Abstract
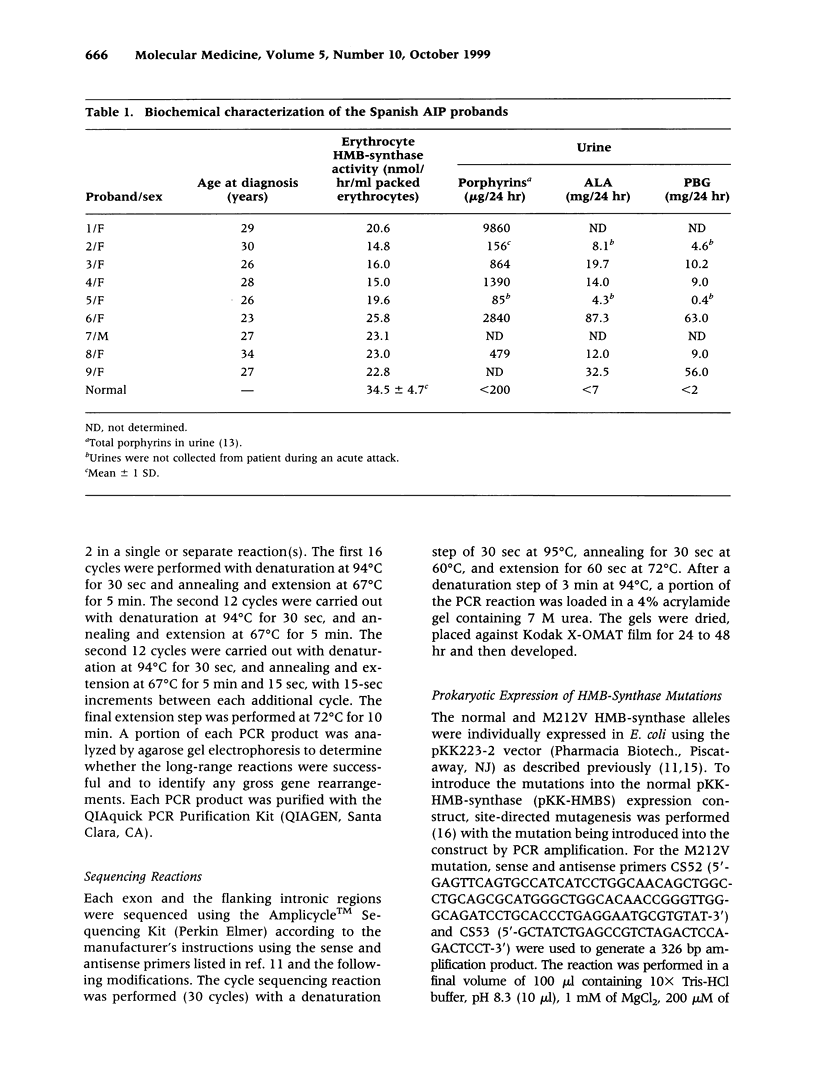
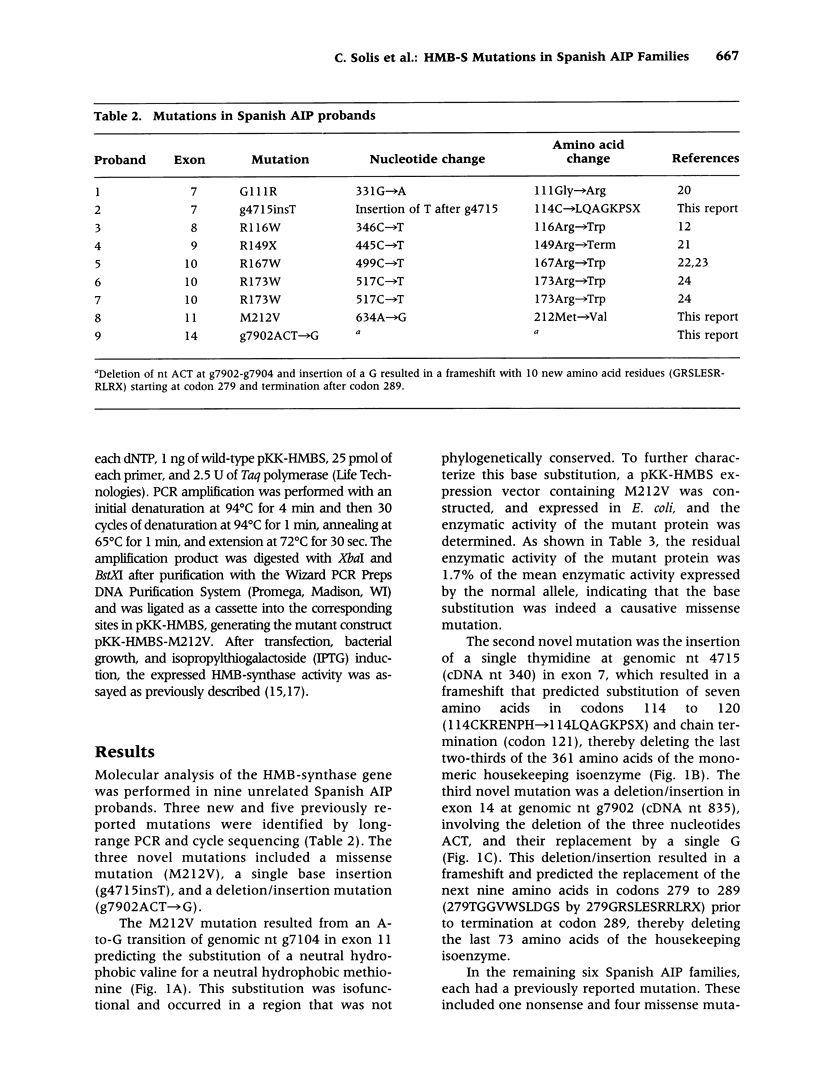
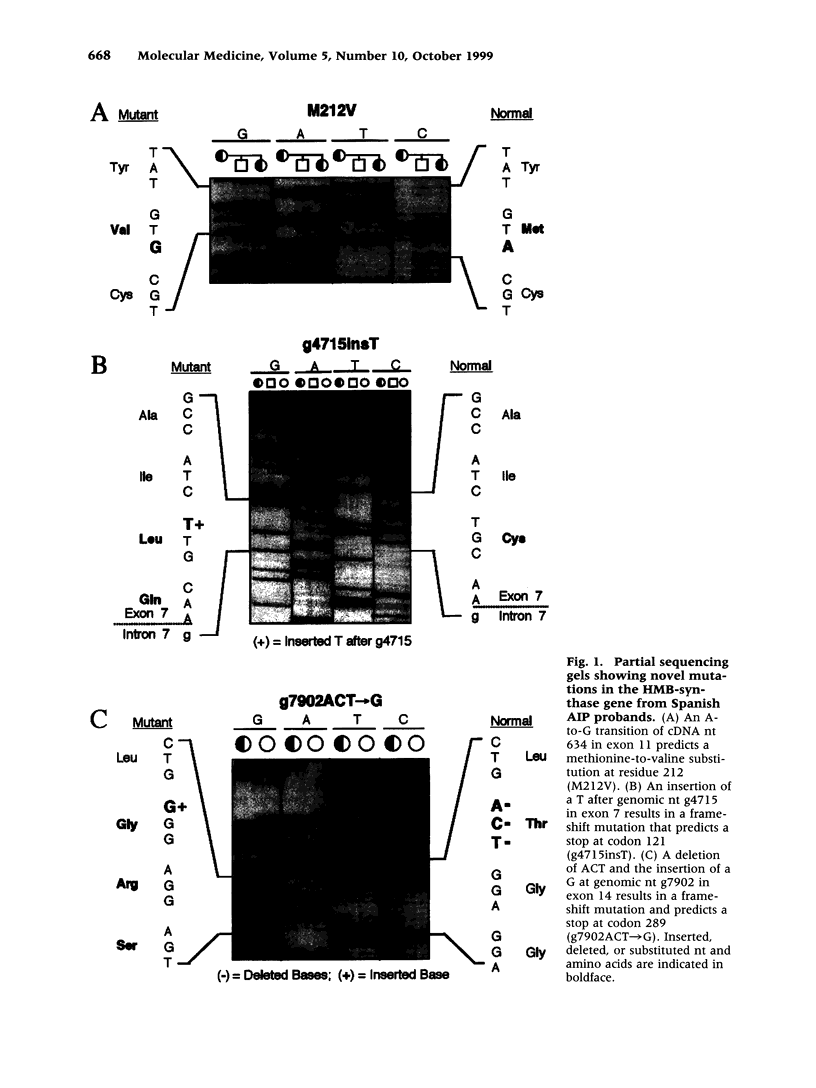
BACKGROUND: Acute intermittent porphyria (AIP), an autosomal dominant inborn error, results from the half-normal activity of the heme biosynthetic enzyme hydroxymethylbilane synthase (EC 4.3.1.8; HMB-synthase). This disease is characterized by acute, life-threatening neurologic attacks that are precipitated by various drugs, hormones, and other factors. The enzymatic and/or biochemical diagnosis of AIP heterozygotes is problematic; therefore, efforts have focused on the identification of HMB-synthase mutations so that heterozygotes can be identified and educated to avoid the precipitating factors. In Spain, the occurrence of AIP has been reported, but the nature of the HMB-synthase mutations causing AIP in Spanish families has not been investigated. Molecular analysis was therefore undertaken in nine unrelated Spanish AIP patients. MATERIALS AND METHODS: Genomic DNA was isolated from affected probands and family members of nine unrelated Spanish families with AIP. The HMB-synthase gene was amplified by long-range PCR and the nucleotide sequence of each exon was determined by cycle sequencing. RESULTS: Three new mutations, a missense, M212V; a single base insertion, g4715insT; and a deletion/insertion, g7902ACT-->G, as well as five previously reported mutations (G111R, R116W, R149X R167W, and R173W) were detected in the Spanish probands. Expression of the novel missense mutation M212V in E. coli revealed that the mutation was causative, having <2% residual activity. CONCLUSIONS: These studies identified the first mutations in the HMB-synthase gene causing AIP in Spanish patients. Three of the mutations were novel, while five previously reported lesions were found in six Spanish families. These findings enable accurate identification and counseling of presymptomatic carriers in these nine unrelated Spanish AIP families and further demonstrate the genetic heterogeneity of mutations causing AIP.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. M., Desnick R. J. Porphobilinogen deaminase: methods and principles of the enzymatic assay. Enzyme. 1982;28(2-3):146–157. doi: 10.1159/000459098. [DOI] [PubMed] [Google Scholar]
