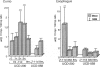Abstract
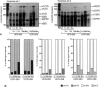
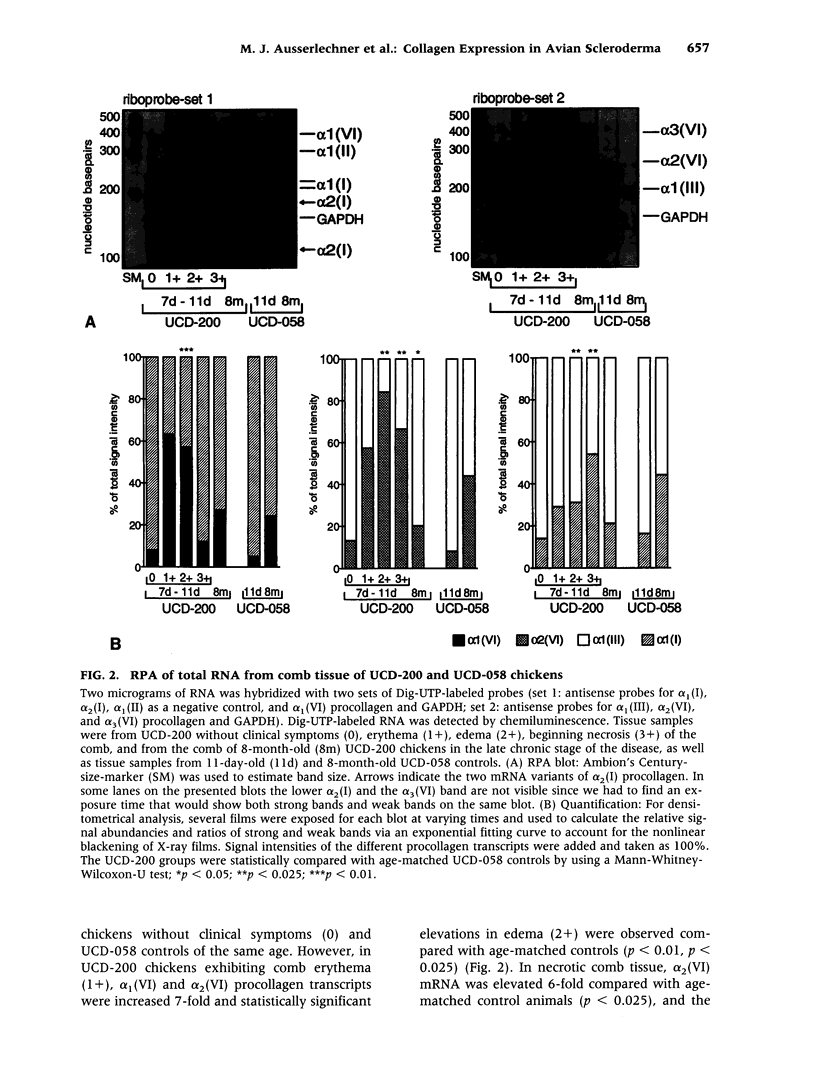
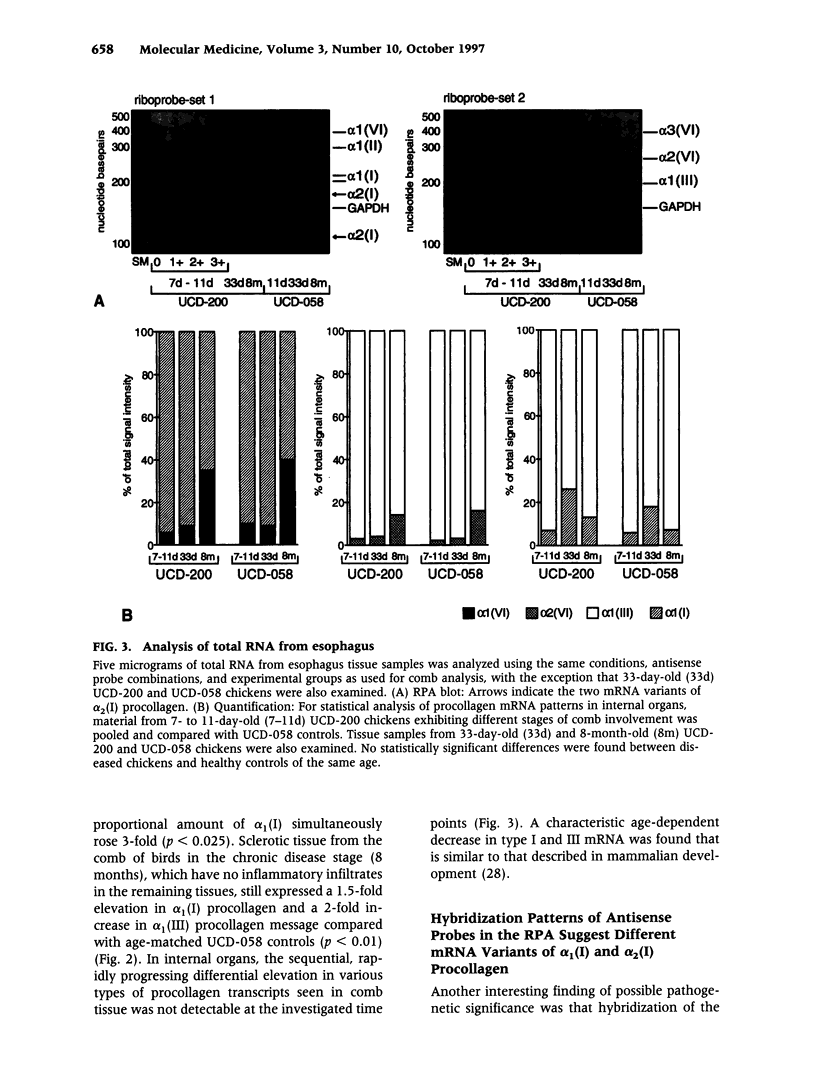
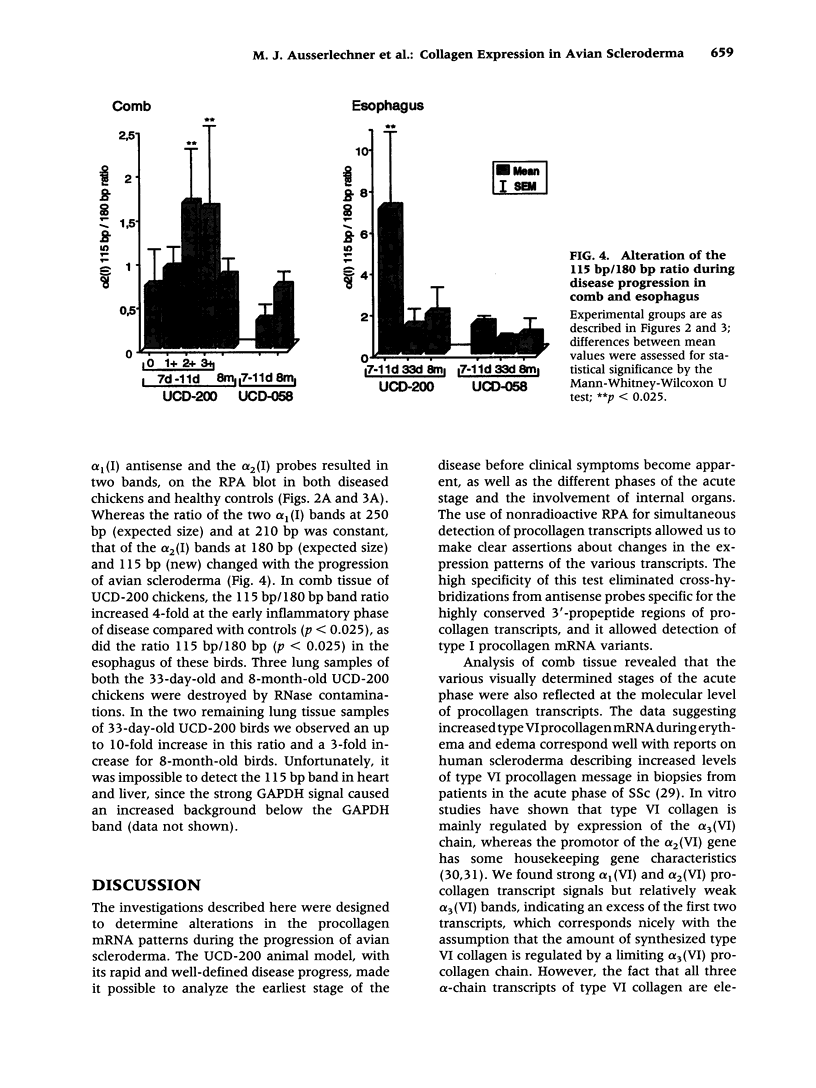
BACKGROUND: Spontaneous animal models of human autoimmune diseases provide the means to study the very first pathogenetic events, which is not possible in their human counterparts. This is particularly true for connective tissue diseases in which clinical symptoms become manifest only after a long and still obscure course of immunologic, inflammatory, and fibrotic processes. University of California at Davis line 200 chickens (UCD-200) develop a hereditary scleroderma-like disease resembling the entire spectrum of human systemic sclerosis, such as early endothelial cell damage, severe lymphocytic infiltration, and accumulation of collagen in skin and internal organs. MATERIALS AND METHODS: In the present study, we investigated mRNA levels of alpha 1(I), alpha 2(I), alpha 1(II), alpha 1(III), alpha 1(VI), alpha 2(VI), and alpha 3(VI) procollagen and GAPDH using digoxigenin-labeled antisense probes in a nonradioactive ribonuclease protection assay (RPA). We analyzed tissue samples from comb, esophagus, heart, lung, and liver of UCD-200 chickens at different stages of the disease, and healthy UCD-058 chickens. RESULTS: During the early inflammatory stage of the disease, the ratios of procollagen types VI/I and types VI/III increased 7-fold in comb tissue, followed by a 3-fold elevation in type I procollagen transcripts in the late acute stage. In the chronic stage, alpha 1(III) procollagen message was increased 2-fold. Additionally, hybridization with the 180 bp alpha 2(I) antisense probe resulted in two bands of 180 bp and 115 bp, respectively, in the RPA. The ratio of these two previously undescribed bands changes in the early stage of the disease both in comb and esophagus. CONCLUSIONS: In an animal model with a spontaneous scleroderma-like disease we found a characteristic, sequential increase in type VI, type I, and type III procollagen transcripts, and we found evidence for the presence and altered ratio of two mRNA variants of alpha 2(I) procollagen, possibly caused by alternative splicing. Comparative analysis of alpha 2(I) procollagen variants in early stages of avian scleroderma and human SSc might provide answers to unresolved questions concerning the molecular basis for generalized fibrosis in scleroderma.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abplanalp H., Gershwin M. E., Johnston E., Reid J. Genetic control of avian scleroderma. Immunogenetics. 1990;31(5-6):291–295. doi: 10.1007/BF02115002. [DOI] [PubMed] [Google Scholar]
