Abstract
The adoptive transfer of cytotoxic T lymphocytes (CTLs) derived from tumor-infiltrating lymphocytes (TIL) along with interleukin 2 (IL-2) into autologous patients with cancer resulted in the objective regression of tumor, indicating that these CTLs recognized cancer rejection antigens on tumor cells. To understand the molecular basis of T cell-mediated antitumor immunity, several groups started to search for such tumor antigens in melanoma as well as in other types of cancers. This led to the subject I will review in this article. A number of tumor antigens were isolated by the use of cDNA expression systems and biochemical approaches. These tumor antigens could be classified into several categories: tissue-specific differentiation antigens, tumor-specific shared antigens, and tumor-specific unique antigens. However, the majority of tumor antigens identified to date are nonmutated, self proteins. This raises important questions regarding the mechanism of antitumor activity and autoimmune disease. The identification of human tumor rejection antigens provides new opportunities for the development of therapeutic strategies against cancer. This review will summarize the current status and progress toward identifying human tumor antigens and their potential applications to cancer treatment.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adema G. J., de Boer A. J., van 't Hullenaar R., Denijn M., Ruiter D. J., Vogel A. M., Figdor C. G. Melanocyte lineage-specific antigens recognized by monoclonal antibodies NKI-beteb, HMB-50, and HMB-45 are encoded by a single cDNA. Am J Pathol. 1993 Dec;143(6):1579–1585. [PMC free article] [PubMed] [Google Scholar]
- Bakker A. B., Schreurs M. W., Tafazzul G., de Boer A. J., Kawakami Y., Adema G. J., Figdor C. G. Identification of a novel peptide derived from the melanocyte-specific gp100 antigen as the dominant epitope recognized by an HLA-A2.1-restricted anti-melanoma CTL line. Int J Cancer. 1995 Jul 4;62(1):97–102. doi: 10.1002/ijc.2910620118. [DOI] [PubMed] [Google Scholar]
- Bakker A. B., Schreurs M. W., de Boer A. J., Kawakami Y., Rosenberg S. A., Adema G. J., Figdor C. G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J Exp Med. 1994 Mar 1;179(3):1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K. F., Atkinson M. J., Reich U., Becker I., Nekarda H., Siewert J. R., Höfler H. E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res. 1994 Jul 15;54(14):3845–3852. [PubMed] [Google Scholar]
- Blake J., Johnston J. V., Hellström K. E., Marquardt H., Chen L. Use of combinatorial peptide libraries to construct functional mimics of tumor epitopes recognized by MHC class I-restricted cytolytic T lymphocytes. J Exp Med. 1996 Jul 1;184(1):121–130. doi: 10.1084/jem.184.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. B., Perry-Lalley D., Robbins P. F., Li Y., el-Gamil M., Rosenberg S. A., Yang J. C. Identification of tyrosinase-related protein 2 as a tumor rejection antigen for the B16 melanoma. J Exp Med. 1997 Feb 3;185(3):453–459. doi: 10.1084/jem.185.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard B., Fuller B. B., Vijayasaradhi S., Houghton A. N. Induction of pigmentation in mouse fibroblasts by expression of human tyrosinase cDNA. J Exp Med. 1989 Jun 1;169(6):2029–2042. doi: 10.1084/jem.169.6.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