- Barker D., Schafer M., White R. Restriction sites containing CpG show a higher frequency of polymorphism in human DNA. Cell. 1984 Jan;36(1):131–138. doi: 10.1016/0092-8674(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Bottomley S. S., Bonkowsky H. L., Kreimer-Birnbaum M. The diagnosis of acute intermittent porphyria. Usefulness and limitations of the erythrocyte uroporphyrinogen I synthase assay. Am J Clin Pathol. 1981 Aug;76(2):133–139. doi: 10.1093/ajcp/76.2.133. [DOI] [PubMed] [Google Scholar]
- Brownlie P. D., Lambert R., Louie G. V., Jordan P. M., Blundell T. L., Warren M. J., Cooper J. B., Wood S. P. The three-dimensional structures of mutants of porphobilinogen deaminase: toward an understanding of the structural basis of acute intermittent porphyria. Protein Sci. 1994 Oct;3(10):1644–1650. doi: 10.1002/pro.5560031004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Astrin K. H., Lee G., Anderson K. E., Desnick R. J. Acute intermittent porphyria: identification and expression of exonic mutations in the hydroxymethylbilane synthase gene. An initiation codon missense mutation in the housekeeping transcript causes "variant acute intermittent porphyria" with normal expression of the erythroid-specific enzyme. J Clin Invest. 1994 Nov;94(5):1927–1937. doi: 10.1172/JCI117543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Siervi A., Rossetti M. V., Parera V. E., Astrin K. H., Aizencang G. I., Glass I. A., Batlle A. M., Desnick R. J. Identification and characterization of hydroxymethylbilane synthase mutations causing acute intermittent porphyria: evidence for an ancestral founder of the common G111R mutation. Am J Med Genet. 1999 Oct 8;86(4):366–375. [PubMed] [Google Scholar]
- Grandchamp B. Acute intermittent porphyria. Semin Liver Dis. 1998;18(1):17–24. doi: 10.1055/s-2007-1007136. [DOI] [PubMed] [Google Scholar]
