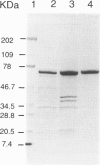
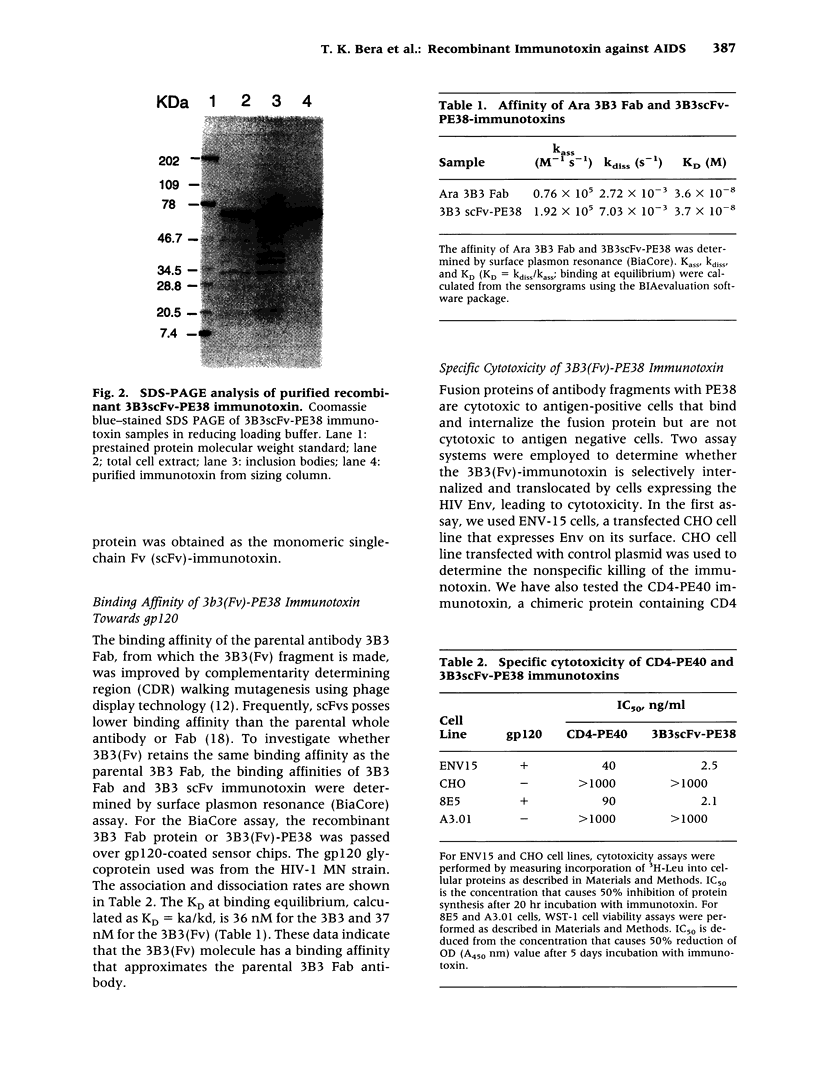
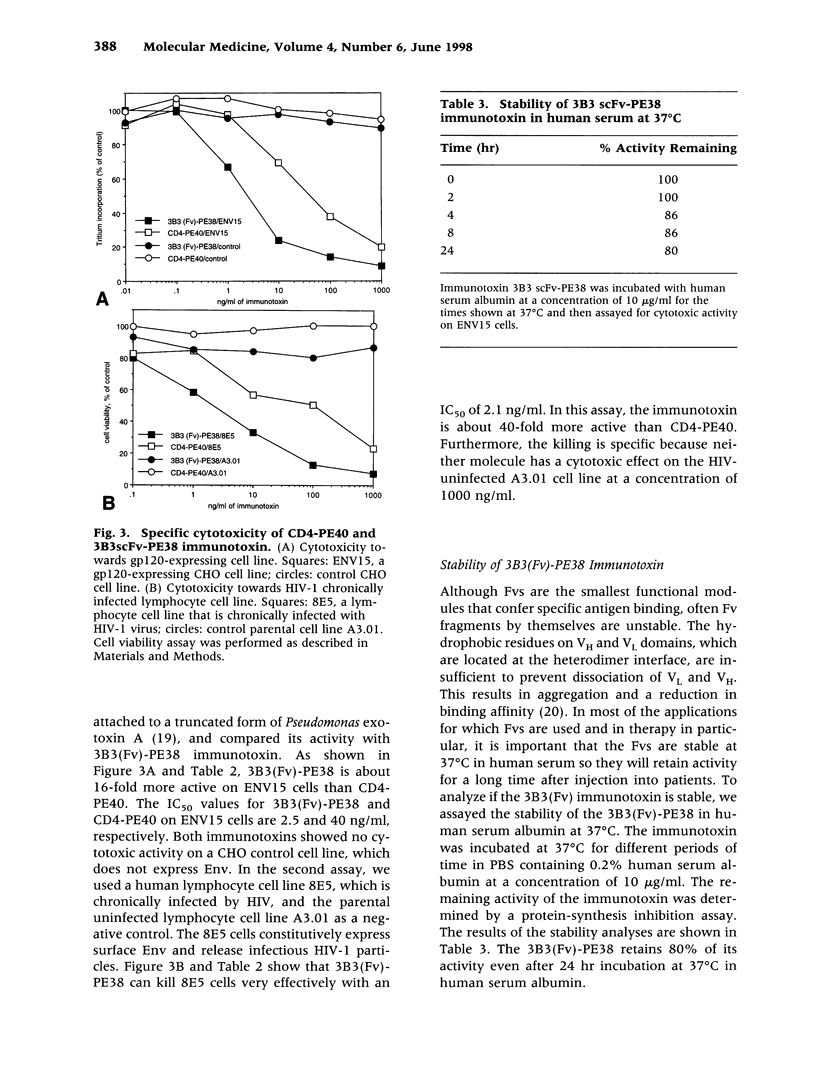
Abstract
BACKGROUND: 3B3 is a high-affinity anti-gp120 antibody that neutralizes a wide range of primary and laboratory isolates of HIV-1. The parental antibody was isolated from a combinatorial phage display library constructed from bone marrow RNA of an HIV-infected individual. We have generated a highly active immunotoxin using the 3B3 single-chain Fv (scFv) which can specifically kill lymphocytes infected by HIV-1. MATERIALS AND METHODS: We used recombinant DNA technology to clone the Fv fragment of 3B3 and produce a single-chain Fv (scFv). 3B3 scFv was then fused to a truncated version of Pseudomonas exotoxin A (PE38), giving rise to a recombinant immunotoxin 3B3(Fv)-PE38 that was expressed in E. coli and purified to near homogeneity. RESULTS: 3B3(Fv)-PE38 binds with the same affinity as the parental Fab antibody to the MN strain of gp120. The immunotoxin specifically kills a gp120-expressing transfected cell line and a chronically HIV-infected lymphocytic cell line. The immunotoxin is very stable at 37 degrees C, retaining 80% of its original activity after 24 hr. CONCLUSIONS: Potent immunotoxins such as 3B3(Fv)-PE38 could be utilized in combination with multidrug cocktails that limit viral replication to help reduce viral reservoirs in patients with AIDS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashorn P., Moss B., Weinstein J. N., Chaudhary V. K., FitzGerald D. J., Pastan I., Berger E. A. Elimination of infectious human immunodeficiency virus from human T-cell cultures by synergistic action of CD4-Pseudomonas exotoxin and reverse transcriptase inhibitors. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8889–8893. doi: 10.1073/pnas.87.22.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas C. F., 3rd, Hu D., Dunlop N., Sawyer L., Cababa D., Hendry R. M., Nara P. L., Burton D. R. In vitro evolution of a neutralizing human antibody to human immunodeficiency virus type 1 to enhance affinity and broaden strain cross-reactivity. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3809–3813. doi: 10.1073/pnas.91.9.