Abstract
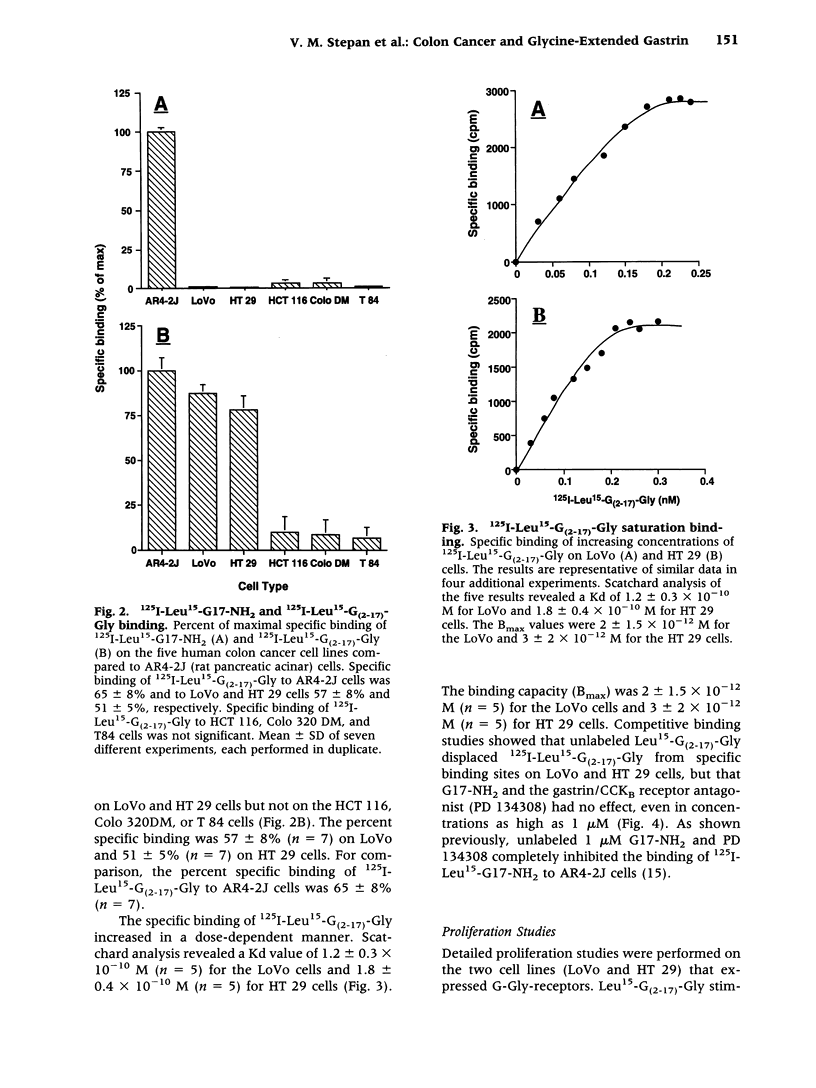
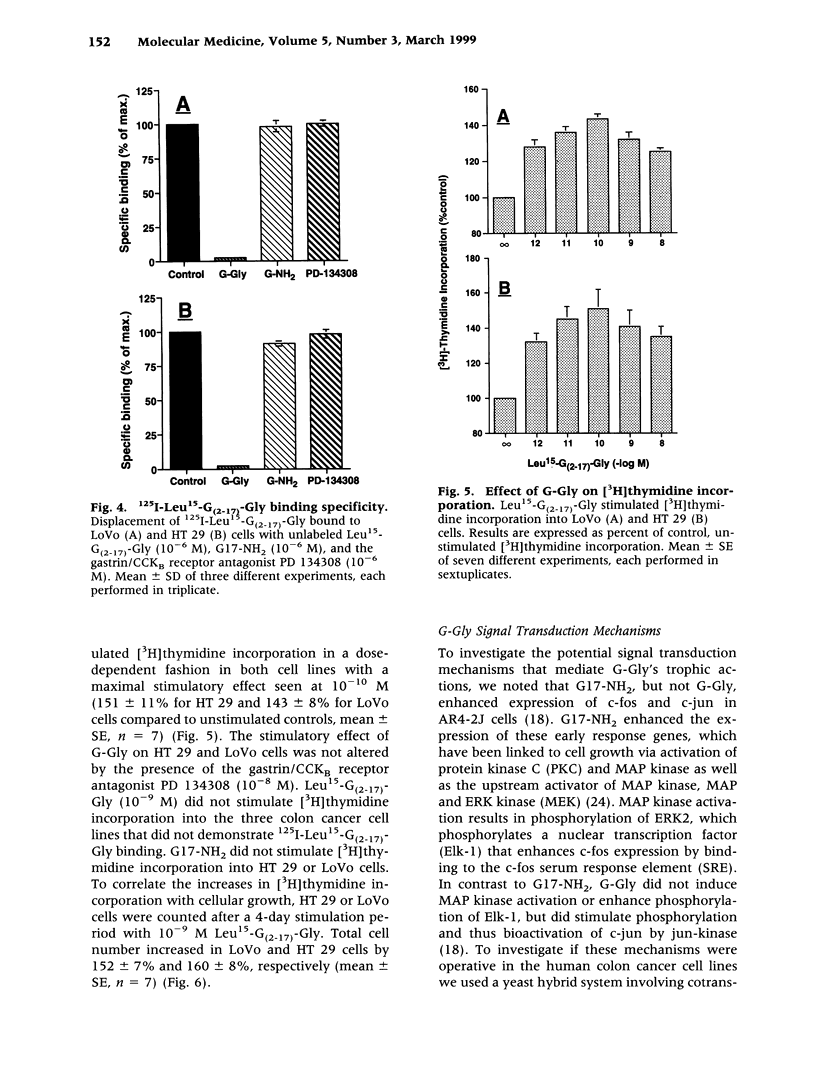
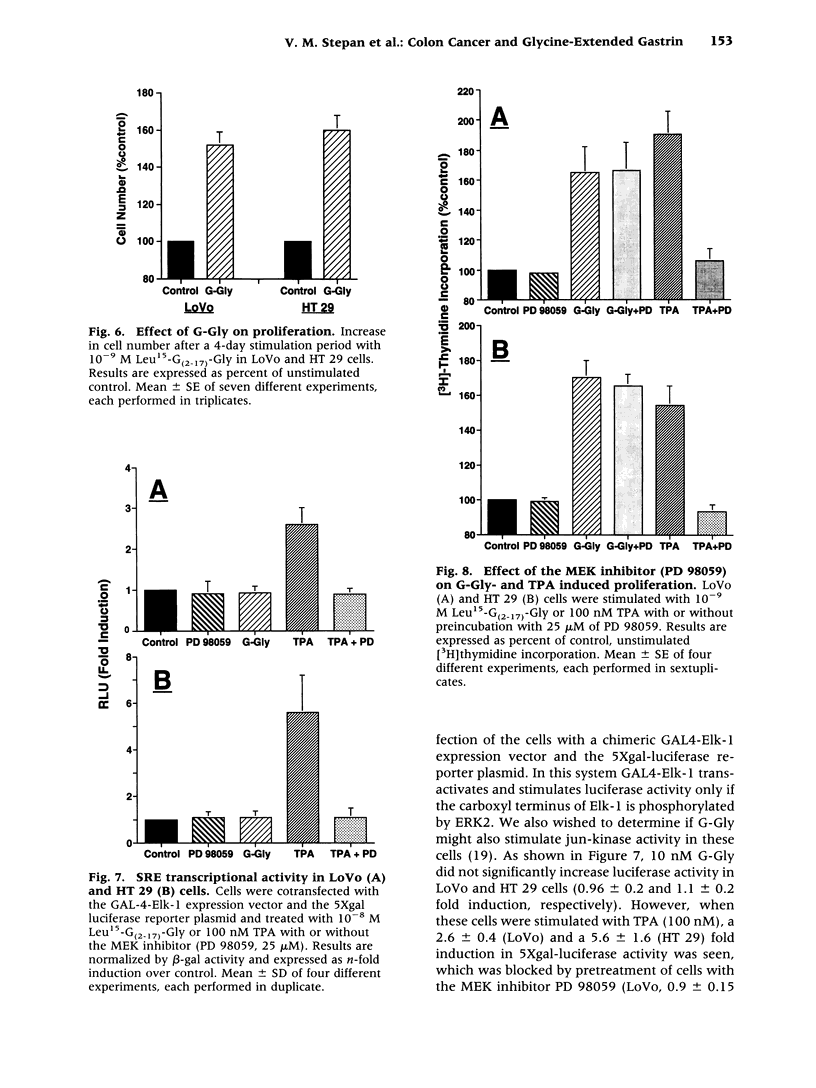
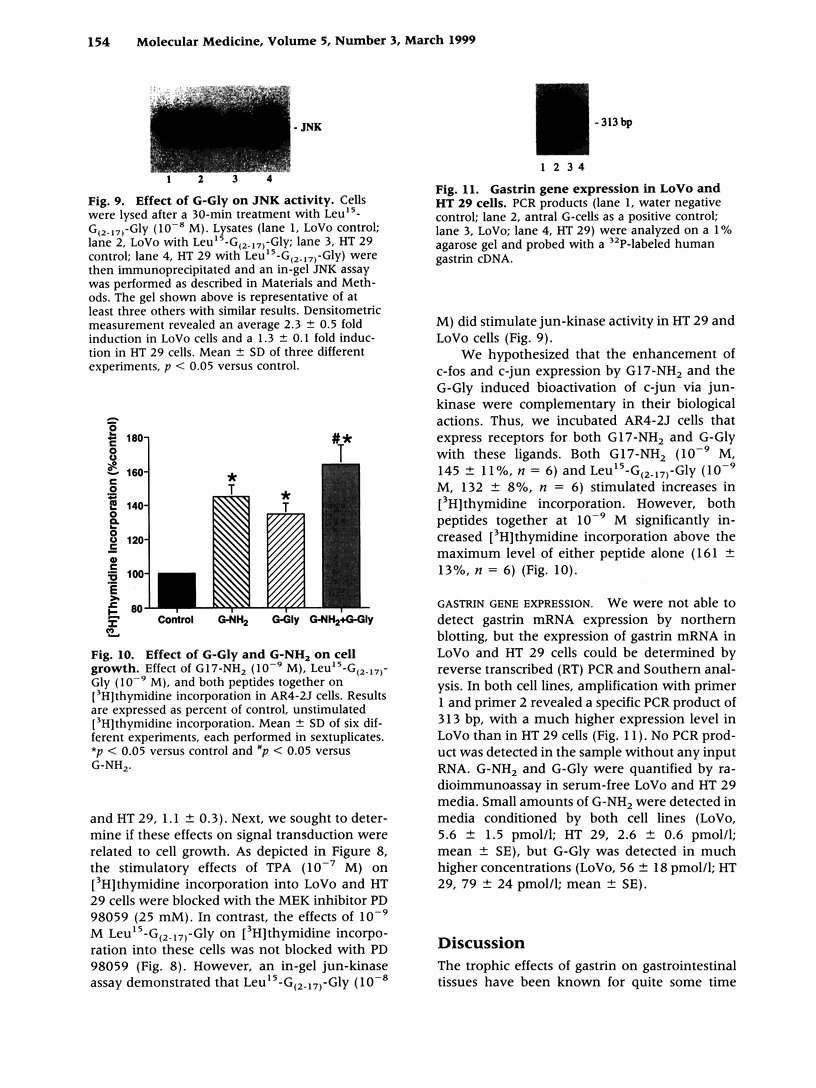
BACKGROUND: Since human colon cancers often contain significant quantities of progastrin-processing intermediates, we sought to explore the possibility that the biosynthetic precursor of fully processed amidated gastrin, glycine-extended gastrin, may exert trophic effects on human colonic cancer cells. MATERIALS AND METHODS: Binding of radiolabeled glycine-extended and amidated gastrins was assessed on five human cancer cell lines: LoVo, HT 29, HCT 116, Colo 320DM, and T 84. Trophic actions of the peptides were assessed by increases in [3H]thymidine incorporation and cell number. Gastrin expression was determined by northern blot and radioimmunoassay. RESULTS: Amidated gastrin did not bind to or stimulate the growth of any of the five cell lines. In contrast, saturable binding of radiolabeled glycine-extended gastrin was seen on LoVo and HT 29 cells that was not inhibited by amidated gastrin (10(-6) M) nor by a gastrin/CCKB receptor antagonist (PD 134308). Glycine-extended gastrin induced a dose-dependent increase in [3H]thymidine uptake in LoVo (143 +/- 8% versus control at 10(-10) M) and HT 29 (151 +/- 11% versus control at 10(-10) M) cells that was not inhibited by PD 134308 or by a mitogen-activated protein (MAP) or ERK kinase (MEK) inhibitor (PD 98509). Glycine-extended gastrin did stimulate jun-kinase activity in LoVo and HT 29 cells. The two cell lines expressed the gastrin gene at low levels and secreted small amounts of amidated gastrin and glycine-extended gastrin into the media. CONCLUSIONS: Glycine-extended gastrin receptors are present on human colon cancer cells that mediate glycine-extended gastrin's trophic effects via a MEK-independent mechanism. This suggests that glycine-extended gastrin and its novel receptors may play a role in colon cancer cell growth.