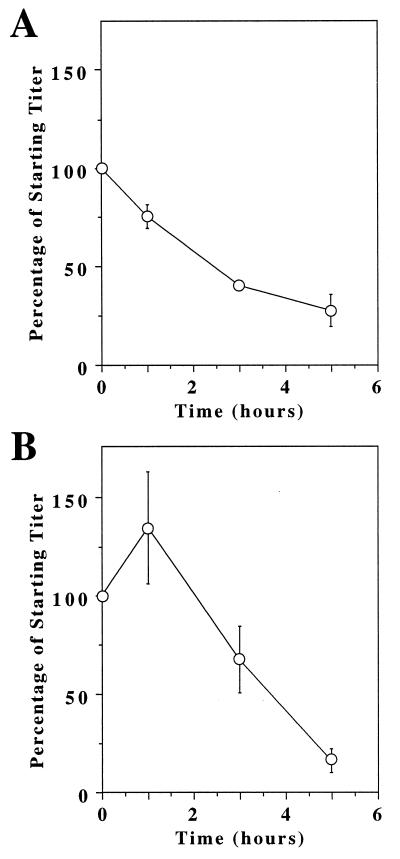
The fact that preloaded virions were infectious, even when produced from virus packaging cells, indicated that these viral complexes must be somewhat stable at 37°C. To investigate their thermostability, preloaded viruses were incubated at 37°C for different periods of time before addition to M5 cells. For control purposes, these experiments were also performed by using native virions that were then used to infect the control M5:S3/T cells. These studies showed that both the native virions (Fig. 3A) and preloaded virions (Fig. 3B) were inactivated when incubated for several hours at 37°C. It is clear from these data, however, that the preloaded virions are at least as stable as native virions when preincubated at 37°C for a short time period.
Figure 3.
MLV-lacZ (ALV-B)/TVB-EGF complexes are stable at 37oC. MLV-lacZ (ALV-B) (A) or MLV-lacZ (ALV-B Env)/TVB-EGF (B) complexes were incubated at 37°C for different time intervals before addition to M5:S3/T cells or to M5 cells, respectively. The number of infected cells was measured by lacZ staining. The results shown reflect the percentage remaining of the starting virus titer (at t = 0). The starting titer of MLV-lacZ (ALV-B) was approximately 2 × 105 lacZ-positive colonies per ml and that of the virion/TVB-EGF complexes was approximately 3 × 104 lacZ-positive colonies per ml.
DISCUSSION
In this report, we have shown that TVB-EGF, a model TVB-ligand bridge protein, can be used to target infection by retroviral vectors to cells that express EGFR. TVB-EGF that was bound to M5 cell surface EGFR before viral challenge efficiently mediated infection by a pseudotyped MLV vector bearing the ALV-B Env protein, whereas there was no obvious infection of B82 cells, which lack EGFR. Indeed, the level of infection obtained with M5 cells was approximately one-half of that seen with control TVB-expressing M5 cells challenged with the same number of native virions.
The most important activity of TVB-EGF was that it also supported efficient viral entry when attached to virions before their addition to cells. These preloaded viruses were produced successfully either by mixing native virions with extracellular supernatants containing TVB-EGF or instead by coexpressing the bridge protein in virus packaging cells. Virions preloaded with TVB-EGF specifically infected M5 and T23 cells with an efficiency that exceeded or closely approximated that seen with control TVB-expressing M5 cells challenged with native virions. In these experiments, the preloaded virions preferentially infected M5 cells as compared with T23 cells (Table 2). The reason for this difference between the two cell types is currently under investigation and might be linked to differences in rates of endocytosis and/or trafficking of kinase-deficient EGFR, as opposed to wild-type forms of EGFR, as discussed previously (20).
In contrast to M5 cells and T23 cells, B82 cells were only infected at a low level by virions preloaded with TVB-EGF. It is extremely unlikely that this small number of infection events was caused by the fact that these virions were preloaded with the retroviral receptor-ligand bridge protein before addition to cells, because similar numbers of infected cells were seen when B82, T23, and M5 cells were challenged with native virions. Instead, it seems that this low level of nonspecific infection is caused by an intrinsic ability of the MLV pseudotyped viruses to enter these cells with a low efficiency.
