Abstract
In this study, we introduce the Micro-Electrode Ion Flux Estimation technique as a sensitive and accurate technique to study systemin-induced changes in ion fluxes from isolated nearly intact plant tissues. Our results demonstrate the effectiveness and value of the Micro-Electrode Ion Flux Estimation technique to monitor and characterize those elicitor-induced ion flux changes from intact tissues. We used the method to monitor the systemin-induced changes in ion fluxes from leaf tissue of various plant species, including wild-type and cu3 mutant tomato (Solanum pimpinellifolium) plants, and confirm previous observations, but now in intact leaf tissue. Upon exposure of leaf tissue of plant species from the subtribe solaneae to systemin, the H+ influx and K+ efflux were transiently strongly increased. Plant species of other clades did not show a response upon systemin exposure. Although it has been reported that the gene containing the cu3 null mutation is identical to the SR160/tBRI1 gene, which encodes the systemin/brassinosteroid receptor and is essential in systemin and brassinosteroid perception, we observed no differences in the response of H+ and K+ fluxes from both wild-type and mutant leaf tissue to systemin. Also, the effects of various pharmacological effectors on systemin-induced flux changes were similar. Moreover, a SR160/tBRI1 transgene-containing tobacco (Nicotiana tabacum) line was insensitive to systemin, whereas both this line and its wild-type predecessor were responsive to the elicitor flg22. Our results support the conclusion that the Cu3 receptor of tomato is not the systemin receptor, and, hence, another receptor is the principal systemin receptor.
To repel herbivores, plants have evolved a wide array of sophisticated defense mechanisms. Several of these mechanisms are inducible upon feeding by the herbivore and not only at the site of feeding but also in plant parts at considerable distance from this site. This systemic induction of defense is attributed to the plant hormone jasmonic acid (JA) or its derivative methyl jasmonate (Farmer et al., 2003; Lorenzo and Solano, 2005). In solaneous plants, the JA-signaling system is fine tuned by the introduction of the peptide hormones, systemin and Hyp-rich systemin, as signaling intermediates (Ryan and Pearce, 2003). Only solaneous plants belonging to the subtribe Solaneae, like tomato (Solanum lycopersicum), potato (Solanum tuberosum), black nightshade (Solanum nigrum), and pepper (Capsicum annuum; Constabel et al., 1998), contain homologs to systemin, whereas Hyp-rich systemins are shown to be present both in tomato and tobacco (Nicotiana tabacum; Ryan and Pearce, 2003).
In tomato, the 18-amino acid peptide, systemin, is released upon feeding and locally induces the defense response. Initially, it was assumed that this peptide was the signal molecule responsible for the systemic dispersal of the defense activation, hence, its name systemin (Pearce et al., 1991; McGurl et al., 1992). Although it has been shown that radioactive systemin, fed to wounded plants, can be recovered from phloem exudate and, therefore, can be transported through the plant (Pearce et al., 1991; Narvaez-Vasquez et al., 1994), the currently preferred view on the systemic dispersal of the wound defense response is that systemin is not the systemic signal. The systemic signal is likely to be derived from the octadecanoid pathway but not necessarily JA itself, as was demonstrated by grafting experiments with JA biosynthesis and JA perception mutants (Li et al., 2002; Lee and Howe, 2003). Systemin, then, functions locally by inducing and amplifying JA and its own production along the vascular tissues and strengthens the systemic response (Ryan and Moura, 2002; Schilmiller and Howe, 2005).
Exposing plants to systemin, either indirectly through wounding or directly by application of a systemin-containing solution, results in a well-defined response. Two phases can be discriminated in this response: an early phase, which consists of changes in ion fluxes across the plasma membrane, simultaneous changes in the cytoplasmic Ca2+ concentrations (Felix and Boller, 1995; Moyen and Johannes, 1996; Meindl et al., 1998; Moyen et al., 1998; Schaller and Oecking, 1999) and an increase in the production of reactive oxygen species (Orozco-Cardenas and Ryan, 1999; Orozco-Cardenas et al., 2001), and a late phase, which consists of changes in gene expression. The induced genes are involved in the production of defensive substances, like proteinase inhibitors, protein-cross linkers, and repellents, in alkaloid synthesis or in signal transduction (secondary messengers, JA synthesis, systemin production). Of a few of these induced genes their relation with defense is less obvious (Ryan, 2000).
