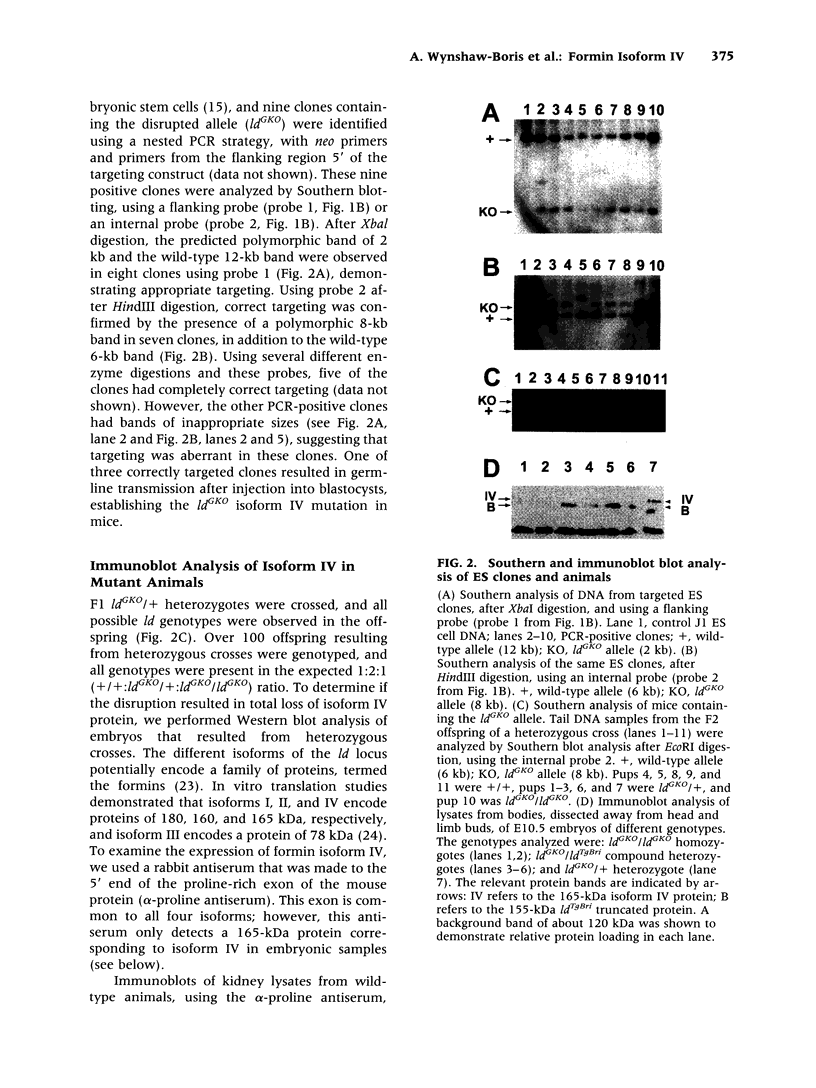
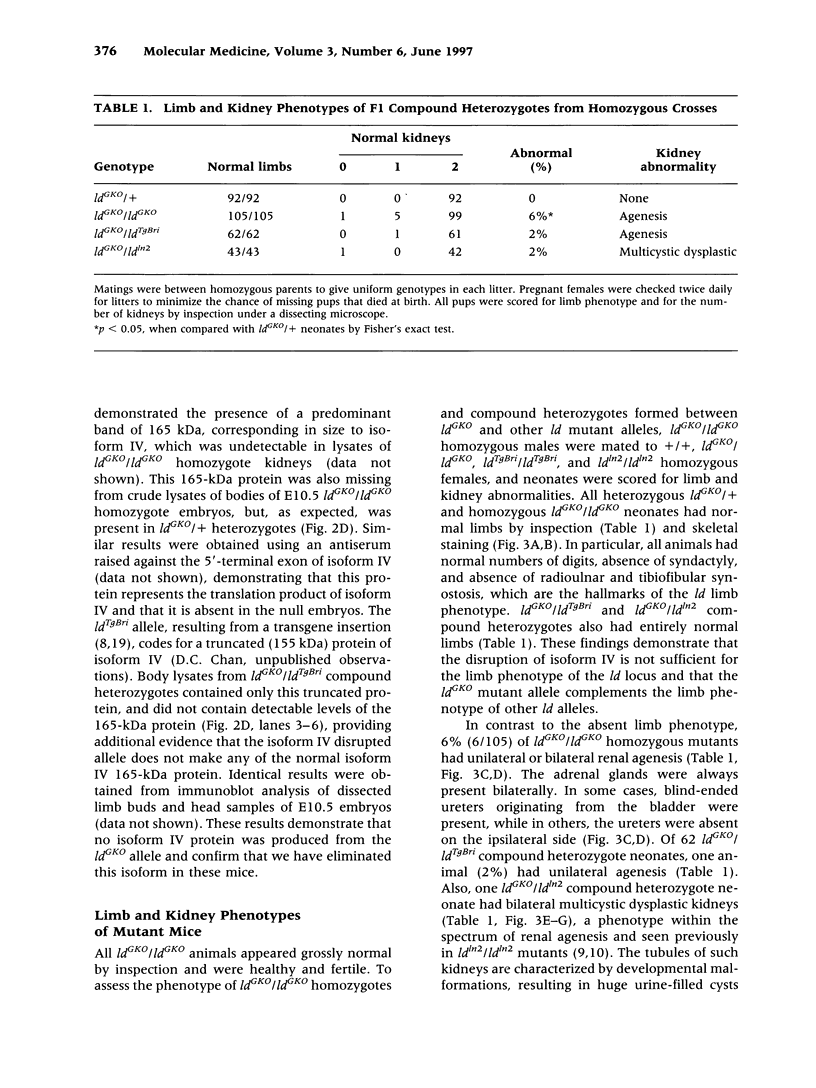
Abstract
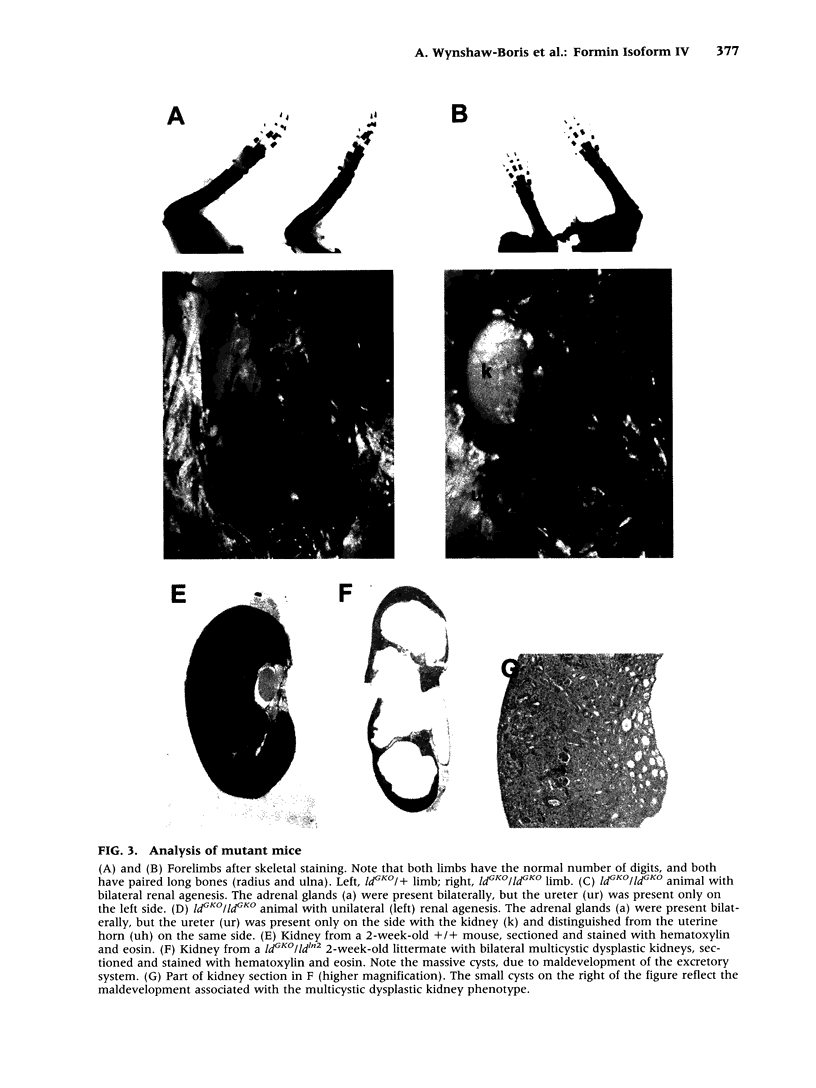
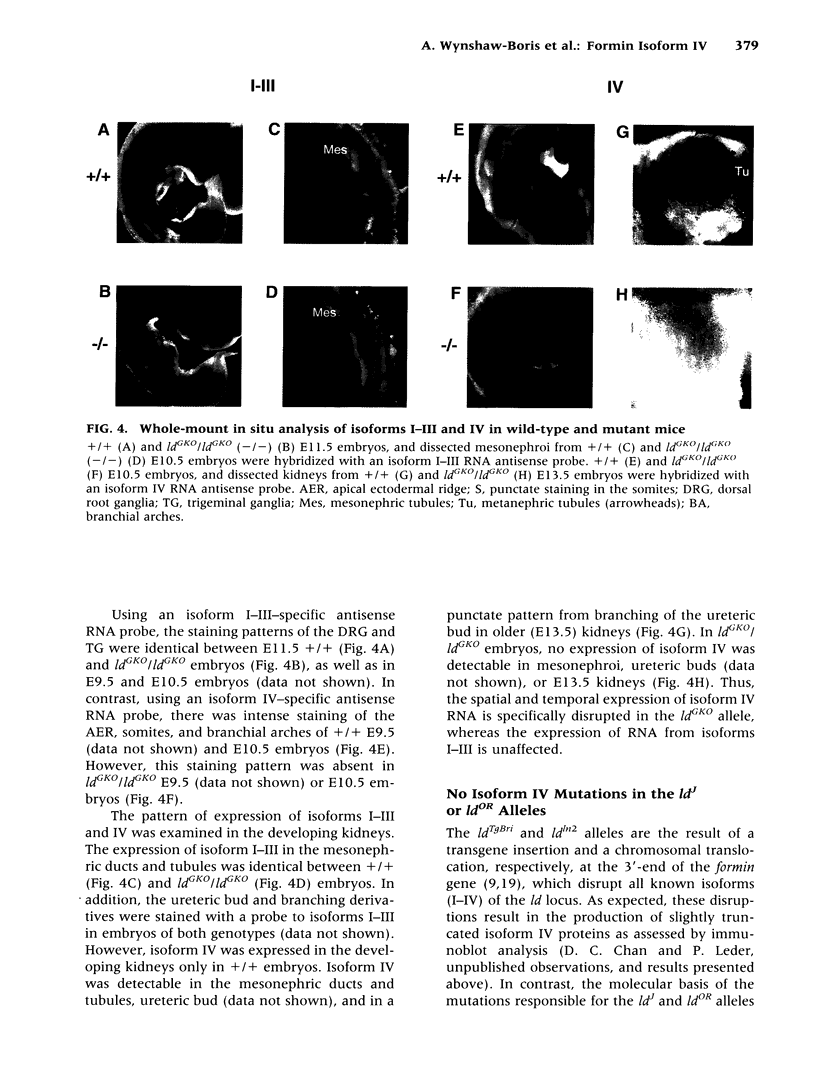
BACKGROUND: Mutations of the murine limb deformity (ld) locus are responsible for a pleiotropic phenotype of completely penetrant limb malformations and incompletely penetrant renal agenesis and/or dysgenesis. The ld locus encodes a complex family of mRNA and protein isoforms. MATERIALS AND METHODS: To examine the role of one of the more prominent of these isoforms, isoform IV, we specifically eliminated it by gene targeting. RESULTS: Unlike other mutant ld mice, homozygous mice bearing this isoform IV disruption display incompletely penetrant renal agenesis, but have perfectly normal limbs. Whole mount in situ hybridization demonstrated that this targeted disruption was specific for isoform IV and did not interfere with the expression of other ld isoforms. The isoform IV-disrupted allele of ld does not complement the renal agenesis phenotype of other ld alleles, in a manner consistent with its penetrance, and like the isoform IV-deficient mice, these compound heterozygotes have normal limbs. Sequence analysis of formin isoform IV in other ld mutant alleles did not detect any amino acid changes relative to the strain of origin of the mutant allele. CONCLUSIONS: Thus, the disruption of isoform IV is sufficient for the renal agenesis phenotype, but not the limb phenotype of ld mutant mice. Structural mutations in this isoform are only one of several genetic mechanisms leading to the renal phenotype, since amino acid changes in this isoform were not detected. These results demonstrate that this gene is limb deformity, and that variable isoform expression may play a role in generating the pleiotropic ld phenotype.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. F., Pritchard-Jones K., Bickmore W. A., Hastie N. D., Bard J. B. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech Dev. 1993 Jan;40(1-2):85–97. doi: 10.1016/0925-4773(93)90090-k. [DOI] [PubMed] [Google Scholar]