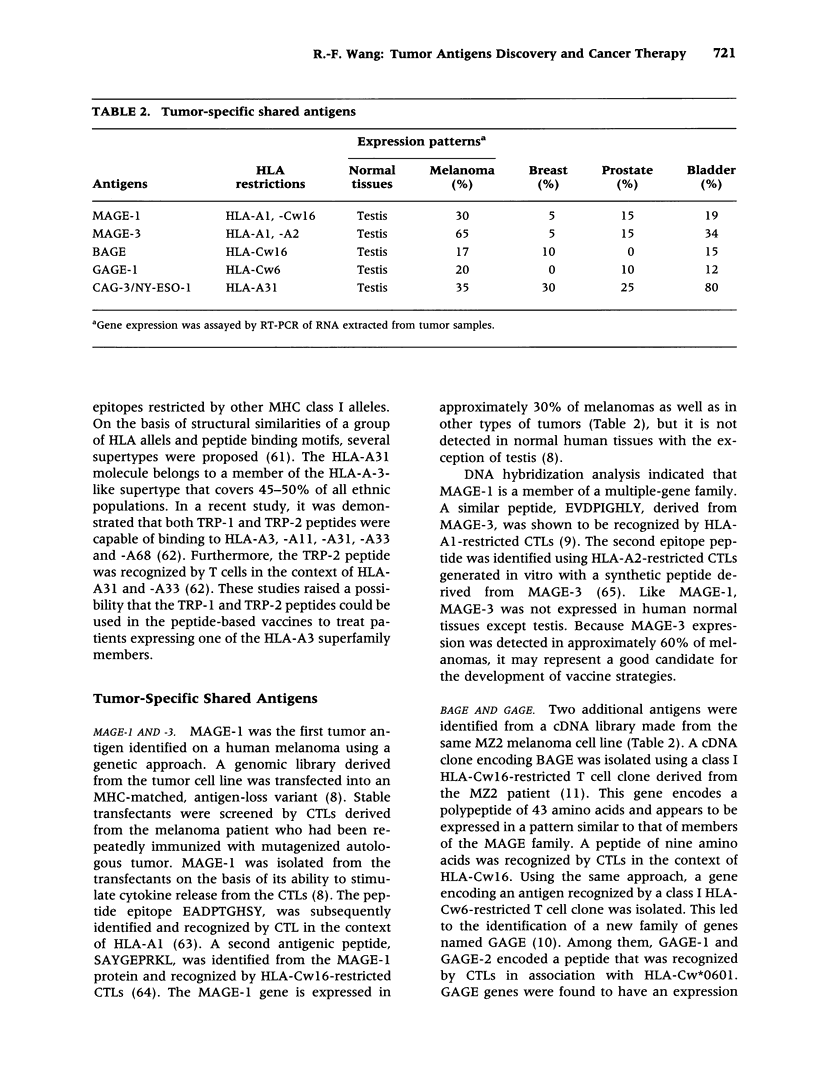
- Boël P., Wildmann C., Sensi M. L., Brasseur R., Renauld J. C., Coulie P., Boon T., van der Bruggen P. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995 Feb;2(2):167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Brichard V. G., Herman J., Van Pel A., Wildmann C., Gaugler B., Wölfel T., Boon T., Lethé B. A tyrosinase nonapeptide presented by HLA-B44 is recognized on a human melanoma by autologous cytolytic T lymphocytes. Eur J Immunol. 1996 Jan;26(1):224–230. doi: 10.1002/eji.1830260135. [DOI] [PubMed] [Google Scholar]
- Brichard V., Van Pel A., Wölfel T., Wölfel C., De Plaen E., Lethé B., Coulie P., Boon T. The tyrosinase gene codes for an antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1993 Aug 1;178(2):489–495. doi: 10.1084/jem.178.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Tsung K., Rao J. B., Chen P. W., Wang M., Rosenberg S. A., Restifo N. P. IL-2 enhances the function of recombinant poxvirus-based vaccines in the treatment of established pulmonary metastases. J Immunol. 1995 May 15;154(10):5282–5292. [PMC free article] [PubMed] [Google Scholar]
- Brändle D., Brasseur F., Weynants P., Boon T., Van den Eynde B. A mutated HLA-A2 molecule recognized by autologous cytotoxic T lymphocytes on a human renal cell carcinoma. J Exp Med. 1996 Jun 1;183(6):2501–2508. doi: 10.1084/jem.183.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystryn J. C., Rigel D., Friedman R. J., Kopf A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987 Aug;123(8):1053–1055. [PubMed] [Google Scholar]
- Celis E., Tsai V., Crimi C., DeMars R., Wentworth P. A., Chesnut R. W., Grey H. M., Sette A., Serra H. M. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2105–2109. doi: 10.1073/pnas.91.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M., Yang R., Hubbell E., Berno A., Huang X. C., Stern D., Winkler J., Lockhart D. J., Morris M. S., Fodor S. P. Accessing genetic information with high-density DNA arrays. Science. 1996 Oct 25;274(5287):610–614. doi: 10.1126/science.274.5287.610. [DOI] [PubMed] [Google Scholar]
- Cheever M. A., Disis M. L., Bernhard H., Gralow J. R., Hand S. L., Huseby E. S., Qin H. L., Takahashi M., Chen W. Immunity to oncogenic proteins. Immunol Rev. 1995 Jun;145:33–59. doi: 10.1111/j.1600-065x.1995.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Chen Y. T., Scanlan M. J., Sahin U., Türeci O., Gure A. O., Tsang S., Williamson B., Stockert E., Pfreundschuh M., Old L. J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulie P. G., Lehmann F., Lethé B., Herman J., Lurquin C., Andrawiss M., Boon T. A mutated intron sequence codes for an antigenic peptide recognized by cytolytic T lymphocytes on a human melanoma. Proc Natl Acad Sci U S A. 1995 Aug 15;92(17):7976–7980. doi: 10.1073/pnas.92.17.7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A. L., Skipper J., Chen Y., Henderson R. A., Darrow T. L., Shabanowitz J., Engelhard V. H., Hunt D. F., Slingluff C. L., Jr Identification of a peptide recognized by five melanoma-specific human cytotoxic T cell lines. Science. 1994 Apr 29;264(5159):716–719. doi: 10.1126/science.7513441. [DOI] [PubMed] [Google Scholar]