Several elements of the signal transduction network that are associated with the systemin response have been isolated and characterized, although several important steps remain elusive. The first step in the cascade is the release of systemin from its proprotein. Systemin is an 18-amino acid peptide that is embedded in a 200-amino acid prosystemin protein (McGurl et al., 1992). The processing and subsequent release of systemin from its precursor has been suggested to result from the mixing of prosystemin with proteolytic enzymes from other compartments as the result of grazing (Ryan, 2000). However, this explains only the initial release and not the subsequent systemin signal amplification by intact cells (Schilmiller and Howe, 2005).
A few years ago, a receptor for systemin was isolated by usage of an azido derivative of systemin, which labeled a 160-kD protein in a microsomal fraction from Solanum peruvianum suspension-cultured cells. After analysis of its mass spectrum and sequencing of tryptic peptides, the SR160 systemin receptor was identified as a receptor-like kinase (Scheer and Ryan, 1999, 2002). Confirmation of the nature of this receptor was obtained by functional expression of the SR160 gene in suspension cells of tobacco, a plant species that does not express a systemin precursor gene or respond to systemin (Scheer et al., 2003). Challenging the transgenic tobacco cell suspension culture with systemin resulted in an alkalinization of the medium similar to the one observed in tomato suspension cells. This alkalinization was not observed in wild-type cells or by challenging the cells with an inactive systemin analog (Scheer et al., 2003). One of the most surprising results of the isolation and characterization of the systemin receptor was its high similarity with the brassinolide receptor (tBRI1) of tomato (Scheer and Ryan, 2002). A second confirmation of the SR160 protein being the systemin receptor came from the cu3 mutant tomato plant. This mutant tomato line has a nonsense mutation in the tBRI1 gene and, consequently, a dwarf phenotype, typical of mutants affected in brassinosteroid perception (Montoya et al., 2002; Choe, 2006). More importantly, this mutant tomato line also shows reduced induction of defense gene expression by systemin (Scheer et al., 2003), which suggests a dual role for the SR160/tBRI1 receptor, namely, in systemin perception and brassinosteroid perception (Szekeres, 2003; Wang and He, 2004). However, recently, Holton et al. (2007) demonstrated that that the tBRI1 receptor is not essential for wound signaling and suggested an additional developmentally regulated systemin receptor.
The structure of the downstream signaling pathway after perception of systemin by SR160/tBRI1 remains largely unknown, although several elements have been implicated. Channels and transporters seem to be involved because of the changes in proton, potassium, and calcium fluxes (Moyen and Johannes, 1996; Meindl et al., 1998; Moyen et al., 1998). The modulation of the H+ fluxes depends on a calcium signal via a Ca2+-dependent protein kinase (Schaller, 1999; Schaller and Oecking, 1999), whereas calmodulin and several mitogen-activated protein kinases have also been implicated in systemin signaling (Stratmann and Ryan, 1997; Bergey and Ryan, 1999; Schaller and Oecking, 1999; Kandoth et al., 2007). Phospholipases are involved either as enzymes in the octadecanoid pathway by providing building blocks for JA synthesis (Narvaez-Vasquez et al., 1999; Schaller, 1999) or by providing signaling intermediates, like inositol 1,4,5-trisphosphate (Moyen et al., 1998). Also, hydrogen peroxide seems to be involved (Orozco-Cardenas and Ryan, 1999; Orozco-Cardenas et al., 2001). Elucidation of the pathway and its structure is hampered by the fact that the systemin pathway exists only in Solanaceae, but recently an analogous system has been characterized in Arabidopsis (Arabidopsis thaliana). The Arabidopsis system, although apparently mechanistically similar to the systemin system, is based on proteins with no close homology to the systemin system. The putative ligand (AtPEP1) and its precursor protein (AtPROPEP1; Huffaker et al., 2006) and its receptor (AtPEPR1), although Leu-rich repeats containing receptor-like kinase, like BRI-1 (Yamaguchi et al., 2006), show no or only a low degree of sequence identity with the systemin-related proteins. The presence of this analogous system in Arabidopsis opens the possibility of genetical dissection of a related signal transduction pathway. Although these systems appear similar, some important differences remain: (1) the systemin system is unique for solaneae (raising questions on its origin), while homologs of AtPROPEP1 are present in a wide variety of plant species; (2) systemin is involved in defense against herbivores, while AtPEP1 is involved in resistance against a root pathogen (although effects on other defenses and resistances of both systems have not been tested); and (3) systemin is perceived by the tBRI receptor, which it shares with brassinosteroids, while AtPEP1 is perceived by an apparently dedicated receptor (AtPEPR1; Szekeres, 2003; Wang and He, 2004; Huffaker et al., 2006; Yamaguchi et al., 2006).