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Brinkmann U., Pai L. H., FitzGerald D. J., Willingham M., Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Pastan I., Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992 Sep;205(2):263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- Burton D. R., Barbas C. F., 3rd, Persson M. A., Koenig S., Chanock R. M., Lerner R. A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton D. R., Pyati J., Koduri R., Sharp S. J., Thornton G. B., Parren P. W., Sawyer L. S., Hendry R. M., Dunlop N., Nara P. L. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994 Nov 11;266(5187):1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- Cavert W., Notermans D. W., Staskus K., Wietgrefe S. W., Zupancic M., Gebhard K., Henry K., Zhang Z. Q., Mills R., McDade H. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science. 1997 May 9;276(5314):960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Davey R. T., Jr, Boenning C. M., Herpin B. R., Batts D. H., Metcalf J. A., Wathen L., Cox S. R., Polis M. A., Kovacs J. A., Falloon J. Use of recombinant soluble CD4 Pseudomonas exotoxin, a novel immunotoxin, for treatment of persons infected with human immunodeficiency virus. J Infect Dis. 1994 Nov;170(5):1180–1188. doi: 10.1093/infdis/170.5.1180. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., 2nd, McKenna P. M., Emini E. A., Chan C. P., Patel M. D., Gupta S. K., Mark G. E., 3rd, Barbas C. F., 3rd, Burton D. R., Conley A. J. Recombinant human monoclonal antibody IgG1b12 neutralizes diverse human immunodeficiency virus type 1 primary isolates. AIDS Res Hum Retroviruses. 1997 May 1;13(7):575–582. doi: 10.1089/aid.1997.13.575. [DOI] [PubMed] [Google Scholar]
- Pai L. H., Wittes R., Setser A., Willingham M. C., Pastan I. Treatment of advanced solid tumors with immunotoxin LMB-1: an antibody linked to Pseudomonas exotoxin. Nat Med. 1996 Mar;2(3):350–353. doi: 10.1038/nm0396-350. [DOI] [PubMed] [Google Scholar]
- Pastan I. H., Pai L. H., Brinkmann U., Fitzgerald D. J. Recombinant toxins: new therapeutic agents for cancer. Ann N Y Acad Sci. 1995 Jun 30;758:345–354. doi: 10.1111/j.1749-6632.1995.tb24840.x. [DOI] [PubMed] [Google Scholar]