- Dadmarz R., Sgagias M. K., Rosenberg S. A., Schwartzentruber D. J. CD4+ T lymphocytes infiltrating human breast cancer recognise autologous tumor in an MHC-class-II restricted fashion. Cancer Immunol Immunother. 1995 Jan;40(1):1–9. doi: 10.1007/BF01517229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRisi J., Penland L., Brown P. O., Bittner M. L., Meltzer P. S., Ray M., Chen Y., Su Y. A., Trent J. M. Use of a cDNA microarray to analyse gene expression patterns in human cancer. Nat Genet. 1996 Dec;14(4):457–460. doi: 10.1038/ng1296-457. [DOI] [PubMed] [Google Scholar]
- Disis M. L., Calenoff E., McLaughlin G., Murphy A. E., Chen W., Groner B., Jeschke M., Lydon N., McGlynn E., Livingston R. B. Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res. 1994 Jan 1;54(1):16–20. [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Takiguchi M., Grahovac B., Gnau V., Stevanović S., Jung G., Rammensee H. G. Peptide motifs of HLA-A1, -A11, -A31, and -A33 molecules. Immunogenetics. 1994;40(3):238–241. doi: 10.1007/BF00167086. [DOI] [PubMed] [Google Scholar]
- Finn O. J., Jerome K. R., Henderson R. A., Pecher G., Domenech N., Magarian-Blander J., Barratt-Boyes S. M. MUC-1 epithelial tumor mucin-based immunity and cancer vaccines. Immunol Rev. 1995 Jun;145:61–89. doi: 10.1111/j.1600-065x.1995.tb00077.x. [DOI] [PubMed] [Google Scholar]
- Fisk B., Blevins T. L., Wharton J. T., Ioannides C. G. Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med. 1995 Jun 1;181(6):2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaugler B., Van den Eynde B., van der Bruggen P., Romero P., Gaforio J. J., De Plaen E., Lethé B., Brasseur F., Boon T. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994 Mar 1;179(3):921–930. doi: 10.1084/jem.179.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P. D. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. doi: 10.1016/s0065-2776(08)60778-6. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993 Oct;5(5):797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Hara I., Takechi Y., Houghton A. N. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995 Nov 1;182(5):1609–1614. doi: 10.1084/jem.182.5.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. A., Nimgaonkar M. T., Watkins S. C., Robbins P. D., Ball E. D., Finn O. J. Human dendritic cells genetically engineered to express high levels of the human epithelial tumor antigen mucin (MUC-1). Cancer Res. 1996 Aug 15;56(16):3763–3770. [PubMed] [Google Scholar]
- Hoschuetzky H., Aberle H., Kemler R. Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol. 1994 Dec;127(5):1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbiers J. G., Nijman H. W., van der Burg S. H., Drijfhout J. W., Kenemans P., van de Velde C. J., Brand A., Momburg F., Kast W. M., Melief C. J. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993 Sep;23(9):2072–2077. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- Ioannides C. G., Fisk B., Jerome K. R., Irimura T., Wharton J. T., Finn O. J. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol. 1993 Oct 1;151(7):3693–3703. [PubMed] [Google Scholar]
- Ioannides C. G., Fisk B., Pollack M. S., Frazier M. L., Taylor Wharton J., Freedman R. S. Cytotoxic T-cell clones isolated from ovarian tumour infiltrating lymphocytes recognize common determinants on non-ovarian tumour clones. Scand J Immunol. 1993 Apr;37(4):413–424. doi: 10.1111/j.1365-3083.1993.tb03312.x. [DOI] [PubMed] [Google Scholar]