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessi D. R., Cuenda A., Cohen P., Dudley D. T., Saltiel A. R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995 Nov 17;270(46):27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Blackmore M., Hirst B. H. Autocrine stimulation of growth of AR4-2J rat pancreatic tumour cells by gastrin. Br J Cancer. 1992 Jul;66(1):32–38. doi: 10.1038/bjc.1992.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boel E., Vuust J., Norris F., Norris K., Wind A., Rehfeld J. F., Marcker K. A. Molecular cloning of human gastrin cDNA: evidence for evolution of gastrin by gene duplication. Proc Natl Acad Sci U S A. 1983 May;80(10):2866–2869. doi: 10.1073/pnas.80.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bold R. J., Ishizuka J., Townsend C. M., Jr, Thompson J. C. Gastrin stimulates growth of human colon cancer cells via a receptor other than CCK-A or CCK-B. Biochem Biophys Res Commun. 1994 Aug 15;202(3):1222–1226. doi: 10.1006/bbrc.1994.2061. [DOI] [PubMed] [Google Scholar]
- Cavigelli M., Dolfi F., Claret F. X., Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995 Dec 1;14(23):5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu M., Nielsen F. C., Franzén L., Rehfeld J. F., Holst J. J., Borch K. Effect of endogenous hypergastrinemia on gastrin receptor expressing human colon carcinoma transplanted to athymic rats. Gastroenterology. 1995 Nov;109(5):1415–1420. doi: 10.1016/0016-5085(95)90625-8. [DOI] [PubMed] [Google Scholar]
- Ciccotosto G. D., McLeish A., Hardy K. J., Shulkes A. Expression, processing, and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995 Oct;109(4):1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- Clark G. J., Westwick J. K., Der C. J. p120 GAP modulates Ras activation of Jun kinases and transformation. J Biol Chem. 1997 Jan 17;272(3):1677–1681. doi: 10.1074/jbc.272.3.1677. [DOI] [PubMed] [Google Scholar]
- Davis R. J. The mitogen-activated protein kinase signal transduction pathway. J Biol Chem. 1993 Jul 15;268(20):14553–14556. [PubMed] [Google Scholar]