- Perelson A. S., Essunger P., Cao Y., Vesanen M., Hurley A., Saksela K., Markowitz M., Ho D. D. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997 May 8;387(6629):188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- Pincus S. H., Cole R. L., Hersh E. M., Lake D., Masuho Y., Durda P. J., McClure J. In vitro efficacy of anti-HIV immunotoxins targeted by various antibodies to the envelope protein. J Immunol. 1991 Jun 15;146(12):4315–4324. [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ramachandran R. V., Katzenstein D. A., Wood R., Batts D. H., Merigan T. C. Failure of short-term CD4-PE40 infusions to reduce virus load in human immunodeficiency virus-infected persons. J Infect Dis. 1994 Oct;170(4):1009–1013. doi: 10.1093/infdis/170.4.1009. [DOI] [PubMed] [Google Scholar]
- Reiter Y., Brinkmann U., Lee B., Pastan I. Engineering antibody Fv fragments for cancer detection and therapy: disulfide-stabilized Fv fragments. Nat Biotechnol. 1996 Oct;14(10):1239–1245. doi: 10.1038/nbt1096-1239. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Thrush G. R., Lark L. R., Clinchy B. C., Vitetta E. S. Immunotoxins: an update. Annu Rev Immunol. 1996;14:49–71. doi: 10.1146/annurev.immunol.14.1.49. [DOI] [PubMed] [Google Scholar]
- Till M. A., Ghetie V., Gregory T., Patzer E. J., Porter J. P., Uhr J. W., Capon D. J., Vitetta E. S. HIV-infected cells are killed by rCD4-ricin A chain. Science. 1988 Nov 25;242(4882):1166–1168. doi: 10.1126/science.2847316. [DOI] [PubMed] [Google Scholar]
- Till M. A., Zolla-Pazner S., Gorny M. K., Patton J. S., Uhr J. W., Vitetta E. S. Human immunodeficiency virus-infected T cells and monocytes are killed by monoclonal human anti-gp41 antibodies coupled to ricin A chain. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1987–1991. doi: 10.1073/pnas.86.6.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trkola A., Pomales A. B., Yuan H., Korber B., Maddon P. J., Allaway G. P., Katinger H., Barbas C. F., 3rd, Burton D. R., Ho D. D. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995 Nov;69(11):6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]