The Micro-Electrode Ion Flux Estimation (MIFE) technique allows the noninvasive and simultaneous monitoring of different ion fluxes from intact tissues with a high spatial and temporal resolution (Shabala et al., 1997; Newman, 2001; Tegg et al., 2005; Vreeburg et al., 2005). In this study, we analyzed the changes in H+ and K+ fluxes from tomato leaf tissues elicited by systemin. We confirm in situ the systemin response, the alkalinization and the accompanying K+ efflux, and analyze the characteristics of these changes. We also show that all plant species tested that are phylogenetically close to tomato displayed the response to systemin (Constabel et al., 1998; Ryan and Pearce, 2003). However, in the cu3 mutant tomato leaves, believed to be insensitive to systemin, identical responses of the H+ an K+ fluxes to systemin were observed as in wild-type tomato. These responses were observed in situ in the first true leaves of plants containing at least three leaves. Also, pharmacological effectors had similar effects on systemin-induced changes in ion fluxes in cu3 and wild-type plants. Flux measurements confirmed the insensitivity of wild-type tobacco to systemin. The tobacco lines containing the SR160/tBRI1 transgene were also unresponsive to systemin. Tobacco, wild type, as well as the transgenic line, were, however, responsive to the general elicitor flg22. The most logical conclusion from our results is that the cu3 tomato plant still contains a functional systemin receptor, which is identical to the receptor of the wild type and is not the Cu3 receptor.
RESULTS
The Effect of Systemin on Proton and Potassium Fluxes in Wild-Type Tomato Leaf Tissue
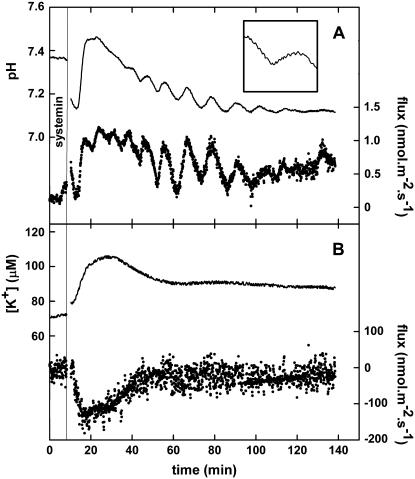
With the MIFE technique, ion fluxes from intact tissues can be monitored. We used this technique to study the effect of the elicitor systemin on H+ and K+ fluxes from tissues of various plant species. Whereas exposing freshly prepared S. peruvianum (accession no. TGRC: LA2172) leaf tissue to systemin did not trigger an effect on the ion fluxes, exposure of the tissue to 100 nm systemin after an 18-h recovery period resulted in an increased H+ influx and K+ efflux. This 18-h recovery period is essential to observe systemin responses in all Solanum species and is related with the presence of the systemin system (F.C. Lanfermeijer, M. Staal, J.T.M. Elzenga, unpublished data). This modification of the fluxes resulted in an alkalinization of the unstirred layer surrounding the cells and an increase of the K+ concentration in this layer (Fig. 1). On average, the change in fluxes became visible 2 min after the addition of systemin and reached its maximum after 5 min. Because of the spatial and temporal resolution of the MIFE, system fluxes can be observed in high detail. These high-resolution observations revealed that in 27% of the experiments, oscillations either in both or in one of the fluxes could be observed. Although these oscillations were frequently triggered by the application of systemin, they were sometimes present during the whole experiment or even only before the application of systemin. The oscillations had a frequency of 0.17 ± 0.04 min−1. H+ influx and K+ efflux are not directly coupled, because the oscillations were sometimes visible in only one of the two simultaneously monitored cation fluxes and because there was no fixed ratio between H+ and K+ flux sizes (results not shown).
