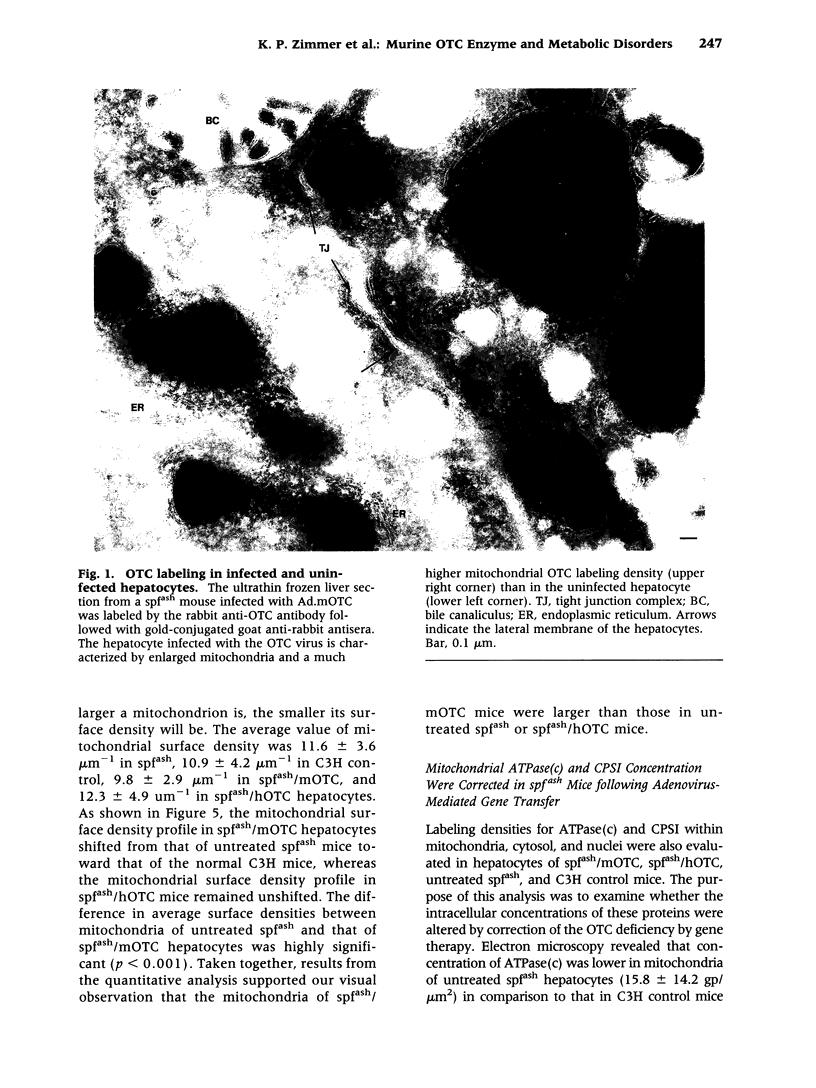
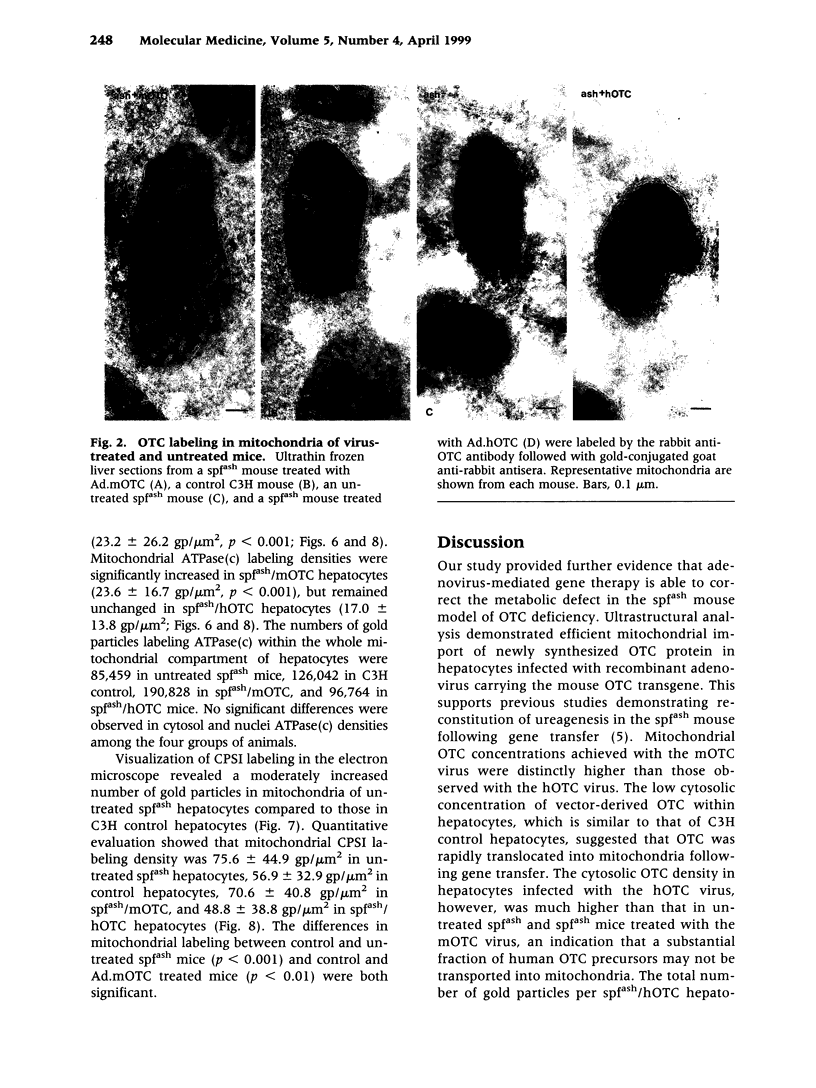
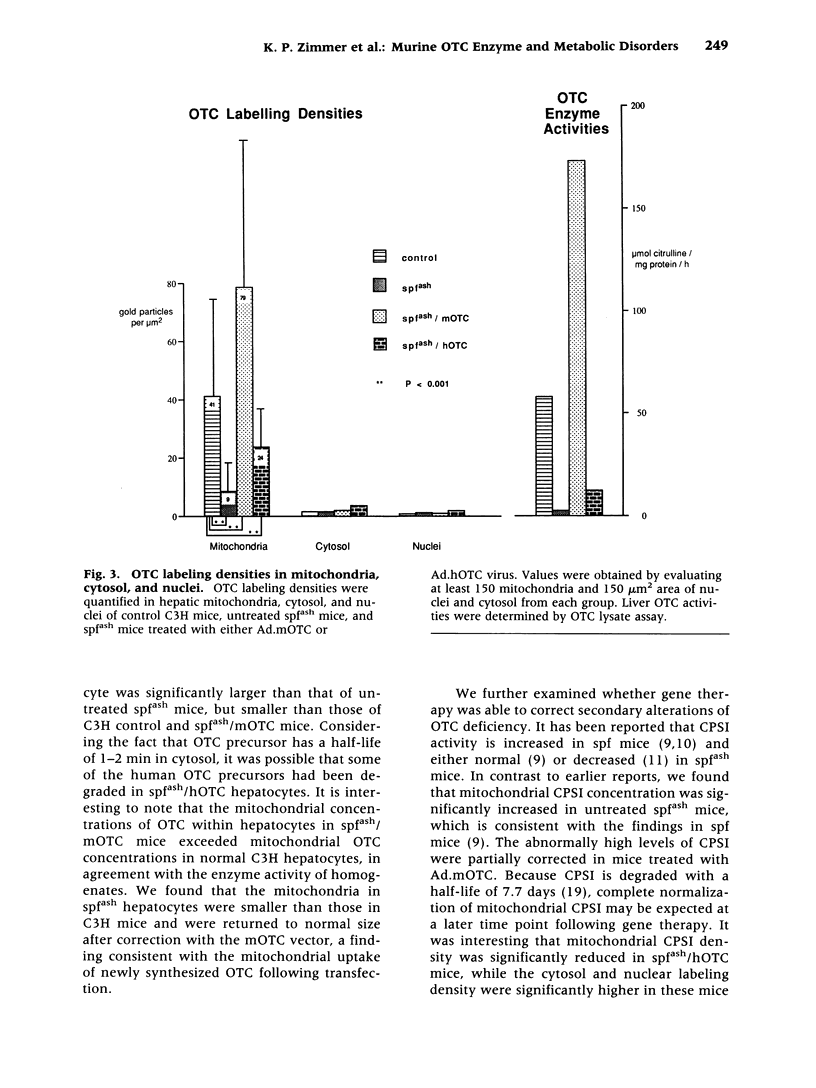
Abstract
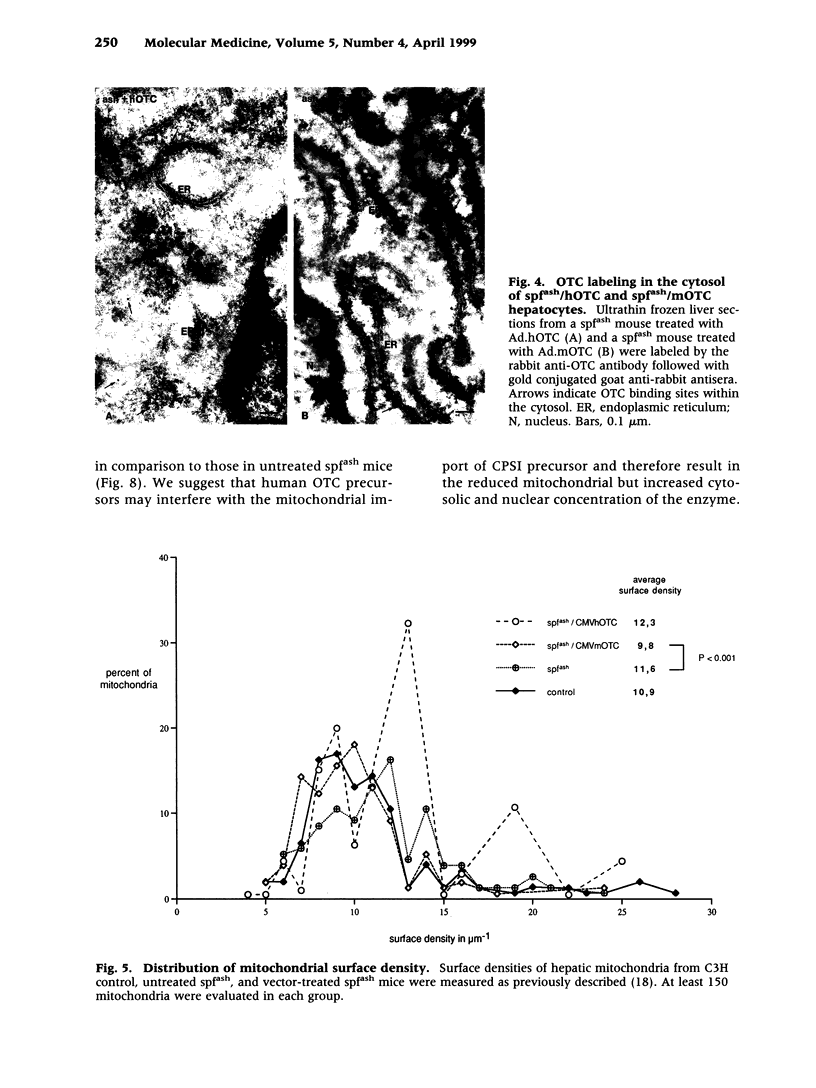
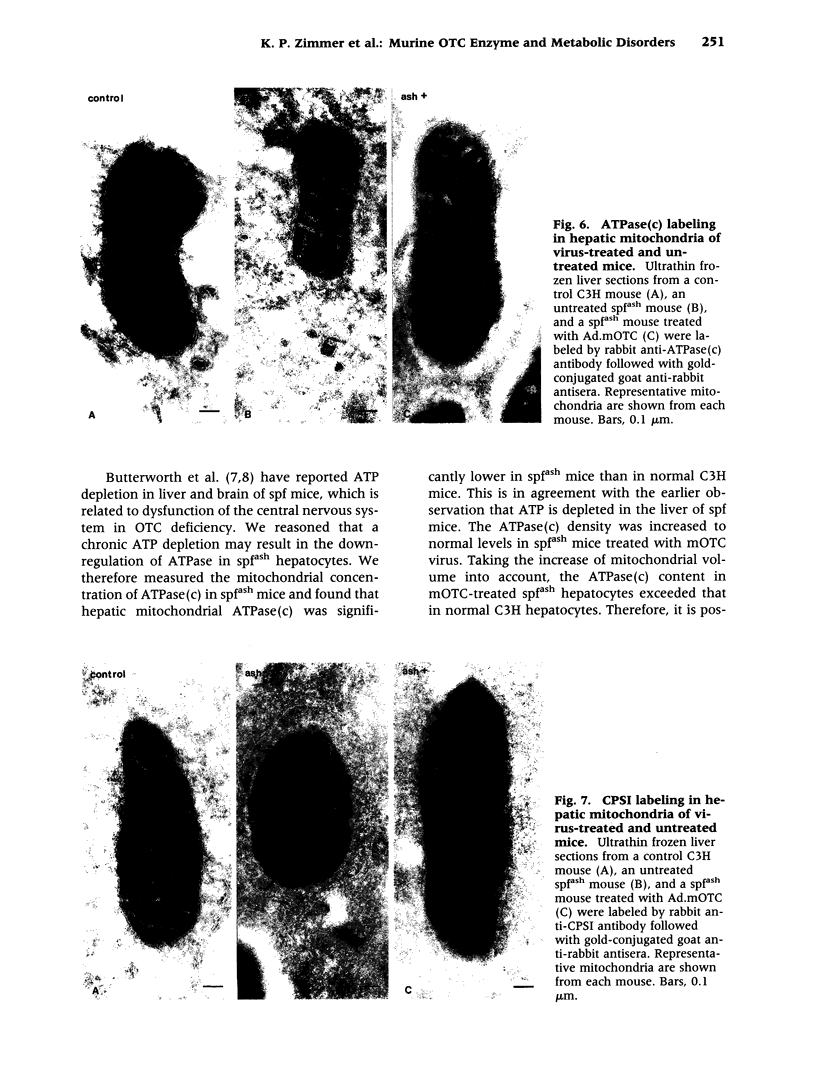
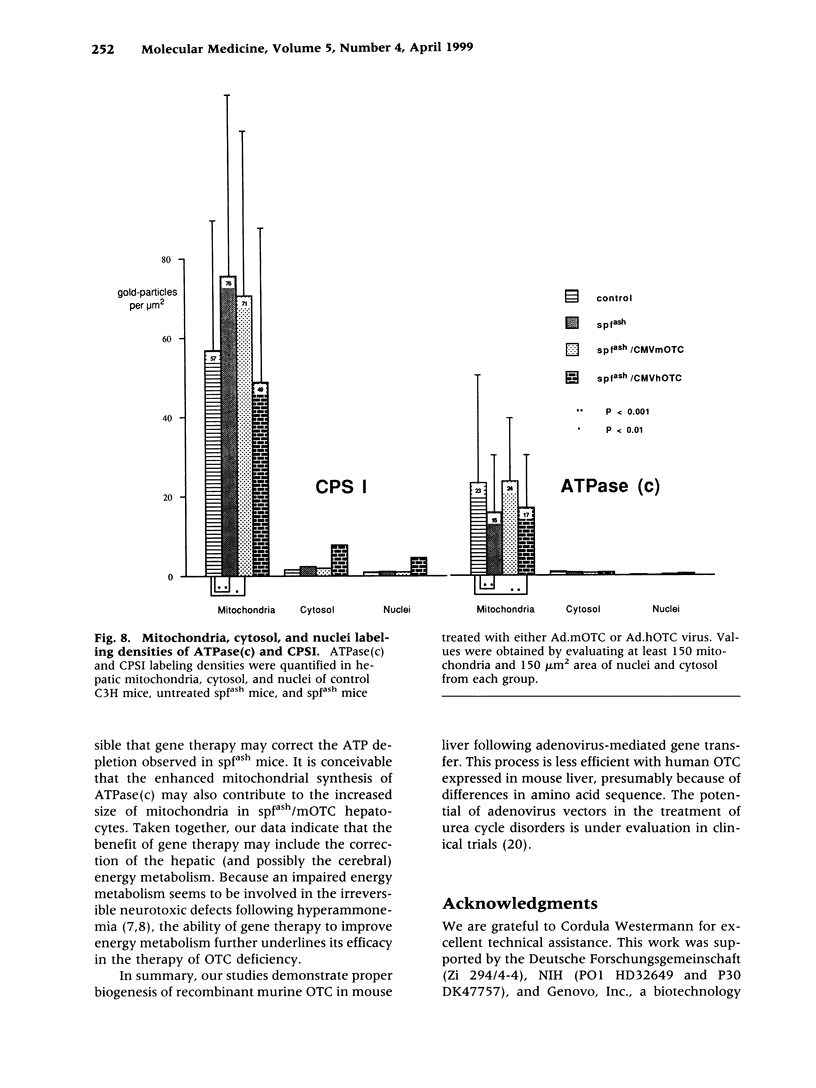
BACKGROUND: The mouse strain sparse fur with abnormal skin and hair (spf(ash)) is a model for the human ornithine transcarbamylase (OTC) deficiency, an X-linked inherited urea cycle disorder. The spf(ash) mouse carries a single base-pair mutation in the OTC gene that leads to the production of OTC enzyme at 10% of the normal level. MATERIALS AND METHODS: Recombinant adenoviruses carrying either mouse (Ad.mOTC) or human (Ad.hOTC) OTC cDNA were injected intravenously into the spf(ash) mice. Expression of OTC enzyme precursor and its translocation to mitochondria in the vector-transduced hepatocytes were analyzed on an ultrastructural level. Liver OTC activity and mitochondrial OTC concentration were significantly increased (300% of normal) in mice treated with Ad.mOTC and were moderately increased in mice receiving