Abstract
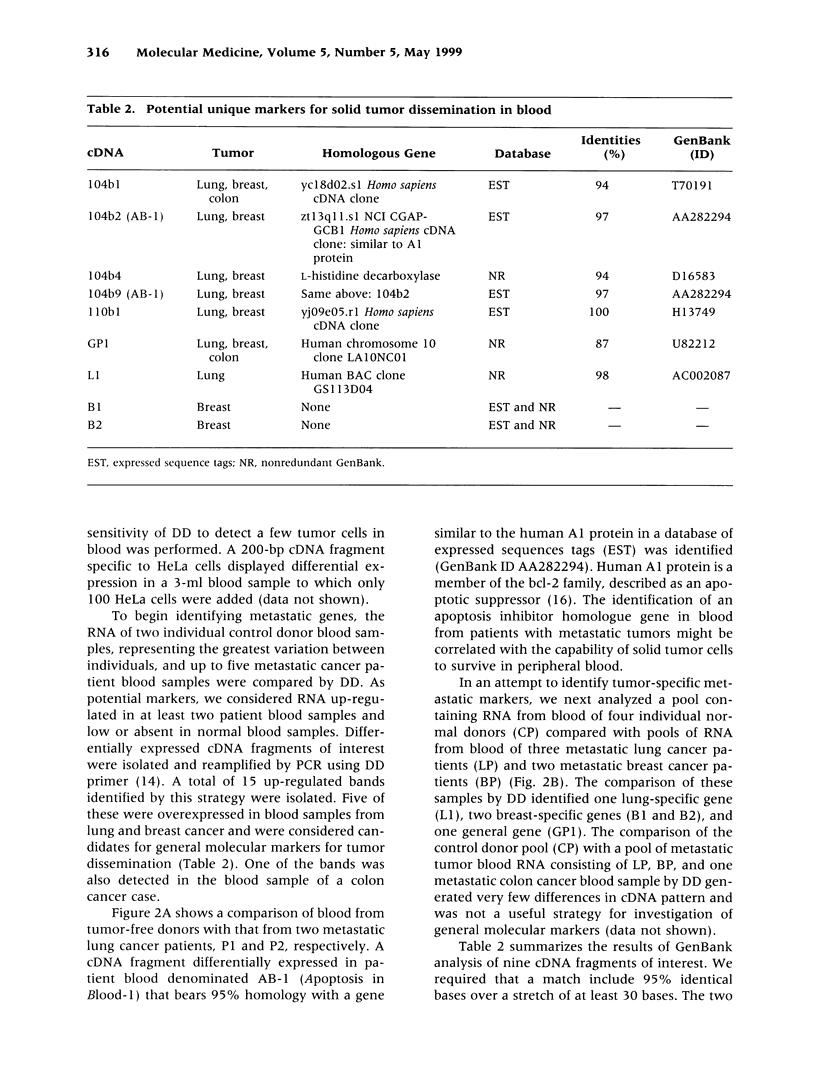
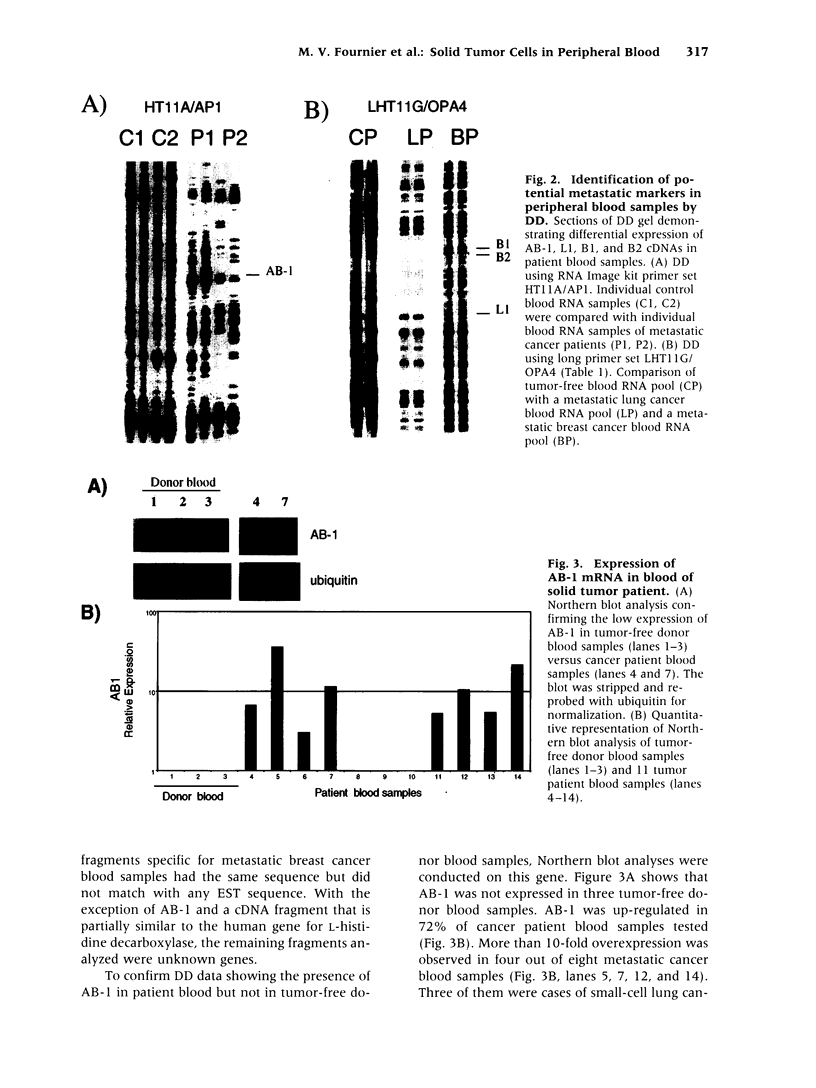
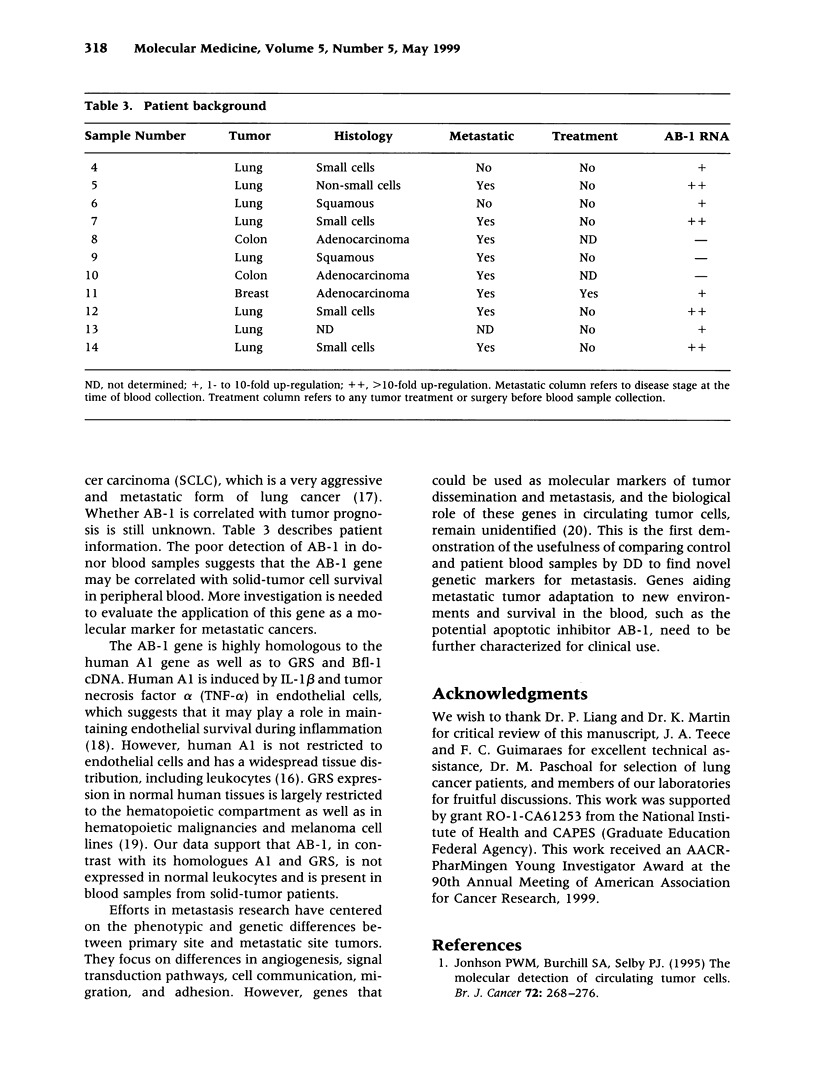
Efforts in metastasis research have centered on the phenotypic and genetic differences between primary site and metastatic site tumors. However, genes that may be used as molecular markers of metastasis in circulating tumor cells remain unidentified. Genes regulating the dissemination and survival of solid tumor cells in the blood, as well as their adaptation to new environments, could be candidates for unique metastatic tumor markers. Differential display (DD) was conducted to compare the blood of tumor-free individuals with the blood of patients with lung, breast, and colon cancers. Twenty-one up-expressed genes in the tumor patient blood samples but none in the tumor-free donor blood samples were identified. Nine of these samples were isolated, amplified, and directly sequenced. A gene AB-1 homologous to a Bcl-2 family member, which might function as an apoptosis inhibitor, was identified. The overexpression of an apoptosis inhibitor in blood from patients with metastatic tumors might be correlated with the capability of solid tumor cells to survive in peripheral blood. This is