- Del Valle J., Sugano K., Yamada T. Progastrin and its glycine-extended posttranslational processing intermediates in human gastrointestinal tissues. Gastroenterology. 1987 Jun;92(6):1908–1912. doi: 10.1016/0016-5085(87)90623-8. [DOI] [PubMed] [Google Scholar]
- DelValle J., Sugano K., Yamada T. Glycine-extended processing intermediates of gastrin and cholecystokinin in human plasma. Gastroenterology. 1989 Nov;97(5):1159–1163. doi: 10.1016/0016-5085(89)91685-5. [DOI] [PubMed] [Google Scholar]
- Dickinson C. J. Relationship of gastrin processing to colon cancer. Gastroenterology. 1995 Oct;109(4):1384–1388. doi: 10.1016/0016-5085(95)90603-7. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991 Feb 1;192(2):262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Hibi M., Lin A., Smeal T., Minden A., Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993 Nov;7(11):2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Higashide S., Gomez G., Greeley G. H., Jr, Townsend C. M., Jr, Thompson J. C. Glycine-extended gastrin potentiates gastrin-stimulated gastric acid secretion in rats. Am J Physiol. 1996 Jan;270(1 Pt 1):G220–G224. doi: 10.1152/ajpgi.1996.270.1.G220. [DOI] [PubMed] [Google Scholar]
- Hilsted L. Glycine-extended gastrin precursors. Regul Pept. 1991 Nov 26;36(3):323–343. doi: 10.1016/0167-0115(91)90067-q. [DOI] [PubMed] [Google Scholar]
- Hoosein N. M., Kiener P. A., Curry R. C., Rovati L. C., McGilbra D. K., Brattain M. G. Antiproliferative effects of gastrin receptor antagonists and antibodies to gastrin on human colon carcinoma cell lines. Cancer Res. 1988 Dec 15;48(24 Pt 1):7179–7183. [PubMed] [Google Scholar]
- Jensen S., Borch K., Hilsted L., Rehfeld J. F. Progastrin processing during antral G-cell hypersecretion in humans. Gastroenterology. 1989 Apr;96(4):1063–1070. doi: 10.1016/0016-5085(89)91624-7. [DOI] [PubMed] [Google Scholar]
- Johnson L. R. Gastrointestinal hormones and their functions. Annu Rev Physiol. 1977;39:135–158. doi: 10.1146/annurev.ph.39.030177.001031. [DOI] [PubMed] [Google Scholar]
- Johnson L. R. New aspects of the trophic action of gastrointestinal hormones. Gastroenterology. 1977 Apr;72(4 PT2):788–792. [PubMed] [Google Scholar]