An unexpected finding of these studies was that virions bound to TVB-EGF were at least as stable as native virions when incubated at 37°C before addition to cells. Based on previous studies of ALV-A Env/TVA interactions, we had expected that MLV-lacZ (ALV-B) pseudotypes preloaded with TVB-EGF might be unstable under these conditions, because TVA binding induces rapid temperature-dependent conformational changes in a soluble ALV-A Env protein that are similar to those expected to give rise to the fusion-active form of the viral glycoprotein (32, 33). In the absence of an appropriate target cell membrane, these receptor-induced structural changes in Env might be expected to inactivate the viral glycoprotein in much the same way seen with low-pH-treated influenza virus hemagglutinin glycoproteins (34). Consistent with this idea, we have not been able to efficiently infect M5 cells with MLV-lacZ (ALV-A) vectors that are preloaded with TVA-EGF (data not shown). Although the precise defect of these TVA-EGF-loaded viruses remains to be established, these data indicate an apparent difference between ALV-A and ALV-B Env-receptor interactions and show that these types of bridge proteins can be useful tools for studying parameters that influence the efficiency of retroviral entry in addition to their use as viral targeting reagents.
Another important result obtained from these studies was that TVB-EGF-loaded MLV vectors bearing ALV-B Env can be stably produced from virus packaging cells while retaining their targeting specificity. This result opens up the possibility of targeting a variety of different retroviral vectors (e.g., those based on ALV, MLV, or lentiviruses) by simply expressing both ALV-B Env and a TVB-ligand bridge protein in cells that produce these viral vectors. This possibility, in turn, could have important implications for the delivery of retroviral vectors to specific cell types in vivo. Previously, it was shown that infection of cancer cells in vivo by MLV vectors could be achieved by direct injection of virus packaging cells (35). The approach described in this study could extend the specificity of infection that can be achieved by this method by introducing cells that produce viral vectors that are preloaded with TVB-ligand bridge proteins with defined target cell specificities.
Acknowledgments
We thank Gordon Gill for providing the mouse L cell lines, John Daley at the Dana–Farber Cancer Institute for assistance with flow cytometry, and Mayra Lorenzo for her assistance in plasmid construction. We also thank John Naughton for help with the figures, and members of the Young lab, Richard Mulligan, and John Collier for critically reading the manuscript. This work was supported by National Institutes of Health Grant CA 70810, by Department of the Army Grant DAMD17-98-1-8488, and by a Harvard Medical School Funds for Discovery Award.
ABBREVIATIONS
- MLV
murine leukemia virus
- ALV
avian leukosis virus
- TVB
subgroup B ALV receptor
- SU
surface envelope protein
- EGF
epidermal growth factor
- EGFR
EGF receptor
- lacZ
β-galactosidase
- GFP
green fluorescent protein
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Neda H, Wu C H, Wu G Y. J Biol Chem. 1991;266:14143–14146. [PubMed] [Google Scholar]
- 2.Roux P, Jeanteur P, Piechaczyk M. Proc Natl Acad Sci USA. 1989;86:9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etienne-Julan M, Roux P, Carillo S, Jeanteur P, Piechaczyk M. J Gen Virol. 1992;73:3251–3255. doi: 10.1099/0022-1317-73-12-3251. [DOI] [PubMed] [Google Scholar]
- 4.Valsesia-Wittmann S, Drynda A, Deleage G, Aumailley M, Heard J M, Danos O, Verdier G, Cosset F L. J Virol. 1994;68:4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valsesia-Wittmann S, Morling F J, Nilson B H, Takeuchi Y, Russell S J, Cosset F L. J Virol. 1996;70:2059–2064. doi: 10.1128/jvi.70.3.2059-2064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasahara N, Dozy A M, Kan Y W. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 7.Han X, Kasahara N, Kan Y W. Proc Natl Acad Sci USA. 1995;92:9747–9751. doi: 10.1073/pnas.92.21.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matano T, Odawara T, Iwamoto A, Yoshikura H. J Gen Virol. 1995;76:3165–3169. doi: 10.1099/0022-1317-76-12-3165. [DOI] [PubMed] [Google Scholar]