Figure 1.
Typical changes in proton and potassium fluxes from S. peruvianum leaf tissue induced by 100 nm systemin. A, pH of the unstirred layer and proton flux. B, K+ concentration of the unstirred layer and K+ flux. Continuous lines, pH or K+ concentration at 10 and 50 μm from the cell wall (left y axis). Dotted lines, Calculated H+ or K+ flux based on the pH at 10 and 50 μm (right y axis). A vertical line indicates the moment of the addition of 100 nm systemin. When the flux becomes more positive, this means either an increase of the influx or a reduction of the efflux. Typical experiments of at least three experiments are shown. The insert in A shows a magnification of the pH trace between 40 and 55 min and between pH values 7.20 and 7.35, which shows the saw-tooth-like trace due to the movement of the electrode through the pH gradient in the unstirred layer.
The systemin-induced changes in H+ and K+ fluxes were transient; after approximately 30 min, the pH and the fluxes returned to approximately the levels present before the addition of systemin, although the presence of oscillations sometimes prevented the exact determination of the length of the transient.
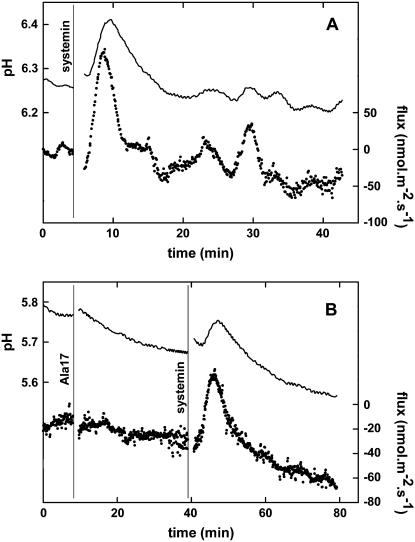
The tomato species Solanum pimpinellifolium (TGRC no. LA1610) responded in a similar way to systemin (Fig. 2A). In this species, the effect of the application of 100 nm of the inactive Ala-17 derivative of systemin was also tested (Pearce et al., 1993). After addition of the Ala-17 derivative, no modulation of ion fluxes were observed, while the subsequent addition of 400 nm of systemin resulted in a change of the H+ flux (Fig. 2B). The size of this change (42.6 ± 14.8 nmol m−2 s−1 [n = 5] at pH 6.1 ± 0.6) is comparable to the change induced by 100 nm systemin in the absence of Ala-17 and is in accordance with competitive inhibition and approximately similar affinities for systemin and the Ala-17 derivative (Scheer and Ryan, 1999).
Figure 2.
A typical change in the proton flux from S. pimpinellifolium leaf tissue induced by 100 nm systemin or by 400 nm systemin after the addition of 100 nm Ala-17. A, 100 nm systemin. B, 400 nm systemin after the addition of 100 nm Ala-17. Continuous line, pH of the unstirred layer at 10 and 50 μm from the cell wall (left y axis). Dotted line, Calculated H+ flux based on the pH at 10 and 50 μm (right y axis). A vertical line indicates the moment of addition of the indicated solute. When the flux becomes more positive, this means either an increase of the influx or a reduction of the efflux. Typical experiments of at least three experiments are shown.