- Kaise M., Muraoka A., Seva C., Takeda H., Dickinson C. J., Yamada T. Glycine-extended progastrin processing intermediates induce H+,K(+)-ATPase alpha-subunit gene expression through a novel receptor. J Biol Chem. 1995 May 12;270(19):11155–11160. doi: 10.1074/jbc.270.19.11155. [DOI] [PubMed] [Google Scholar]
- Kochman M. L., DelValle J., Dickinson C. J., Boland C. R. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem Biophys Res Commun. 1992 Dec 15;189(2):1165–1169. doi: 10.1016/0006-291x(92)92326-s. [DOI] [PubMed] [Google Scholar]
- Kusyk C. J., McNiel N. O., Johnson L. R. Stimulation of growth of a colon cancer cell line by gastrin. Am J Physiol. 1986 Nov;251(5 Pt 1):G597–G601. doi: 10.1152/ajpgi.1986.251.5.G597. [DOI] [PubMed] [Google Scholar]
- Marais R., Wynne J., Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993 Apr 23;73(2):381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- Matsumoto M., Park J., Sugano K., Yamada T. Biological activity of progastrin posttranslational processing intermediates. Am J Physiol. 1987 Mar;252(3 Pt 1):G315–G319. doi: 10.1152/ajpgi.1987.252.3.G315. [DOI] [PubMed] [Google Scholar]
- McGregor D. B., Jones R. D., Karlin D. A., Romsdahl M. M. Trophic effects of gastrin on colorectal neoplasms in the rat. Ann Surg. 1982 Feb;195(2):219–223. doi: 10.1097/00000658-198202000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A., Lin A., Smeal T., Dérijard B., Cobb M., Davis R., Karin M. c-Jun N-terminal phosphorylation correlates with activation of the JNK subgroup but not the ERK subgroup of mitogen-activated protein kinases. Mol Cell Biol. 1994 Oct;14(10):6683–6688. doi: 10.1128/mcb.14.10.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A. A truncated isoform of human CCK-B/gastrin receptor generated by alternative usage of a novel exon. Biochem Biophys Res Commun. 1995 Mar 8;208(1):230–237. doi: 10.1006/bbrc.1995.1328. [DOI] [PubMed] [Google Scholar]