- Grandchamp B., De Verneuil H., Beaumont C., Chretien S., Walter O., Nordmann Y. Tissue-specific expression of porphobilinogen deaminase. Two isoenzymes from a single gene. Eur J Biochem. 1987 Jan 2;162(1):105–110. doi: 10.1111/j.1432-1033.1987.tb10548.x. [DOI] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., Lee J. S., Te Velde K., Deybach J. C., Nordmann Y., Grandchamp B. High prevalence of a point mutation in the porphobilinogen deaminase gene in Dutch patients with acute intermittent porphyria. Hum Genet. 1993 Mar;91(2):128–130. doi: 10.1007/BF00222712. [DOI] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., Voortman G., Te Velde K., Nordmann Y., Grandchamp B. High frequency of mutations in exon 10 of the porphobilinogen deaminase gene in patients with a CRIM-positive subtype of acute intermittent porphyria. Am J Hum Genet. 1992 Sep;51(3):660–665. [PMC free article] [PubMed] [Google Scholar]
- Gu X. F., de Rooij F., de Baar E., Bruyland M., Lissens W., Nordmann Y., Grandchamp B. Two novel mutations of the porphobilinogen deaminase gene in acute intermittent porphyria. Hum Mol Genet. 1993 Oct;2(10):1735–1736. doi: 10.1093/hmg/2.10.1735. [DOI] [PubMed] [Google Scholar]
- Kauppinen R., Mustajoki S., Pihlaja H., Peltonen L., Mustajoki P. Acute intermittent porphyria in Finland: 19 mutations in the porphobilinogen deaminase gene. Hum Mol Genet. 1995 Feb;4(2):215–222. doi: 10.1093/hmg/4.2.215. [DOI] [PubMed] [Google Scholar]
- Lee J. S., Anvret M. Identification of the most common mutation within the porphobilinogen deaminase gene in Swedish patients with acute intermittent porphyria. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10912–10915. doi: 10.1073/pnas.88.23.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Lundin G., Lannfelt L., Forsell L., Picat C., Grandchamp B., Anvret M. Genetic heterogeneity of the porphobilinogen deaminase gene in Swedish families with acute intermittent porphyria. Hum Genet. 1991 Aug;87(4):484–488. doi: 10.1007/BF00197173. [DOI] [PubMed] [Google Scholar]
- Llewellyn D. H., Smyth S. J., Elder G. H., Hutchesson A. C., Rattenbury J. M., Smith M. F. Homozygous acute intermittent porphyria: compound heterozygosity for adjacent base transitions in the same codon of the porphobilinogen deaminase gene. Hum Genet. 1992 Apr;89(1):97–98. doi: 10.1007/BF00207051. [DOI] [PubMed] [Google Scholar]