Ad.hOTC (34% of normal). The concentration and subcellular location of OTC and associated enzymes were studied by electron microscope immunolocalization and quantitative morphometry. RESULTS: Cytosolic OTC concentration remained unchanged in Ad.mOTC-injected mice but was significantly increased in mice receiving Ad.hOTC, suggesting a block of mitochondria translocation for the human OTC precursor. Mitochondrial ATPase subunit c [ATPase(c)] was significantly reduced and mitochondrial carbamy delta phosphate synthetase I (CPSI) was significantly elevated in spf(ash) mice relative to C3H. In Ad.mOTC-treated mice, the hepatic mitochondrial concentration of ATPase(c) was completely normalized and the CPSI concentration was partially corrected. CONCLUSIONS: Taken together, we conclude that newly synthesized mouse OTC enzyme was efficiently imported into mitochondria following vector-mediated gene delivery in spf(ash) mice, correcting secondary metabolic alterations.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann C., Colombo J. P. Increase of tryptophan and 5-hydroxyindole acetic acid in the brain of ornithine carbamoyltransferase deficient sparse-fur mice. Pediatr Res. 1984 Apr;18(4):372–375. doi: 10.1203/00006450-198404000-00014. [DOI] [PubMed] [Google Scholar]
- Cohen N. S., Cheung C. W., Raijman L. Altered enzyme activities and citrulline synthesis in liver mitochondria from ornithine carbamoyltransferase-deficient sparse-furash mice. Biochem J. 1989 Jan 1;257(1):251–257. doi: 10.1042/bj2570251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois N., Cavard C., Chasse J. F., Kamoun P., Briand P. Compared expression levels of ornithine transcarbamylase and carbamylphosphate synthetase in liver and small intestine of normal and mutant mice. Biochim Biophys Acta. 1988 Sep 7;950(3):321–328. doi: 10.1016/0167-4781(88)90128-5. [DOI] [PubMed] [Google Scholar]
- Hodges P. E., Rosenberg L. E. The spfash mouse: a missense mutation in the ornithine transcarbamylase gene also causes aberrant mRNA splicing. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4142–4146. doi: 10.1073/pnas.86.11.