- Nemeth J., Taylor B., Pauwels S., Varro A., Dockray G. J. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993 Jan;34(1):90–95. doi: 10.1136/gut.34.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nègre F., Fagot-Revurat P., Bouisson M., Rehfeld J. F., Vaysse N., Pradayrol L. Autocrine stimulation of AR4-2J rat pancreatic tumor cell growth by glycine-extended gastrin. Int J Cancer. 1996 May 29;66(5):653–658. doi: 10.1002/(SICI)1097-0215(19960529)66:5<653::AID-IJC12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Oscarson J. E., Veen H. F., Ross J. S., Malt R. A. Dimethylhydrazine-induced colonic neoplasia: dissociation from endogenous gastrin levels. Surgery. 1982 May;91(5):525–530. [PubMed] [Google Scholar]
- Rehfeld J. F. Gastrin and colorectal cancer: a never-ending dispute? Gastroenterology. 1995 Apr;108(4):1307–1310. doi: 10.1016/0016-5085(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F. Characterization of preprocholecystokinin products in the porcine cerebral cortex. Evidence of different processing pathways. J Biol Chem. 1986 May 5;261(13):5832–5840. [PubMed] [Google Scholar]
- Scemama J. L., Fourmy D., Zahidi A., Pradayrol L., Susini C., Ribet A. Characterisation of gastrin receptors on a rat pancreatic acinar cell line (AR42J). A possible model for studying gastrin mediated cell growth and proliferation. Gut. 1987;28 (Suppl):233–236. doi: 10.1136/gut.28.suppl.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seva C., Dickinson C. J., Yamada T. Growth-promoting effects of glycine-extended progastrin. Science. 1994 Jul 15;265(5170):410–412. doi: 10.1126/science.8023165. [DOI] [PubMed] [Google Scholar]
- Singh P., Owlia A., Espeijo R., Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin (CCK)-A and CCK-B receptors. J Biol Chem. 1995 Apr 14;270(15):8429–8438. doi: 10.1074/jbc.270.15.8429. [DOI] [PubMed] [Google Scholar]
- Singh P., Owlia A., Varro A., Dai B., Rajaraman S., Wood T. Gastrin gene expression is required for the proliferation and tumorigenicity of human colon cancer cells. Cancer Res. 1996 Sep 15;56(18):4111–4115. [PubMed] [Google Scholar]