- 9.Cosset F L, Morling F J, Takeuchi Y, Weiss R A, Collins M K, Russell S J. J Virol. 1995;69:6314–6322. doi: 10.1128/jvi.69.10.6314-6322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilson B H, Morling F J, Cosset F L, Russell S J. Gene Ther. 1996;3:280–286. [PubMed] [Google Scholar]
- 11.Chu T H, Dornburg R. J Virol. 1995;69:2659–2663. doi: 10.1128/jvi.69.4.2659-2663.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu T H, Martinez I, Sheay W C, Dornburg R. Gene Ther. 1994;1:292–299. [PubMed] [Google Scholar]
- 13.Somia N V, Zoppe M, Verma I M. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell S J, Hawkins R E, Winter G. Nucleic Acids Res. 1993;21:1081–1085. doi: 10.1093/nar/21.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marin M, Noel D, Valsesia-Wittman S, Brockly F, Etienne-Julan M, Russell S, Cosset F L, Piechaczyk M. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ager S, Nilson B H, Morling F J, Peng K W, Cosset F L, Russell S J. Hum Gene Ther. 1996;7:2157–2164. doi: 10.1089/hum.1996.7.17-2157. [DOI] [PubMed] [Google Scholar]
- 17.Jiang A, Chu T H, Nocken F, Cichutek K, Dornburg R. J Virol. 1998;72:10148–10156. doi: 10.1128/jvi.72.12.10148-10156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosset F L, Russell S J. Gene Ther. 1996;3:946–956. [PubMed] [Google Scholar]
- 19.Verma I M, Somia N. Nature (London) 1997;389:239–242. doi: 10.1038/38410. [DOI] [PubMed] [Google Scholar]
- 20.Snitkovsky S, Young J A. Proc Natl Acad Sci USA. 1998;95:7063–7068. doi: 10.1073/pnas.95.12.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gullick W J. Biochem Soc Symp. 1998;63:193–198. [PubMed] [Google Scholar]
- 22.Brojatsch J, Naughton J, Rolls M M, Zingler K, Young J A. Cell. 1996;87:845–855. doi: 10.1016/s0092-8674(00)81992-3. [DOI] [PubMed] [Google Scholar]
- 23.Ory D S, Neugeboren B A, Mulligan R C. Proc Natl Acad Sci USA. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W S, Lazar C S, Poenie M, Tsien R Y, Gill G N, Rosenfeld M G. Nature (London) 1987;328:820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- 25.Glenney J R, Jr, Chen W S, Lazar C S, Walton G M, Zokas L M, Rosenfeld M G, Gill G N. Cell. 1988;52:675–684. doi: 10.1016/0092-8674(88)90405-9. [DOI] [PubMed] [Google Scholar]
- 26.Adkins H B, Brojatsch J, Naughton J, Rolls M M, Pesola J M, Young J A. Proc Natl Acad Sci USA. 1997;94:11617–11622. doi: 10.1073/pnas.94.21.11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landau N R, Littman D R. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wool-Lewis R J, Bates P. J Virol. 1998;72:3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith E J, Brojatsch J, Naughton J, Young J A. J Virol. 1998;72:3501–3503. doi: 10.1128/jvi.72.4.3501-3503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern D F, Hare D L, Cecchini M A, Weinberg R A. Science. 1987;235:321–324. doi: 10.1126/science.3492043. [DOI] [PubMed] [Google Scholar]
- 31.Runnebaum I B. Anticancer Res. 1997;17:2887–2890. [PubMed] [Google Scholar]
- 32.Hernandez L D, Peters R J, Delos S E, Young J A, Agard D A, White J M. J Cell Biol. 1997;139:1455–1464. doi: 10.1083/jcb.139.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damico R L, Crane J, Bates P. Proc Natl Acad Sci USA. 1998;95:2580–2585. doi: 10.1073/pnas.95.5.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wharton S A, Calder L J, Ruigrok R W, Skehel J J, Steinhauer D A, Wiley D C. EMBO J. 1995;14:240–246. doi: 10.1002/j.1460-2075.1995.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurford R K, Jr, Dranoff G, Mulligan R C, Tepper R I. Nat Genet. 1995;10:430–435. doi: 10.1038/ng0895-430. [DOI] [PubMed] [Google Scholar]