- Irvine K. R., Rao J. B., Rosenberg S. A., Restifo N. P. Cytokine enhancement of DNA immunization leads to effective treatment of established pulmonary metastases. J Immunol. 1996 Jan 1;156(1):238–245. [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Cervantes C., Solano F., Kobayashi T., Urabe K., Hearing V. J., Lozano J. A., García-Borrón J. C. A new enzymatic function in the melanogenic pathway. The 5,6-dihydroxyindole-2-carboxylic acid oxidase activity of tyrosinase-related protein-1 (TRP1). J Biol Chem. 1994 Jul 8;269(27):17993–18000. [PubMed] [Google Scholar]
- Jung S., Schluesener H. J. Human T lymphocytes recognize a peptide of single point-mutated, oncogenic ras proteins. J Exp Med. 1991 Jan 1;173(1):273–276. doi: 10.1084/jem.173.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Rivoltini L., Topalian S. L., Miki T., Rosenberg S. A. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3515–3519. doi: 10.1073/pnas.91.9.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C. H., Robbins P. F., Sakaguchi K., Appella E., Yannelli J. R., Adema G. J., Miki T., Rosenberg S. A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994 Jul 5;91(14):6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Jennings C., Sakaguchi K., Kang X., Southwood S., Robbins P. F., Sette A., Appella E., Rosenberg S. A. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995 Apr 15;154(8):3961–3968. [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Sakaguchi K., Robbins P. F., Rivoltini L., Yannelli J. R., Appella E., Rosenberg S. A. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994 Jul 1;180(1):347–352. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi J., Kato J., Sasaki K., Fujii S., Watanabe N., Niitsu Y. Loss of E-cadherin-dependent cell-cell adhesion due to mutation of the beta-catenin gene in a human cancer cell line, HSC-39. Mol Cell Biol. 1995 Mar;15(3):1175–1181. doi: 10.1128/mcb.15.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korinek V., Barker N., Morin P. J., van Wichen D., de Weger R., Kinzler K. W., Vogelstein B., Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997 Mar 21;275(5307):1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Linehan D. C., Goedegebuure P. S., Peoples G. E., Rogers S. O., Eberlein T. J. Tumor-specific and HLA-A2-restricted cytolysis by tumor-associated lymphocytes in human metastatic breast cancer. J Immunol. 1995 Nov 1;155(9):4486–4491. [PubMed] [Google Scholar]
- Magarian-Blander J., Domenech N., Finn O. J. Specific and effective T-cell recognition of cells transfected with a truncated human mucin cDNA. Ann N Y Acad Sci. 1993 Aug 12;690:231–243. doi: 10.1111/j.1749-6632.1993.tb44012.x. [DOI] [PubMed] [Google Scholar]
- Malarkannan S., Afkarian M., Shastri N. A rare cryptic translation product is presented by Kb major histocompatibility complex class I molecule to alloreactive T cells. J Exp Med. 1995 Dec 1;182(6):1739–1750. doi: 10.1084/jem.182.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboim O., Berke G., Fridkin M., Feldman M., Eisenstein M., Eisenbach L. CTL induction by a tumour-associated antigen octapeptide derived from a murine lung carcinoma. Nature. 1994 May 5;369(6475):67–71. doi: 10.1038/369067a0. [DOI] [PubMed] [Google Scholar]