the first demonstration of the usefulness of comparing control and patient blood samples by DD to find novel potential genetic markers identifying metastasis in the blood. http://link.springer-ny. com/link/service/journals/00020/bibs/5n5p313.html
Full text
PDF

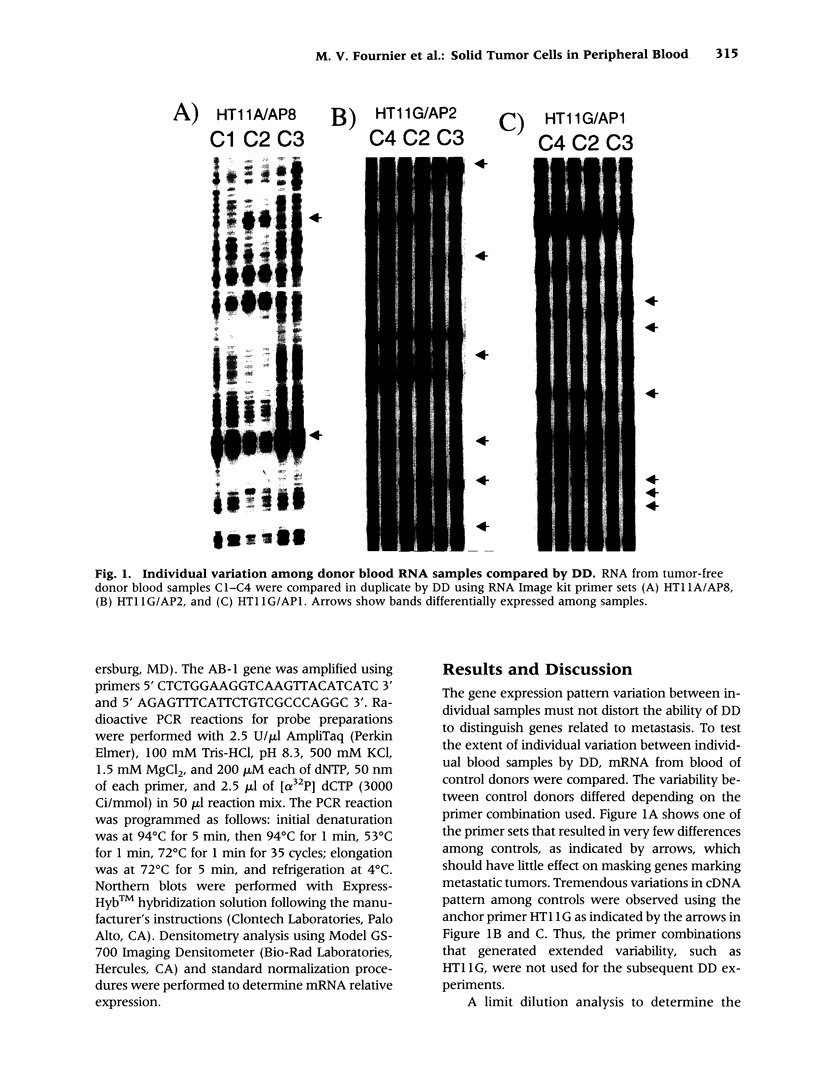

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dong Z., Staroselsky A. H., Qi X., Xie K., Fidler I. J. Inverse correlation between expression of inducible nitric oxide synthase activity and production of metastasis in K-1735 murine melanoma cells. Cancer Res. 1994 Feb 1;54(3):789–793. [PubMed] [Google Scholar]
- Fidler I. J. Macrophages and metastasis--a biological approach to cancer therapy. Cancer Res. 1985 Oct;45(10):4714–4726. [PubMed] [Google Scholar]
- Glaves D. Correlation between circulating cancer cells and incidence of metastases. Br J Cancer. 1983 Nov;48(5):665–673. doi: 10.1038/bjc.1983.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna N., Fidler I. J. Role of natural killer cells in the destruction of circulating tumor emboli. J Natl Cancer Inst. 1980 Oct;65(4):801–809. doi: 10.1093/jnci/65.4.801. [DOI] [PubMed] [Google Scholar]