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Kanazawa M., Terada K., Kato S., Mori M. HSDJ, a human homolog of DnaJ, is farnesylated and is involved in protein import into mitochondria. J Biochem. 1997 May;121(5):890–895. doi: 10.1093/oxfordjournals.jbchem.a021670. [DOI] [PubMed] [Google Scholar]
- Kominami E., Ezaki J., Muno D., Ishido K., Ueno T., Wolfe L. S. Specific storage of subunit c of mitochondrial ATP synthase in lysosomes of neuronal ceroid lipofuscinosis (Batten's disease). J Biochem. 1992 Feb;111(2):278–282. doi: 10.1093/oxfordjournals.jbchem.a123749. [DOI] [PubMed] [Google Scholar]
- Lee J. T., Nussbaum R. L. An arginine to glutamine mutation in residue 109 of human ornithine transcarbamylase completely abolishes enzymatic activity in Cos1 cells. J Clin Invest. 1989 Dec;84(6):1762–1766. doi: 10.1172/JCI114360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Cell-free translation of carbamyl phosphate synthetase I and ornithine transcarbamylase messenger RNAs of rat liver. Effect of dietary protein and fasting on translatable mRNA levels. J Biol Chem. 1981 Apr 25;256(8):4127–4132. [PubMed] [Google Scholar]