- Markus N. R., Rosenberg S. A., Topalian S. L. Analysis of cytokine secretion by melanoma-specific CD4+ T lymphocytes. J Interferon Cytokine Res. 1995 Aug;15(8):739–746. doi: 10.1089/jir.1995.15.739. [DOI] [PubMed] [Google Scholar]
- Mattes M. J., Thomson T. M., Old L. J., Lloyd K. O. A pigmentation-associated, differentiation antigen of human melanoma defined by a precipitating antibody in human serum. Int J Cancer. 1983 Dec 15;32(6):717–721. doi: 10.1002/ijc.2910320610. [DOI] [PubMed] [Google Scholar]
- Morin P. J., Sparks A. B., Korinek V., Barker N., Clevers H., Vogelstein B., Kinzler K. W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997 Mar 21;275(5307):1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Nanda N. K., Sercarz E. E. Induction of anti-self-immunity to cure cancer. Cell. 1995 Jul 14;82(1):13–17. doi: 10.1016/0092-8674(95)90047-0. [DOI] [PubMed] [Google Scholar]
- Nijman H. W., Houbiers J. G., van der Burg S. H., Vierboom M. P., Kenemans P., Kast W. M., Melief C. J. Characterization of cytotoxic T lymphocyte epitopes of a self-protein, p53, and a non-self-protein, influenza matrix: relationship between major histocompatibility complex peptide binding affinity and immune responsiveness to peptides. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):121–126. [PubMed] [Google Scholar]
- Nijman H. W., Van der Burg S. H., Vierboom M. P., Houbiers J. G., Kast W. M., Melief C. J. p53, a potential target for tumor-directed T cells. Immunol Lett. 1994 May;40(2):171–178. doi: 10.1016/0165-2478(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Nordlund J. J., Kirkwood J. M., Forget B. M., Milton G., Albert D. M., Lerner A. B. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983 Nov;9(5):689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- Peoples G. E., Goedegebuure P. S., Smith R., Linehan D. C., Yoshino I., Eberlein T. J. Breast and ovarian cancer-specific cytotoxic T lymphocytes recognize the same HER2/neu-derived peptide. Proc Natl Acad Sci U S A. 1995 Jan 17;92(2):432–436. doi: 10.1073/pnas.92.2.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle D. E., Zindy F., Ashmun R. A., Sherr C. J. Alternative reading frames of the INK4a tumor suppressor gene encode two unrelated proteins capable of inducing cell cycle arrest. Cell. 1995 Dec 15;83(6):993–1000. doi: 10.1016/0092-8674(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Rammensee H. G., Friede T., Stevanoviíc S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41(4):178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- Reeves M. E., Royal R. E., Lam J. S., Rosenberg S. A., Hwu P. Retroviral transduction of human dendritic cells with a tumor-associated antigen gene. Cancer Res. 1996 Dec 15;56(24):5672–5677. [PubMed] [Google Scholar]
- Richards J. M., Mehta N., Ramming K., Skosey P. Sequential chemoimmunotherapy in the treatment of metastatic melanoma. J Clin Oncol. 1992 Aug;10(8):1338–1343. doi: 10.1200/JCO.1992.10.8.1338. [DOI] [PubMed] [Google Scholar]
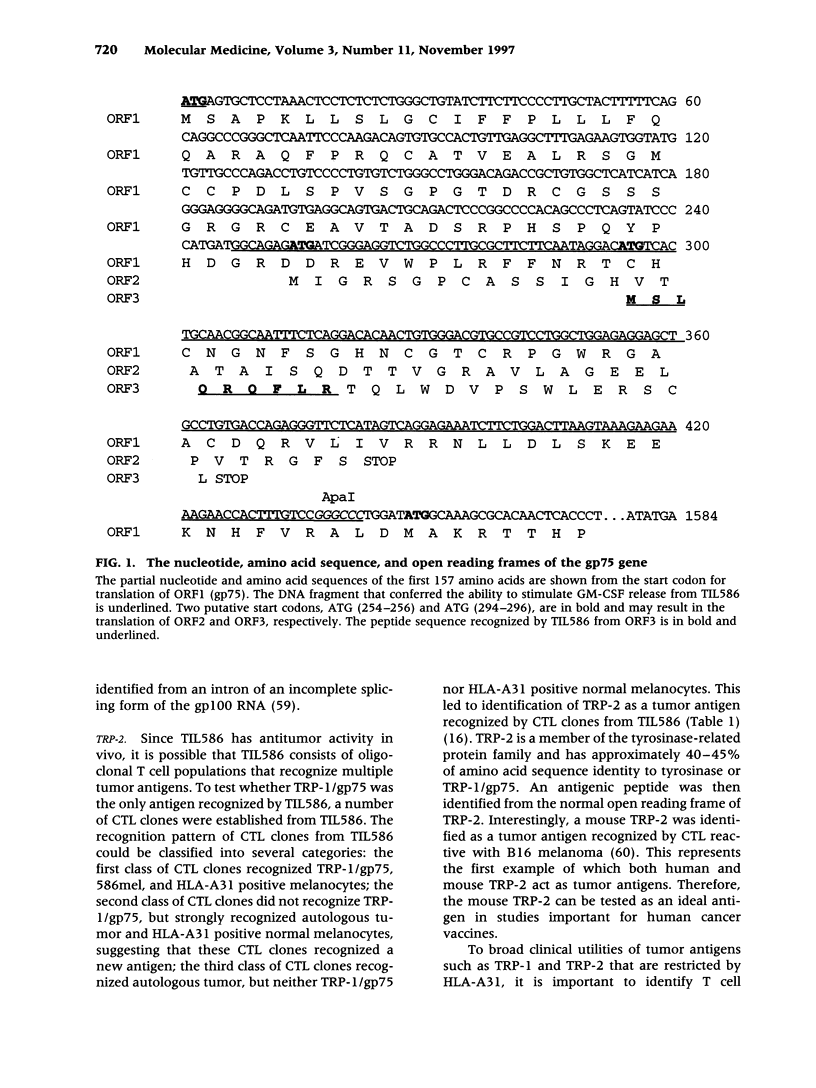
- Robbins P. F., El-Gamil M., Li Y. F., Fitzgerald E. B., Kawakami Y., Rosenberg S. A. The intronic region of an incompletely spliced gp100 gene transcript encodes an epitope recognized by melanoma-reactive tumor-infiltrating lymphocytes. J Immunol. 1997 Jul 1;159(1):303–308. [PubMed] [Google Scholar]