- Bankier A., de Campo M., Newell R., Rogers J. G., Danks D. M. A pedigree study of perinatally lethal renal disease. J Med Genet. 1985 Apr;22(2):104–111. doi: 10.1136/jmg.22.2.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battin J., Lacombe D., Leng J. J. Familial occurrence of hereditary renal adysplasia with müllerian anomalies. Clin Genet. 1993 Jan;43(1):23–24. doi: 10.1111/j.1399-0004.1993.tb04420.x. [DOI] [PubMed] [Google Scholar]
- Deng C. X., Wynshaw-Boris A., Shen M. M., Daugherty C., Ornitz D. M., Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994 Dec 15;8(24):3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L., Kuo A., Leder P. A variant limb deformity transcript expressed in the embryonic mouse limb defines a novel formin. Genes Dev. 1992 Jan;6(1):29–37. doi: 10.1101/gad.6.1.29. [DOI] [PubMed] [Google Scholar]
- Johnson R. L., Riddle R. D., Laufer E., Tabin C. Sonic hedgehog: a key mediator of anterior-posterior patterning of the limb and dorso-ventral patterning of axial embryonic structures. Biochem Soc Trans. 1994 Aug;22(3):569–574. doi: 10.1042/bst0220569. [DOI] [PubMed] [Google Scholar]
- Kleinebrecht J., Selow J., Winkler W. The mouse mutant limb-deformity (ld). Anat Anz. 1982;152(4):313–324. [PubMed] [Google Scholar]
- Kreidberg J. A., Sariola H., Loring J. M., Maeda M., Pelletier J., Housman D., Jaenisch R. WT-1 is required for early kidney development. Cell. 1993 Aug 27;74(4):679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kuhlman J., Niswander L. Limb deformity proteins: role in mesodermal induction of the apical ectodermal ridge. Development. 1997 Jan;124(1):133–139. doi: 10.1242/dev.124.1.133. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor T. H., Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992 Jun 12;69(6):915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Maas R., Elfering S., Glaser T., Jepeal L. Deficient outgrowth of the ureteric bud underlies the renal agenesis phenotype in mice manifesting the limb deformity (ld) mutation. Dev Dyn. 1994 Mar;199(3):214–228. doi: 10.1002/aja.1001990306. [DOI] [PubMed] [Google Scholar]