- Mori M., Morita T., Ikeda F., Amaya Y., Tatibana M., Cohen P. P. Synthesis, intracellular transport, and processing of the precursors for mitochondrial ornithine transcarbamylase and carbamoyl-phosphate synthetase I in isolated hepatocytes. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6056–6060. doi: 10.1073/pnas.78.10.6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper S. E., Wilson J. M., Yudkoff M., Robinson M. B., Ye X., Batshaw M. L. Developing adenoviral-mediated in vivo gene therapy for ornithine transcarbamylase deficiency. J Inherit Metab Dis. 1998;21 (Suppl 1):119–137. doi: 10.1023/a:1005369926784. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L., Qureshi I. A., Butterworth R. F. Effect of L-carnitine on cerebral and hepatic energy metabolites in congenitally hyperammonemic sparse-fur mice and its role during benzoate therapy. Metabolism. 1993 Aug;42(8):1039–1046. doi: 10.1016/0026-0495(93)90020-o. [DOI] [PubMed] [Google Scholar]
- Ratnakumari L., Qureshi I. A., Butterworth R. F. Effects of congenital hyperammonemia on the cerebral and hepatic levels of the intermediates of energy metabolism in spf mice. Biochem Biophys Res Commun. 1992 Apr 30;184(2):746–751. doi: 10.1016/0006-291x(92)90653-3. [DOI] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wallace R., Knecht E., Grisolía S. Turnover of rat liver ornithine transcarbamylase. FEBS Lett. 1986 Nov 24;208(2):427–430. doi: 10.1016/0014-5793(86)81062-6. [DOI] [PubMed] [Google Scholar]
- Ye X., Robinson M. B., Batshaw M. L., Furth E. E., Smith I., Wilson J. M. Prolonged metabolic correction in adult ornithine transcarbamylase-deficient mice with adenoviral vectors. J Biol Chem. 1996 Feb 16;271(7):3639–3646. doi: 10.1074/jbc.271.7.3639. [DOI] [PubMed] [Google Scholar]
- Zimmer K. P., Matsuda I., Matsuura T., Mori M., Colombo J. P., Fahimi H. D., Koch H. G., Ullrich K., Harms E. Ultrastructural, immunocytochemical and stereological investigation of hepatocytes in a patient with the mutation of the ornithine transcarbamylase gene. Eur J Cell Biol. 1995 May;67(1):73–83. [PubMed] [Google Scholar]