- Robbins P. F., El-Gamil M., Li Y. F., Kawakami Y., Loftus D., Appella E., Rosenberg S. A. A mutated beta-catenin gene encodes a melanoma-specific antigen recognized by tumor infiltrating lymphocytes. J Exp Med. 1996 Mar 1;183(3):1185–1192. doi: 10.1084/jem.183.3.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Kawakami Y., Stevens E., Yannelli J. R., Rosenberg S. A. Recognition of tyrosinase by tumor-infiltrating lymphocytes from a patient responding to immunotherapy. Cancer Res. 1994 Jun 15;54(12):3124–3126. [PubMed] [Google Scholar]
- Robbins P. F., el-Gamil M., Li Y. F., Topalian S. L., Rivoltini L., Sakaguchi K., Appella E., Kawakami Y., Rosenberg S. A. Cloning of a new gene encoding an antigen recognized by melanoma-specific HLA-A24-restricted tumor-infiltrating lymphocytes. J Immunol. 1995 Jun 1;154(11):5944–5950. [PubMed] [Google Scholar]
- Rosenberg S. A. Karnofsky Memorial Lecture. The immunotherapy and gene therapy of cancer. J Clin Oncol. 1992 Feb;10(2):180–199. doi: 10.1200/JCO.1992.10.2.180. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Packard B. S., Aebersold P. M., Solomon D., Topalian S. L., Toy S. T., Simon P., Lotze M. T., Yang J. C., Seipp C. A. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988 Dec 22;319(25):1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Spiess P., Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science. 1986 Sep 19;233(4770):1318–1321. doi: 10.1126/science.3489291. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Robbins P., El-Gamil M., Albert I., Porfiri E., Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997 Mar 21;275(5307):1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Schena M., Shalon D., Davis R. W., Brown P. O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995 Oct 20;270(5235):467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- Schwartz S., Felber B. K., Pavlakis G. N. Mechanism of translation of monocistronic and multicistronic human immunodeficiency virus type 1 mRNAs. Mol Cell Biol. 1992 Jan;12(1):207–219. doi: 10.1128/mcb.12.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensi M., Traversari C., Radrizzani M., Salvi S., Maccalli C., Mortarini R., Rivoltini L., Farina C., Nicolini G., Wölfel T. Cytotoxic T-lymphocyte clones from different patients display limited T-cell-receptor variable-region gene usage in HLA-A2-restricted recognition of the melanoma antigen Melan-A/MART-1. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5674–5678. doi: 10.1073/pnas.92.12.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Shaw M. W., Choppin P. W., Lamb R. A. A previously unrecognized influenza B virus glycoprotein from a bicistronic mRNA that also encodes the viral neuraminidase. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4879–4883. doi: 10.1073/pnas.80.16.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Roberts J. M. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995 May 15;9(10):1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- Sidney J., Grey H. M., Kubo R. T., Sette A. Practical, biochemical and evolutionary implications of the discovery of HLA class I supermotifs. Immunol Today. 1996 Jun;17(6):261–266. doi: 10.1016/0167-5699(96)80542-1. [DOI] [PubMed] [Google Scholar]