- McLeod M. J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980 Dec;22(3):299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Moore M. W., Klein R. D., Fariñas I., Sauer H., Armanini M., Phillips H., Reichardt L. F., Ryan A. M., Carver-Moore K., Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- Niswander L., Jeffrey S., Martin G. R., Tickle C. A positive feedback loop coordinates growth and patterning in the vertebrate limb. Nature. 1994 Oct 13;371(6498):609–612. doi: 10.1038/371609a0. [DOI] [PubMed] [Google Scholar]
- Niswander L., Martin G. R. FGF-4 and BMP-2 have opposite effects on limb growth. Nature. 1993 Jan 7;361(6407):68–71. doi: 10.1038/361068a0. [DOI] [PubMed] [Google Scholar]
- Niswander L., Martin G. R. Mixed signals from the AER: FGF-4 and Bmp-2 have opposite effects on limb growth. Prog Clin Biol Res. 1993;383B:625–633. [PubMed] [Google Scholar]
- Niswander L., Tickle C., Vogel A., Booth I., Martin G. R. FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. Cell. 1993 Nov 5;75(3):579–587. doi: 10.1016/0092-8674(93)90391-3. [DOI] [PubMed] [Google Scholar]
- Niswander L., Tickle C., Vogel A., Martin G. Function of FGF-4 in limb development. Mol Reprod Dev. 1994 Sep;39(1):83–89. doi: 10.1002/mrd.1080390113. [DOI] [PubMed] [Google Scholar]
- Pachnis V., Mankoo B., Costantini F. Expression of the c-ret proto-oncogene during mouse embryogenesis. Development. 1993 Dec;119(4):1005–1017. doi: 10.1242/dev.119.4.1005. [DOI] [PubMed] [Google Scholar]
- Parr B. A., McMahon A. P. Dorsalizing signal Wnt-7a required for normal polarity of D-V and A-P axes of mouse limb. Nature. 1995 Mar 23;374(6520):350–353. doi: 10.1038/374350a0. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Schalling M., Buckler A. J., Rogers A., Haber D. A., Housman D. Expression of the Wilms' tumor gene WT1 in the murine urogenital system. Genes Dev. 1991 Aug;5(8):1345–1356. doi: 10.1101/gad.5.8.1345. [DOI] [PubMed] [Google Scholar]
