Abstract
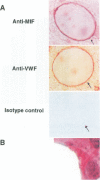
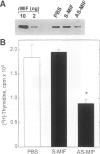
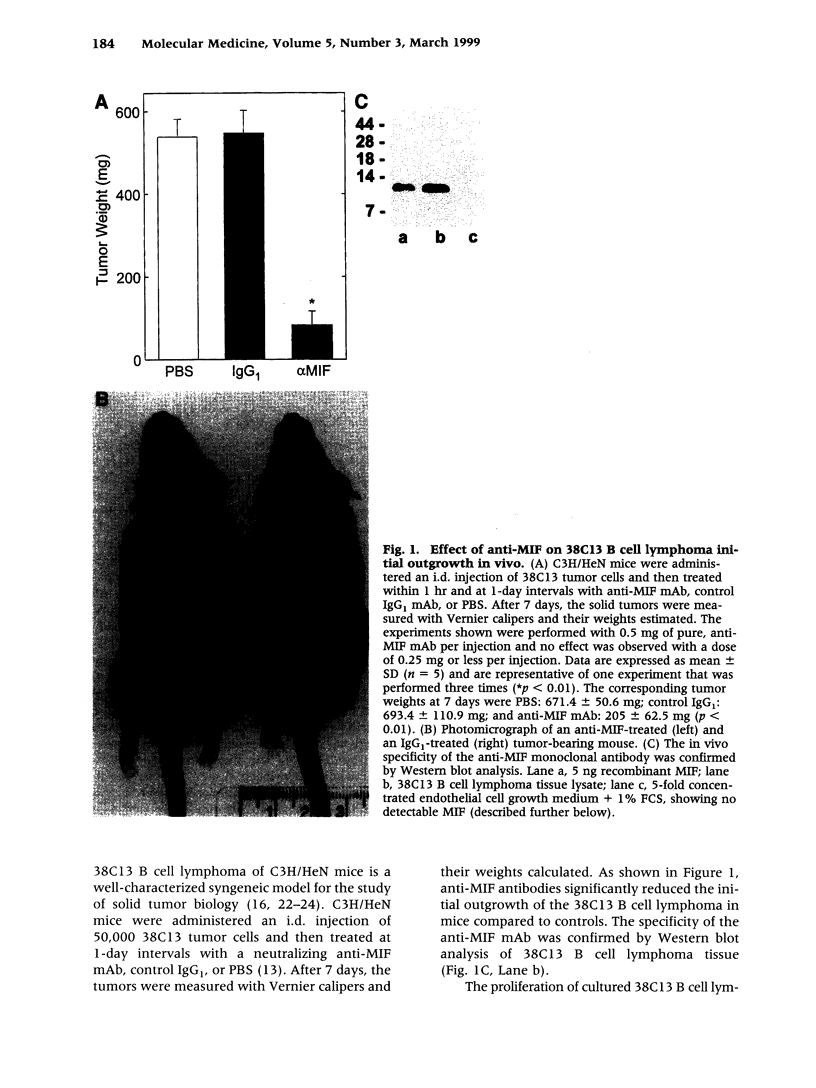
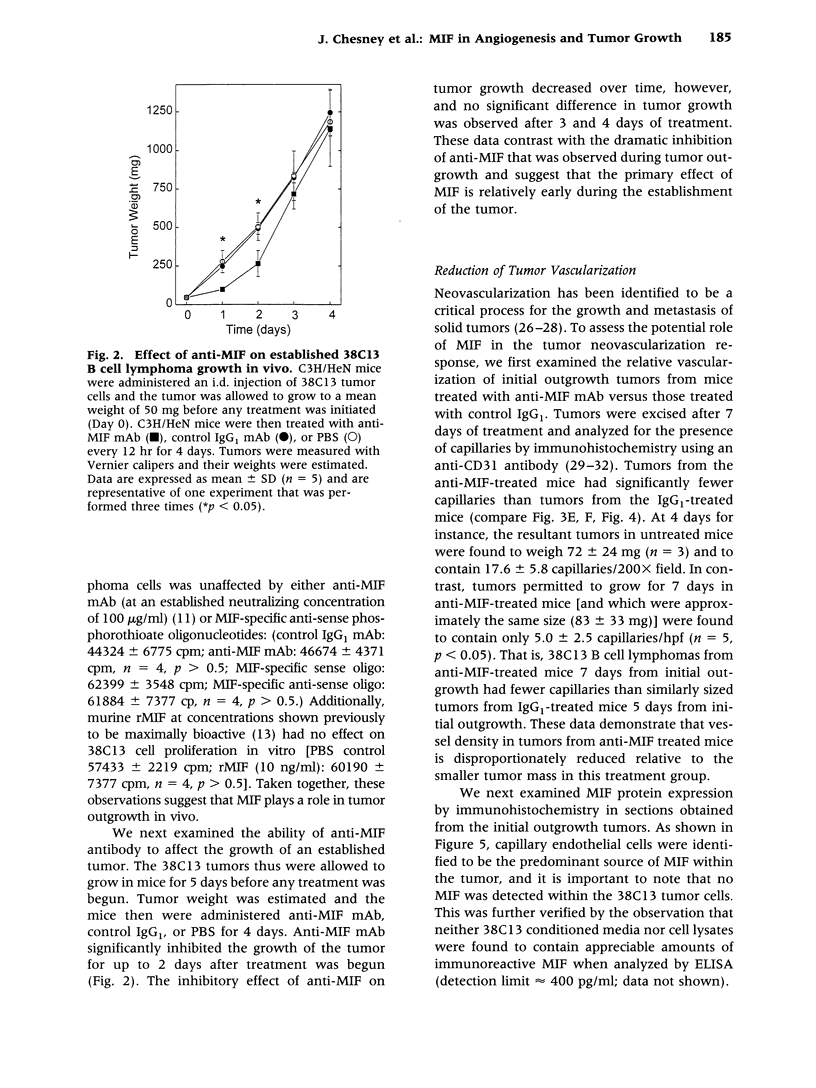
BACKGROUND: Macrophage migration inhibitory factor (MIF) has been shown to counterregulate glucocorticoid action and to play an essential role in the activation of macrophages and T cells in vivo. MIF also may function as an autocrine growth factor in certain cell systems. We have explored the role of MIF in the growth of the 38C13 B cell lymphoma in C3H/HeN mice, a well-characterized syngeneic model for the study of solid tumor biology. MATERIALS AND METHODS: Tumor-bearing mice were treated with a neutralizing anti-MIF monoclonal antibody and the tumor response assessed grossly and histologically. Tumor capillaries were enumerated by immunohistochemistry and analyzed for MIF expression. The effect of MIF on endothelial cell proliferation was studied in vitro, utilizing both specific antibody and antisense oligonucleotide constructs. The role of MIF in angiogenesis also was examined in a standard Matrigel model of new blood vessel formation in vivo. RESULTS: The administration of anti-MIF monoclonal antibodies to mice was found to reduce significantly the growth and the vascularization of the 38C13 B cell lymphoma. By immunohistochemistry, MIF was expressed predominantly within the tumor-associated neovasculature. Cultured microvascular endothelial cells, but not 38C13 B cells, produced MIF protein and required its activity for proliferation in vitro. Anti-MIF monoclonal antibody also was found to markedly inhibit the neovascularization response elicited by Matrigel implantation. CONCLUSION: These data significantly expand the role of MIF in host responses, and suggest a new target for the development of anti-neoplastic agents that inhibit tumor neovascularization.