The Effect of Systemin on Ion Fluxes from Leaf Tissue of Other Plant Species
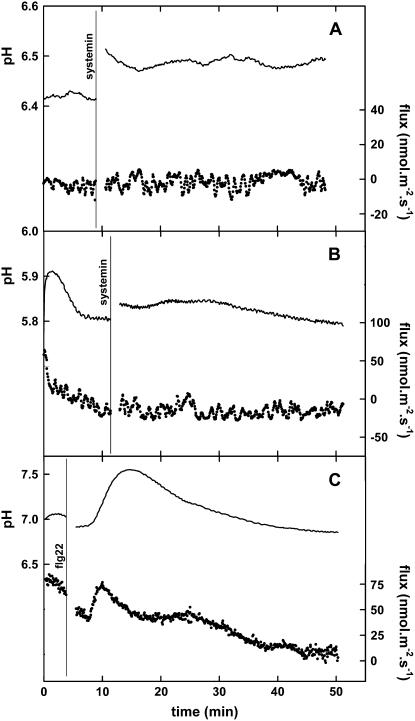
Only plant species from the Solaneae subtribe of the Solanaceae plant family are sensitive to systemin; therefore, several plant species were tested for their responsiveness to systemin. Changes in the proton fluxes from leaf tissue of S. tuberosum var. karnico, Solanum jasminoides, and S. nigrum demonstrated that these species respond in a similar manner to systemin as S. peruvianum and S. pimpinellifolium (Fig. 3). On the other hand, as expected for systemin-insensitive species, pH and H+ fluxes from Arabidopsis, Pisum sativum, Beta vulgaris (Fig. 3), and tobacco (Fig. 4A) leaf tissue did not respond to the addition of systemin with changes after 2 to 3 min.
Figure 3.
Typical changes in the proton fluxes from leaf tissue of various plant species induced by 100 nm systemin. The vertical line indicates the moment of the addition of the 100 nm systemin, except for S. jasminoides, where 10 nm systemin was used. When the flux becomes more positive, this means either an increase of the influx or a reduction of the efflux. The pH at the moment of addition of systemin was between 5.5 and 5.8 in all experiments. Typical experiments of at least three experiments are shown.
Figure 4.
Typical changes in the proton fluxes from tobacco leaf tissue without or with the 35S∷SR160/tBRI1 transgene induced by 100 nm systemin or 10 nm flagellin. A, The response of wild-type tobacco to 100 nm systemin. B, The response of tobacco containing the 35S∷SR160/tBRI1 transgene to 100 nm systemin. C, The response of wild-type tobacco to 10 nm flagellin. Continuous line, pH of the unstirred layer at 10 and 50 μm from the cell wall (left y axis). Dotted line, Calculated H+ flux based on the pH at 10 and 50 μm (right y axis). A vertical line indicates the moment of the addition of the indicated solute. When the flux becomes more positive, this means either an increase of the influx or a reduction of the efflux. Typical experiments of at least three experiments are shown.
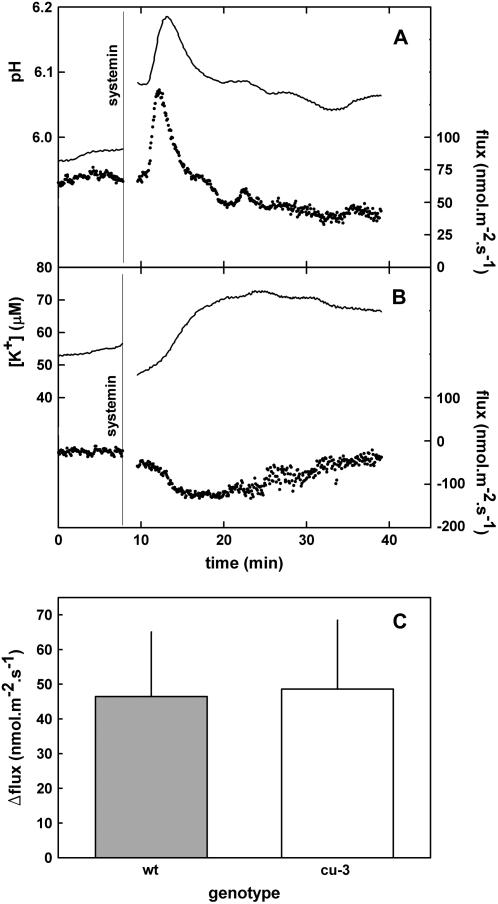
The Effect of Systemin on Proton and Potassium-Ion Fluxes in cu3 Tomato Leaf Tissue
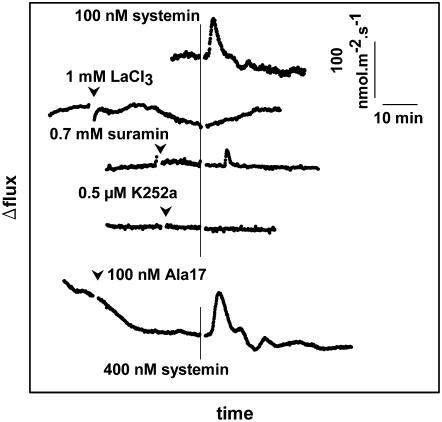
Challenging the assumed systemin-insensitive cu3 mutant of S. pimpinellifolium (TGRC no. LA2398) with systemin also resulted in changes in the H+ and K+ fluxes (Fig. 5). Moreover, the affected fluxes had characteristics similar to those from wild-type tissues and kinetics were comparable; when measured at a comparable initial pH, the sizes of the changes of the flux were similar (compare Figs. 2A and 