- Pichel J. G., Shen L., Sheng H. Z., Granholm A. C., Drago J., Grinberg A., Lee E. J., Huang S. P., Saarma M., Hoffer B. J. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- Roodhooft A. M., Birnholz J. C., Holmes L. B. Familial nature of congenital absence and severe dysgenesis of both kidneys. N Engl J Med. 1984 May 24;310(21):1341–1345. doi: 10.1056/NEJM198405243102101. [DOI] [PubMed] [Google Scholar]
- Rosen B., Beddington R. S. Whole-mount in situ hybridization in the mouse embryo: gene expression in three dimensions. Trends Genet. 1993 May;9(5):162–167. doi: 10.1016/0168-9525(93)90162-b. [DOI] [PubMed] [Google Scholar]
- Schuchardt A., D'Agati V., Larsson-Blomberg L., Costantini F., Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature. 1994 Jan 27;367(6461):380–383. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- Stark K., Vainio S., Vassileva G., McMahon A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994 Dec 15;372(6507):679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- Sánchez M. P., Silos-Santiago I., Frisén J., He B., Lira S. A., Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996 Jul 4;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- Tabin C. The initiation of the limb bud: growth factors, Hox genes, and retinoids. Cell. 1995 Mar 10;80(5):671–674. doi: 10.1016/0092-8674(95)90343-7. [DOI] [PubMed] [Google Scholar]
- Torres M., Gómez-Pardo E., Dressler G. R., Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995 Dec;121(12):4057–4065. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- Tybulewicz V. L., Crawford C. E., Jackson P. K., Bronson R. T., Mulligan R. C. Neonatal lethality and lymphopenia in mice with a homozygous disruption of the c-abl proto-oncogene. Cell. 1991 Jun 28;65(7):1153–1163. doi: 10.1016/0092-8674(91)90011-m. [DOI] [PubMed] [Google Scholar]