- Skipper J. C., Hendrickson R. C., Gulden P. H., Brichard V., Van Pel A., Chen Y., Shabanowitz J., Wolfel T., Slingluff C. L., Jr, Boon T. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996 Feb 1;183(2):527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skipper J. C., Kittlesen D. J., Hendrickson R. C., Deacon D. D., Harthun N. L., Wagner S. N., Hunt D. F., Engelhard V. H., Slingluff C. L., Jr Shared epitopes for HLA-A3-restricted melanoma-reactive human CTL include a naturally processed epitope from Pmel-17/gp100. J Immunol. 1996 Dec 1;157(11):5027–5033. [PubMed] [Google Scholar]
- Skipper J., Stauss H. J. Identification of two cytotoxic T lymphocyte-recognized epitopes in the Ras protein. J Exp Med. 1993 May 1;177(5):1493–1498. doi: 10.1084/jem.177.5.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiropoulou C. F., Nichol S. T. A small highly basic protein is encoded in overlapping frame within the P gene of vesicular stomatitis virus. J Virol. 1993 Jun;67(6):3103–3110. doi: 10.1128/jvi.67.6.3103-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Chapman P. B., Yang S. Y., Hara I., Vijayasaradhi S., Houghton A. N. Reactivity of autologous CD4+ T lymphocytes against human melanoma. Evidence for a shared melanoma antigen presented by HLA-DR15. J Immunol. 1995 Jan 15;154(2):772–779. [PubMed] [Google Scholar]
- Theobald M., Biggs J., Dittmer D., Levine A. J., Sherman L. A. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):11993–11997. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson T. M., Mattes M. J., Roux L., Old L. J., Lloyd K. O. Pigmentation-associated glycoprotein of human melanomas and melanocytes: definition with a mouse monoclonal antibody. J Invest Dermatol. 1985 Aug;85(2):169–174. doi: 10.1111/1523-1747.ep12276608. [DOI] [PubMed] [Google Scholar]
- Thomson T. M., Real F. X., Murakami S., Cordon-Cardo C., Old L. J., Houghton A. N. Differentiation antigens of melanocytes and melanoma: analysis of melanosome and cell surface markers of human pigmented cells with monoclonal antibodies. J Invest Dermatol. 1988 Apr;90(4):459–466. doi: 10.1111/1523-1747.ep12460906. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Gonzales M. I., Parkhurst M., Li Y. F., Southwood S., Sette A., Rosenberg S. A., Robbins P. F. Melanoma-specific CD4+ T cells recognize nonmutated HLA-DR-restricted tyrosinase epitopes. J Exp Med. 1996 May 1;183(5):1965–1971. doi: 10.1084/jem.183.5.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S. L., Rivoltini L., Mancini M., Markus N. R., Robbins P. F., Kawakami Y., Rosenberg S. A. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9461–9465. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian S. L., Rivoltini L., Mancini M., Ng J., Hartzman R. J., Rosenberg S. A. Melanoma-specific CD4+ T lymphocytes recognize human melanoma antigens processed and presented by Epstein-Barr virus-transformed B cells. Int J Cancer. 1994 Jul 1;58(1):69–79. doi: 10.1002/ijc.2910580113. [DOI] [PubMed] [Google Scholar]