Full text
PDF




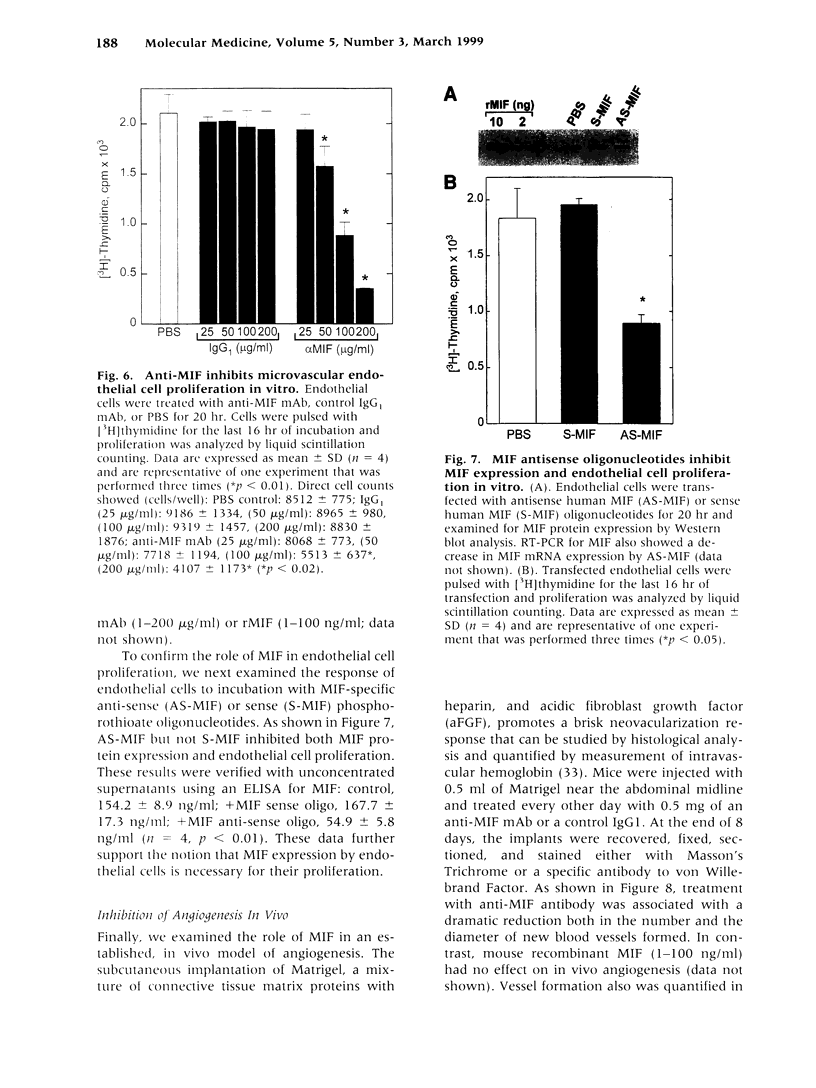
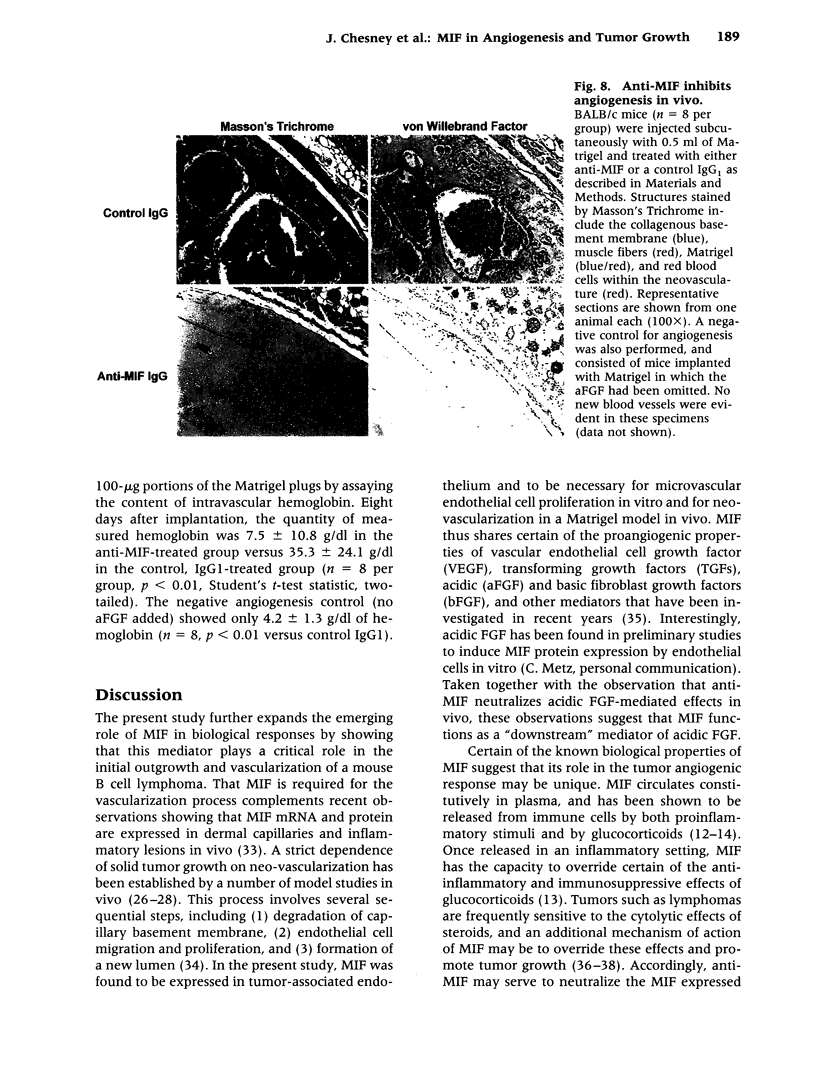


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arenberg D. A., Kunkel S. L., Polverini P. J., Glass M., Burdick M. D., Strieter R. M. Inhibition of interleukin-8 reduces tumorigenesis of human non-small cell lung cancer in SCID mice. J Clin Invest. 1996 Jun 15;97(12):2792–2802. doi: 10.1172/JCI118734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacher M., Meinhardt A., Lan H. Y., Mu W., Metz C. N., Chesney J. A., Calandra T., Gemsa D., Donnelly T., Atkins R. C. Migration inhibitory factor expression in experimentally induced endotoxemia. Am J Pathol. 1997 Jan;150(1):235–246. [PMC free article] [PubMed] [Google Scholar]
- Bacher M., Metz C. N., Calandra T., Mayer K., Chesney J., Lohoff M., Gemsa D., Donnelly T., Bucala R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7849–7854. doi: 10.1073/pnas.93.15.7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard J. A., Lyons R. M., Moses H. L. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990 Jun 1;1032(1):79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- Basham T. Y., Palladino M. A., Badger C. C., Bernstein I. D., Levy R., Merigan T. C. Comparison of combinations of interferons with tumor specific and nonspecific monoclonal antibodies as therapy for murine B- and T-cell lymphomas. Cancer Res. 1988 Aug 1;48(15):4196–4200. [PubMed] [Google Scholar]
- Bergman Y., Haimovich J. Characterization of a carcinogen-induced murine B lymphocyte cell line of C3H/eB origin. Eur J Immunol. 1977 Jul;7(7):413–417. doi: 10.1002/eji.1830070702. [DOI] [PubMed] [Google Scholar]
- Bernhagen J., Bacher M., Calandra T., Metz C. N., Doty S. B., Donnelly T., Bucala R. An essential role for macrophage migration inhibitory factor in the tuberculin delayed-type hypersensitivity reaction. J Exp Med. 1996 Jan 1;183(1):277–282. doi: 10.1084/jem.183.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J., Calandra T., Mitchell R. A., Martin S. B., Tracey K. J., Voelter W., Manogue K. R., Cerami A., Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993 Oct 21;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bernhagen J., Mitchell R. A., Calandra T., Voelter W., Cerami A., Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994 Nov 29;33(47):14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Baldwin H. S., DeLisser H., Mickanin C., Shen H. M., Kennedy G., Chen A., Edelman J. M., Albelda S. M. Cell adhesion receptors and early mammalian heart development: an overview. C R Acad Sci III. 1993 Sep;316(9):838–859. [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Metz C. N., Spiegel L. A., Bacher M., Donnelly T., Cerami A., Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995 Sep 7;377(6544):68–71. doi: 10.1038/377068a0. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Mitchell R. A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994 Jun 1;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra T., Spiegel L. A., Metz C. N., Bucala R. Macrophage migration inhibitory factor is a critical mediator of the activation of immune cells by exotoxins of Gram-positive bacteria. Proc Natl Acad Sci U S A. 1998 Sep 15;95(19):11383–11388. doi: 10.1073/pnas.95.19.11383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. J., Esserman L., Levy R. Immunotherapy of established murine B cell lymphoma. Combination of idiotype immunization and cyclophosphamide. J Immunol. 1988 Nov 1;141(9):3227–3233. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLisser H. M., Newman P. J., Albelda S. M. Platelet endothelial cell adhesion molecule (CD31). Curr Top Microbiol Immunol. 1993;184:37–45. doi: 10.1007/978-3-642-78253-4_3. [DOI] [PubMed] [Google Scholar]
- Fiorentino M. V. Lymphomas in the elderly. Leukemia. 1991;5 (Suppl 1):79–85. [PubMed] [Google Scholar]
- Folkman J., Cotran R. Relation of vascular proliferation to tumor growth. Int Rev Exp Pathol. 1976;16:207–248. [PubMed] [Google Scholar]
- Folkman J., D'Amore P. A. Blood vessel formation: what is its molecular basis? Cell. 1996 Dec 27;87(7):1153–1155. doi: 10.1016/s0092-8674(00)81810-3. [DOI] [PubMed] [Google Scholar]
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? G.H.A. Clowes memorial Award lecture. Cancer Res. 1986 Feb;46(2):467–473. [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Leapman S. B., Cotran R. S., Folkman J. Tumor dormancy in vivo by prevention of neovascularization. J Exp Med. 1972 Aug 1;136(2):261–276. doi: 10.1084/jem.136.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss P. E. New perspectives in the treatment of non-Hodgkin's lymphoma. Semin Oncol. 1992 Dec;19(6 Suppl 12):23–29. [PubMed] [Google Scholar]
- Kaminski M. S., Kitamura K., Maloney D. G., Levy R. Idiotype vaccination against murine B cell lymphoma. Inhibition of tumor immunity by free idiotype protein. J Immunol. 1987 Feb 15;138(4):1289–1296. [PubMed] [Google Scholar]