- Bocchieri M. H., Henriksen P. D., Kasturi K. N., Muryoi T., Bona C. A., Jimenez S. A. Evidence for autoimmunity in the tight skin mouse model of systemic sclerosis. Arthritis Rheum. 1991 May;34(5):599–605. doi: 10.1002/art.1780340512. [DOI] [PubMed] [Google Scholar]
- Bonaldo P., Russo V., Bucciotti F., Bressan G. M., Colombatti A. Alpha 1 chain of chick type VI collagen. The complete cDNA sequence reveals a hybrid molecule made of one short collagen and three von Willebrand factor type A-like domains. J Biol Chem. 1989 Apr 5;264(10):5575–5580. [PubMed] [Google Scholar]
- Bonaldo P., Russo V., Bucciotti F., Doliana R., Colombatti A. Structural and functional features of the alpha 3 chain indicate a bridging role for chicken collagen VI in connective tissues. Biochemistry. 1990 Feb 6;29(5):1245–1254. doi: 10.1021/bi00457a021. [DOI] [PubMed] [Google Scholar]
- Botstein G. R., Sherer G. K., Leroy E. C. Fibroblast selection in scleroderma. An alternative model of fibrosis. Arthritis Rheum. 1982 Feb;25(2):189–195. doi: 10.1002/art.1780250212. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Duncan M. R., Wilson T. J., Van De Water J., Berman B., Boyd R., Wick G., Gershwin M. E. Cultured fibroblasts in avian scleroderma, an autoimmune fibrotic disease, display an activated phenotype. J Autoimmun. 1992 Oct;5(5):603–615. doi: 10.1016/0896-8411(92)90157-l. [DOI] [PubMed] [Google Scholar]
- Gershwin M. E., Abplanalp H., Castles J. J., Ikeda R. M., van der Water J., Eklund J., Haynes D. Characterization of a spontaneous disease of white leghorn chickens resembling progressive systemic sclerosis (scleroderma). J Exp Med. 1981 Jun 1;153(6):1640–1659. doi: 10.1084/jem.153.6.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruschwitz M. S., Moormann S., Krömer G., Sgonc R., Dietrich H., Boeck G., Gershwin M. E., Boyd R., Wick G. Phenotypic analysis of skin infiltrates in comparison with peripheral blood lymphocytes, spleen cells and thymocytes in early avian scleroderma. J Autoimmun. 1991 Aug;4(4):577–593. doi: 10.1016/0896-8411(91)90178-f. [DOI] [PubMed] [Google Scholar]