- Johnson P. W., Burchill S. A., Selby P. J. The molecular detection of circulating tumour cells. Br J Cancer. 1995 Aug;72(2):268–276. doi: 10.1038/bjc.1995.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsan A., Yee E., Harlan J. M. Endothelial cell death induced by tumor necrosis factor-alpha is inhibited by the Bcl-2 family member, A1. J Biol Chem. 1996 Nov 1;271(44):27201–27204. doi: 10.1074/jbc.271.44.27201. [DOI] [PubMed] [Google Scholar]
- Karsan A., Yee E., Kaushansky K., Harlan J. M. Cloning of human Bcl-2 homologue: inflammatory cytokines induce human A1 in cultured endothelial cells. Blood. 1996 Apr 15;87(8):3089–3096. [PubMed] [Google Scholar]
- Kenny J. J., Knobloch T. J., Augustus M., Carter K. C., Rosen C. A., Lang J. C. GRS, a novel member of the Bcl-2 gene family, is highly expressed in multiple cancer cell lines and in normal leukocytes. Oncogene. 1997 Feb 27;14(8):997–1001. doi: 10.1038/sj.onc.1200898. [DOI] [PubMed] [Google Scholar]
- Liang P., Averboukh L., Pardee A. B. Distribution and cloning of eukaryotic mRNAs by means of differential display: refinements and optimization. Nucleic Acids Res. 1993 Jul 11;21(14):3269–3275. doi: 10.1093/nar/21.14.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Saidel M. G., Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976 Mar;36(3):889–894. [PubMed] [Google Scholar]