- Lang R. A., Burgess A. W. Autocrine growth factors and tumourigenic transformation. Immunol Today. 1990 Jul;11(7):244–249. doi: 10.1016/0167-5699(90)90098-t. [DOI] [PubMed] [Google Scholar]
- Muul L. M., Spiess P. J., Director E. P., Rosenberg S. A. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol. 1987 Feb 1;138(3):989–995. [PubMed] [Google Scholar]
- Passaniti A., Taylor R. M., Pili R., Guo Y., Long P. V., Haney J. A., Pauly R. R., Grant D. S., Martin G. R. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab Invest. 1992 Oct;67(4):519–528. [PubMed] [Google Scholar]
- Roberts A. B., Thompson N. L., Heine U., Flanders C., Sporn M. B. Transforming growth factor-beta: possible roles in carcinogenesis. Br J Cancer. 1988 Jun;57(6):594–600. doi: 10.1038/bjc.1988.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren E., Bucala R., Aman P., Jacobsson L., Odh G., Metz C. N., Rorsman H. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Mol Med. 1996 Jan;2(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- Sun H. W., Bernhagen J., Bucala R., Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1996 May 28;93(11):5191–5196. doi: 10.1073/pnas.93.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taetle R., Rosen F., Abramson I., Venditti J., Howell S. Use of nude mouse xenografts as preclinical drug screens: in vivo activity of established chemotherapeutic agents against melanoma and ovarian carcinoma xenografts. Cancer Treat Rep. 1987 Mar;71(3):297–304. [PubMed] [Google Scholar]
- Vecchi A., Garlanda C., Lampugnani M. G., Resnati M., Matteucci C., Stoppacciaro A., Schnurch H., Risau W., Ruco L., Mantovani A. Monoclonal antibodies specific for endothelial cells of mouse blood vessels. Their application in the identification of adult and embryonic endothelium. Eur J Cell Biol. 1994 Apr;63(2):247–254. [PubMed] [Google Scholar]
- Waeber G., Calandra T., Roduit R., Haefliger J. A., Bonny C., Thompson N., Thorens B., Temler E., Meinhardt A., Bacher M. Insulin secretion is regulated by the glucose-dependent production of islet beta cell macrophage migration inhibitory factor. Proc Natl Acad Sci U S A. 1997 Apr 29;94(9):4782–4787. doi: 10.1073/pnas.94.9.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Muller W. A. Molecular cloning and adhesive properties of murine platelet/endothelial cell adhesion molecule 1. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5569–5573. doi: 10.1073/pnas.90.12.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zackheim H. S. Treatment of cutaneous T-cell lymphoma. Semin Dermatol. 1994 Sep;13(3):207–215. [PubMed] [Google Scholar]