- Gruschwitz M. S., Shoenfeld Y., Krupp M., Gershwin M. E., Penner E., Brezinschek H. P., Wick G. Antinuclear antibody profile in UCD line 200 chickens: a model for progressive systemic sclerosis. Int Arch Allergy Immunol. 1993;100(4):307–313. doi: 10.1159/000236430. [DOI] [PubMed] [Google Scholar]
- Haynes D. C., Gershwin M. E. Diversity of autoantibodies in avian scleroderma. An inherited fibrotic disease of White Leghorn chickens. J Clin Invest. 1984 Jun;73(6):1557–1568. doi: 10.1172/JCI111362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann M., Aumailley M., Hatamochi A., Chu M. L., Timpl R., Krieg T. Down-regulation of alpha 3(VI) chain expression by gamma-interferon decreases synthesis and deposition of collagen type VI. Eur J Biochem. 1989 Jul 1;182(3):719–726. doi: 10.1111/j.1432-1033.1989.tb14884.x. [DOI] [PubMed] [Google Scholar]
- Jimenez S. A., Williams C. J., Myers J. C., Bashey R. I. Increased collagen biosynthesis and increased expression of type I and type III procollagen genes in tight skin (TSK) mouse fibroblasts. J Biol Chem. 1986 Jan 15;261(2):657–662. [PubMed] [Google Scholar]
- Koller E., Trueb B. Characterization of the chicken alpha 1(VI) collagen promoter. Eur J Biochem. 1992 Sep 15;208(3):769–774. doi: 10.1111/j.1432-1033.1992.tb17246.x. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M. Activation of dermal connective tissue in scleroderma. Ann Med. 1993 Dec;25(6):511–518. [PubMed] [Google Scholar]
- Kähäri V. M., Heino J., Vuorio E. Interleukin-1 increases collagen production and mRNA levels in cultured skin fibroblasts. Biochim Biophys Acta. 1987 Jul 6;929(2):142–147. doi: 10.1016/0167-4889(87)90169-8. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M., Sandberg M., Kalimo H., Vuorio T., Vuorio E. Identification of fibroblasts responsible for increased collagen production in localized scleroderma by in situ hybridization. J Invest Dermatol. 1988 May;90(5):664–670. doi: 10.1111/1523-1747.ep12560826. [DOI] [PubMed] [Google Scholar]
- LeRoy E. C. The connective tissue in scleroderma. Coll Relat Res. 1981 Apr;1(3):301–308. doi: 10.1016/s0174-173x(81)80007-6. [DOI] [PubMed] [Google Scholar]
- Mays P. K., Bishop J. E., Laurent G. J. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988 Nov 30;45(3):203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Mäkelä J. K., Vuorio E. Type I collagen messenger RNA levels in experimental granulation tissue and silicosis in rats. Med Biol. 1986;64(1):15–22. [PubMed] [Google Scholar]