5A), oscillations could sometimes be observed in both tissues, and the changes could not be triggered by Ala-17 (Fig. 6). Concentration dependency appeared similar in both wild type and cu3, because in leaf tissue from both plants, 0.1 nm of systemin already could trigger a considerable and comparably sized response (data not shown). Further characterization of the concentration dependency of the response is technically difficult with the MIFE system, as only one concentration of systemin can be tested on a tissue sample and variability between experiments is too large for a detailed kinetic analysis.
Figure 5.
Typical systemin-induced changes in proton and potassium fluxes from cu3 S. pimpinellifolium leaf tissue. A, pH of the unstirred layer and proton flux. B, K+ concentration of the unstirred layer and K+ flux. Continuous lines, pH or K+ concentration at 10 and 50 μm from the cell wall (left y axis). Dotted lines, Calculated H+ or K+ flux based on the pH and [K+] at 10 and 50 μm (right y axis). A vertical line indicates the moment of the addition of systemin. When the flux becomes more positive, this means either an increase of the influx or a reduction of the efflux. Typical experiments of at least three experiments are shown. C, Average size (with sd) of the amplitude of the first oscillation of the systemin-induced proton-flux change in wild-type and cu3 S. pimpinellifolium plants. The amplitudes were calculated as the difference between the flux sizes before the addition of systemin and at the top of the oscillation. The number of observations is 13 and 11 for wild type and cu3, respectively. The pH of the medium at the moment of systemin application was 6.0 ± 0.3 and 6.0 ± 0.2 for the experiments with wild type or cu3, respectively.
Figure 6.
The effects of various pharmaceuticals on the systemin-induced changes in the proton flux from cu3 S. pimpinellifolium leaf tissue. To facilitate comparison, the control proton flux from 5A is also shown. A vertical line indicates the moment of the addition of systemin (100 or 400 nm in the experiment with the Ala-17 derivative). Arrowheads indicate the addition of the indicated pharmaceuticals. When the flux becomes more positive, this means either an increase of the influx or a reduction of the efflux. The pH at the moment of addition of systemin was between 5.8 and 6.0 for all experiments. Typical experiments of at least three experiments are shown.
The presence of a developmentally regulated additional systemin receptor system could be excluded, because the effect of systemin was observed in the first true leaves from plants with at least three to five leaves.