- Martin K. J., Pardee A. B. Principles of differential display. Methods Enzymol. 1999;303:234–258. doi: 10.1016/s0076-6879(99)03016-5. [DOI] [PubMed] [Google Scholar]
- Raj G. V., Moreno J. G., Gomella L. G. Utilization of polymerase chain reaction technology in the detection of solid tumors. Cancer. 1998 Apr 15;82(8):1419–1442. doi: 10.1002/(sici)1097-0142(19980415)82:8<1419::aid-cncr1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. Biomechanical interactions of cancer cells with the microvasculature during hematogenous metastasis. Cancer Metastasis Rev. 1992 Nov;11(3-4):227–235. doi: 10.1007/BF01307179. [DOI] [PubMed] [Google Scholar]
- Xie K., Huang S., Dong Z., Juang S. H., Gutman M., Xie Q. W., Nathan C., Fidler I. J. Transfection with the inducible nitric oxide synthase gene suppresses tumorigenicity and abrogates metastasis by K-1735 murine melanoma cells. J Exp Med. 1995 Apr 1;181(4):1333–1343. doi: 10.1084/jem.181.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Ooi S. L., Pardee A. B. New primer strategy improves precision of differential display. Biotechniques. 1995 May;18(5):842-6, 848, 850. [PubMed] [Google Scholar]