- Singh P., Xu Z., Dai B., Rajaraman S., Rubin N., Dhruva B. Incomplete processing of progastrin expressed by human colon cancer cells: role of noncarboxyamidated gastrins. Am J Physiol. 1994 Mar;266(3 Pt 1):G459–G468. doi: 10.1152/ajpgi.1994.266.3.G459. [DOI] [PubMed] [Google Scholar]
- Smith J. P., Stock E. A., Wotring M. G., McLaughlin P. J., Zagon I. S. Characterization of the CCK-B/gastrin-like receptor in human colon cancer. Am J Physiol. 1996 Sep;271(3 Pt 2):R797–R805. doi: 10.1152/ajpregu.1996.271.3.R797. [DOI] [PubMed] [Google Scholar]
- Sugano K., Aponte G. W., Yamada T. Identification and characterization of glycine-extended post-translational processing intermediates of progastrin in porcine stomach. J Biol Chem. 1985 Sep 25;260(21):11724–11729. [PubMed] [Google Scholar]
- Sugano K., Park J., Dobbins W. O., Yamada T. Glycine-extended progastrin processing intermediates: accumulation and cosecretion with gastrin. Am J Physiol. 1987 Oct;253(4 Pt 1):G502–G507. doi: 10.1152/ajpgi.1987.253.4.G502. [DOI] [PubMed] [Google Scholar]
- Tatsuta M., Yamamura H., Iishi H., Noguchi S., Ichii M., Taniguchi H. Gastrin has no promoting effect on chemically induced colonic tumors in Wistar rats. Eur J Cancer Clin Oncol. 1985 Jun;21(6):741–744. doi: 10.1016/0277-5379(85)90272-x. [DOI] [PubMed] [Google Scholar]
- Thorburn C. M., Friedman G. D., Dickinson C. J., Vogelman J. H., Orentreich N., Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998 Aug;115(2):275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- Todisco A., Takeuchi Y., Seva C., Dickinson C. J., Yamada T. Gastrin and glycine-extended progastrin processing intermediates induce different programs of early gene activation. J Biol Chem. 1995 Nov 24;270(47):28337–28341. doi: 10.1074/jbc.270.47.28337. [DOI] [PubMed] [Google Scholar]
- Treisman R. Journey to the surface of the cell: Fos regulation and the SRE. EMBO J. 1995 Oct 16;14(20):4905–4913. doi: 10.1002/j.1460-2075.1995.tb00173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Solinge W. W., Nielsen F. C., Friis-Hansen L., Falkmer U. G., Rehfeld J. F. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993 Apr;104(4):1099–1107. doi: 10.1016/0016-5085(93)90279-l. [DOI] [PubMed] [Google Scholar]