- Vogt T. F., Jackson-Grusby L., Rush J., Leder P. Formins: phosphoprotein isoforms encoded by the mouse limb deformity locus. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5554–5558. doi: 10.1073/pnas.90.12.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt T. F., Jackson-Grusby L., Wynshaw-Boris A. J., Chan D. C., Leder P. The same genomic region is disrupted in two transgene-induced limb deformity alleles. Mamm Genome. 1992;3(8):431–437. doi: 10.1007/BF00356152. [DOI] [PubMed] [Google Scholar]
- Wang C. C., Chan D. C., Leder P. The mouse formin (Fmn) gene: genomic structure, novel exons, and genetic mapping. Genomics. 1997 Feb 1;39(3):303–311. doi: 10.1006/geno.1996.4519. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Generoso W. M., Russell L. B., Cain K. T., Cacheiro N. L., Bultman S. J., Selby P. B., Dickinson M. E., Hogan B. L., Rutledge J. C. Molecular and genetic characterization of a radiation-induced structural rearrangement in mouse chromosome 2 causing mutations at the limb deformity and agouti loci. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2588–2592. doi: 10.1073/pnas.87.7.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Maas R. L., Zeller R., Vogt T. F., Leder P. 'Formins': proteins deduced from the alternative transcripts of the limb deformity gene. Nature. 1990 Aug 30;346(6287):850–853. doi: 10.1038/346850a0. [DOI] [PubMed] [Google Scholar]
- Woychik R. P., Stewart T. A., Davis L. G., D'Eustachio P., Leder P. An inherited limb deformity created by insertional mutagenesis in a transgenic mouse. Nature. 1985 Nov 7;318(6041):36–40. doi: 10.1038/318036a0. [DOI] [PubMed] [Google Scholar]
- Zeller R., Jackson-Grusby L., Leder P. The limb deformity gene is required for apical ectodermal ridge differentiation and anteroposterior limb pattern formation. Genes Dev. 1989 Oct;3(10):1481–1492. doi: 10.1101/gad.3.10.1481. [DOI] [PubMed] [Google Scholar]