- Perlish J. S., Lemlich G., Fleischmajer R. Identification of collagen fibrils in scleroderma skin. J Invest Dermatol. 1988 Jan;90(1):48–54. doi: 10.1111/1523-1747.ep12462561. [DOI] [PubMed] [Google Scholar]
- Scharffetter K., Lankat-Buttgereit B., Krieg T. Localization of collagen mRNA in normal and scleroderma skin by in-situ hybridization. Eur J Clin Invest. 1988 Feb;18(1):9–17. doi: 10.1111/j.1365-2362.1988.tb01158.x. [DOI] [PubMed] [Google Scholar]
- Sgonc R., Gruschwitz M. S., Dietrich H., Recheis H., Gershwin M. E., Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996 Aug 1;98(3):785–792. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trüeb B., Schaeren-Wiemers N., Schreier T., Winterhalter K. H. Molecular cloning of chicken type VI collagen. Primary structure of the subunit alpha 2(VI)-pepsin. J Biol Chem. 1989 Jan 5;264(1):136–140. [PubMed] [Google Scholar]
- Van de Water J., Boyd R., Wick G., Gershwin M. E. The immunologic and genetic basis of avian scleroderma, an inherited fibrotic disease of line 200 chickens. Int Rev Immunol. 1994;11(3):273–282. doi: 10.3109/08830189409061732. [DOI] [PubMed] [Google Scholar]
- Vuorio E., Sandell L., Kravis D., Sheffield V. C., Vuorio T., Dorfman A., Upholt W. B. Construction and partial characterization of two recombinant cDNA clones for procollagen from chicken cartilage. Nucleic Acids Res. 1982 Feb 25;10(4):1175–1192. doi: 10.1093/nar/10.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Kühn K., de Crombrugghe B. A conserved nucleotide sequence, coding for a segment of the C-propeptide, is found at the same location in different collagen genes. Nucleic Acids Res. 1983 May 11;11(9):2733–2744. doi: 10.1093/nar/11.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Water J., Gershwin M. E., Abplanalp H., Wick G., von der Mark K. Serial observations and definition of mononuclear cell infiltrates in avian scleroderma, an inherited fibrotic disease of chickens. Arthritis Rheum. 1984 Jul;27(7):807–815. doi: 10.1002/art.1780270712. [DOI] [PubMed] [Google Scholar]
- van de Water J., Haapanen L., Boyd R., Abplanalp H., Gershwin M. E. Identification of T cells in early dermal lymphocytic infiltrates in avian scleroderma. Arthritis Rheum. 1989 Aug;32(8):1031–1040. doi: 10.1002/anr.1780320813. [DOI] [PubMed] [Google Scholar]