- Louie G. V., Brownlie P. D., Lambert R., Cooper J. B., Blundell T. L., Wood S. P., Warren M. J., Woodcock S. C., Jordan P. M. Structure of porphobilinogen deaminase reveals a flexible multidomain polymerase with a single catalytic site. Nature. 1992 Sep 3;359(6390):33–39. doi: 10.1038/359033a0. [DOI] [PubMed] [Google Scholar]
- Lundin G., Lee J. S., Thunell S., Anvret M. Genetic investigation of the porphobilinogen deaminase gene in Swedish acute intermittent porphyria families. Hum Genet. 1997 Jul;100(1):63–66. doi: 10.1007/s004390050466. [DOI] [PubMed] [Google Scholar]
- Mgone C. S., Lanyon W. G., Moore M. R., Louie G. V., Connor J. M. Identification of five novel mutations in the porphobilinogen deaminase gene. Hum Mol Genet. 1994 May;3(5):809–811. doi: 10.1093/hmg/3.5.809. [DOI] [PubMed] [Google Scholar]
- Mustajoki P. Normal erythrocyte uroporphyrinogen I synthase in a kindred with acute intermittent porphyria. Ann Intern Med. 1981 Aug;95(2):162–166. doi: 10.7326/0003-4819-95-2-162. [DOI] [PubMed] [Google Scholar]
- Puy H., Deybach J. C., Lamoril J., Robreau A. M., Da Silva V., Gouya L., Grandchamp B., Nordmann Y. Molecular epidemiology and diagnosis of PBG deaminase gene defects in acute intermittent porphyria. Am J Hum Genet. 1997 Jun;60(6):1373–1383. doi: 10.1086/515455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. F., Bishop D. F., Desnick R. J. Human uroporphyrinogen III synthase: molecular cloning, nucleotide sequence, and expression of a full-length cDNA. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7049–7053. doi: 10.1073/pnas.85.19.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner C. A., Yoo H. W., Roberts A. G., Desnick R. J. Congenital erythropoietic porphyria: identification and expression of exonic mutations in the uroporphyrinogen III synthase gene. J Clin Invest. 1992 Feb;89(2):693–700. doi: 10.1172/JCI115637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S., Lambert R., Jordan P. M. Molecular basis of acute intermittent porphyria. Mol Med Today. 1995 Aug;1(5):232–239. doi: 10.1016/s1357-4310(95)91513-3. [DOI] [PubMed] [Google Scholar]
- Yoo H. W., Warner C. A., Chen C. H., Desnick R. J. Hydroxymethylbilane synthase: complete genomic sequence and amplifiable polymorphisms in the human gene. Genomics. 1993 Jan;15(1):21–29. doi: 10.1006/geno.1993.1005. [DOI] [PubMed] [Google Scholar]