- Topalian S. L., Solomon D., Avis F. P., Chang A. E., Freerksen D. L., Linehan W. M., Lotze M. T., Robertson C. N., Seipp C. A., Simon P. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988 May;6(5):839–853. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- Traversari C., van der Bruggen P., Luescher I. F., Lurquin C., Chomez P., Van Pel A., De Plaen E., Amar-Costesec A., Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992 Nov 1;176(5):1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Elsas A., Nijman H. W., Van der Minne C. E., Mourer J. S., Kast W. M., Melief C. J., Schrier P. I. Induction and characterization of cytotoxic T-lymphocytes recognizing a mutated p21ras peptide presented by HLA-A*0201. Int J Cancer. 1995 May 4;61(3):389–396. doi: 10.1002/ijc.2910610319. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B., Peeters O., De Backer O., Gaugler B., Lucas S., Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995 Sep 1;182(3):689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visseren M. J., van Elsas A., van der Voort E. I., Ressing M. E., Kast W. M., Schrier P. I., Melief C. J. CTL specific for the tyrosinase autoantigen can be induced from healthy donor blood to lyse melanoma cells. J Immunol. 1995 Apr 15;154(8):3991–3998. [PubMed] [Google Scholar]
- Wang R. F., Appella E., Kawakami Y., Kang X., Rosenberg S. A. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996 Dec 1;184(6):2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R. F., Robbins P. F., Kawakami Y., Kang X. Q., Rosenberg S. A. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995 Feb 1;181(2):799–804. doi: 10.1084/jem.181.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölfel T., Hauer M., Schneider J., Serrano M., Wölfel C., Klehmann-Hieb E., De Plaen E., Hankeln T., Meyer zum Büschenfelde K. H., Beach D. A p16INK4a-insensitive CDK4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science. 1995 Sep 1;269(5228):1281–1284. doi: 10.1126/science.7652577. [DOI] [PubMed] [Google Scholar]
- Wölfel T., Van Pel A., Brichard V., Schneider J., Seliger B., Meyer zum Büschenfelde K. H., Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994 Mar;24(3):759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]
- Yoshino I., Peoples G. E., Goedegebuure P. S., Maziarz R., Eberlein T. J. Association of HER2/neu expression with sensitivity to tumor-specific CTL in human ovarian cancer. J Immunol. 1994 Mar 1;152(5):2393–2400. [PubMed] [Google Scholar]
- Young J. W., Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med. 1996 Jan 1;183(1):7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bruggen P., Bastin J., Gajewski T., Coulie P. G., Boël P., De Smet C., Traversari C., Townsend A., Boon T. A peptide encoded by human gene MAGE-3 and presented by HLA-A2 induces cytolytic T lymphocytes that recognize tumor cells expressing MAGE-3. Eur J Immunol. 1994 Dec;24(12):3038–3043. doi: 10.1002/eji.1830241218. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P., Szikora J. P., Boël P., Wildmann C., Somville M., Sensi M., Boon T. Autologous cytolytic T lymphocytes recognize a MAGE-1 nonapeptide on melanomas expressing HLA-Cw*1601. Eur J Immunol. 1994 Sep;24(9):2134–2140. doi: 10.1002/eji.1830240930. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P., Traversari C., Chomez P., Lurquin C., De Plaen E., Van den Eynde B., Knuth A., Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991 Dec 13;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]