- Varro A., Voronina S., Dockray G. J. Pathways of processing of the gastrin precursor in rat antral mucosa. J Clin Invest. 1995 Apr;95(4):1642–1649. doi: 10.1172/JCI117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. C., Koh T. J., Varro A., Cahill R. J., Dangler C. A., Fox J. G., Dockray G. J. Processing and proliferative effects of human progastrin in transgenic mice. J Clin Invest. 1996 Oct 15;98(8):1918–1929. doi: 10.1172/JCI118993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. A., Durrant L. G., Crosbie J. D., Morris D. L. The in vitro growth response of primary human colorectal and gastric cancer cells to gastrin. Int J Cancer. 1989 Apr 15;43(4):692–696. doi: 10.1002/ijc.2910430425. [DOI] [PubMed] [Google Scholar]
- Westwick J. K., Bielawska A. E., Dbaibo G., Hannun Y. A., Brenner D. A. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995 Sep 29;270(39):22689–22692. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- Westwick J. K., Weitzel C., Minden A., Karin M., Brenner D. A. Tumor necrosis factor alpha stimulates AP-1 activity through prolonged activation of the c-Jun kinase. J Biol Chem. 1994 Oct 21;269(42):26396–26401. [PubMed] [Google Scholar]
- Whitmarsh A. J., Shore P., Sharrocks A. D., Davis R. J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995 Jul 21;269(5222):403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- Wiborg O., Berglund L., Boel E., Norris F., Norris K., Rehfeld J. F., Marcker K. A., Vuust J. Structure of a human gastrin gene. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1067–1069. doi: 10.1073/pnas.81.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsett O. E., Townsend C. M., Jr, Glass E. J., Thompson J. C. Gastrin stimulates growth of colon cancer. Surgery. 1986 Mar;99(3):302–307. [PubMed] [Google Scholar]
- Winsett O. E., Townsend C. M., Jr, Glass E. J., Thompson J. C. Gastrin stimulates growth of colon cancer. Surgery. 1986 Mar;99(3):302–307. [PubMed] [Google Scholar]