Effects of Pharmaceuticals on the Systemin-Induced Fluxes
To compare the characteristics of the signal transduction cascade of the systemin response of both wild-type and cu3 tomato plants, the effect of effectors, known to interfere with the systemin response, were studied. Suramin at a concentration of 100 nm, 0.5 μm K252a, and 1 mm LaCl3 were added 10 min before the addition of systemin. Suramin inhibits ligand-receptor interactions (Stratmann et al., 2000; Yamaguchi et al., 2006) and has been shown to inhibit the systemin-induced alkalization in tomato (Stratmann et al., 2000); it is therefore considered to be indicative for the involvement of receptor-like kinases in the perception of systemin. In standard assay medium, suramin did not show any effect on the systemin-induced alkalization. However, when flux measurements were performed in media with a higher salt concentration (Murashige and Skoog medium or assay medium supplemented with 20 mm of KCl), suramin inhibited the systemin-induced alkalization both in cu3 (Fig. 6) and wild-type (data not shown) tomato leaf tissue. This necessity of high salt in the media suggests that for the negatively charged suramin to affect ligand-receptor interactions, it needs to be able pass the cell wall. K252a is a general protein kinase inhibitor that has also been shown to inhibit systemin-induced alkalization. The addition of this K252a inhibits the systemin-induced alkalization by cu3 (Fig. 6) and wild-type (data not shown) leaf tissue.
LaCl3 is an inorganic blocker of calcium and nonselective cationic channels, and at a concentration of 1 mm this compound blocked the ability of systemin to modulate ion fluxes in both cu3 (Fig. 6) and wild-type (data not shown) leaf tissue. The acidification resulting from the addition of La3+ is a consequence of a La3+ being a 3-fold positively charged ion. It will displace H+ ions from the cell wall constituents, which results in a nonvectorial acidification of the unstirred layer.
The Effect of Systemin and flg22 on H+ Fluxes from Leaf Tissue of 35S∷SR160/tBRI1-Containing Tobacco
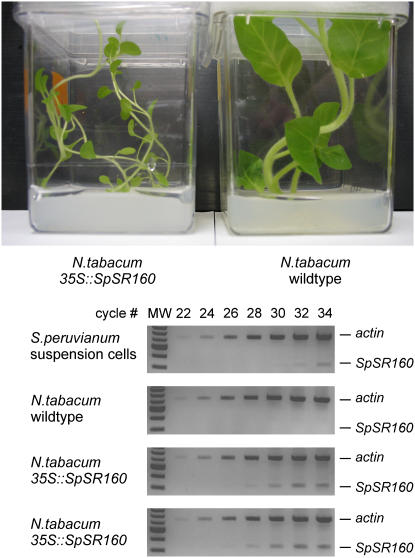
The insensitivity of Nicotianae to systemin has been exploited to demonstrate the functionality of the SR160 receptor as the systemin receptor by producing the protein in tobacco (Scheer et al., 2003). These experiments were performed with suspension cell cultures of a primary transformant. Seeds from this primary transformant were harvested and cultured until a homozygous line was obtained by the group of Dr. C.A. Ryan (personal communication). Under normal conditions, the observed phenotypical difference between wild-type and the SR160-containing tobacco was subtle elongation of the petioles in the transformed plants. However, when grown in vitro, on agar and in a closed box, the differences between the two genotypes were exaggerated and consistent with a disturbed brassinosteroid signal transduction (Fig. 7). The presence of the SpSR160 gene in kanamycin-resistant plants (100% resistant) was confirmed by PCR (data not shown), while expression of the transgene was confirmed by semiquantitative PCR (Fig. 7).
Figure 7.
Estimation of transcript levels of the tomato (S. peruvianum) BRI1 transgene in tobacco plants. Top, The phenotypical difference between wild-type and tobacco plants containing the 35S∷SpSR160 construct when grown in vitro. Bottom, Semiquantitative reverse transcription-PCR was performed to estimate the transcript levels of SpBRI1 in wild-type tobacco plants, and tobacco plants transformed with SpBRI1 (35S∷SpSR160; generated and provided by J. Scheer and C. A. Ryan; two individual plants). For comparison, SpBRI1 levels were estimated in S. peruvianum suspension-cultured cells. Primers specifically amplified SpBRI1, but not NtBRI1. ACTIN transcript levels are shown as a loading control. Molecular weight marker (1-kb ladder, New England Biolabs: only the markers from 0.5 til 5.0 kb are visible) is shown in the first lanes of each gel. Samples were taken after 22, 24, 26, 28, 30, 32, and 34 cycles.
Prepared leaf tissue from these plants and wild-type plants was used to assess their responsiveness to systemin. As expected, systemin did not induce changes in the H+ flux from wild-type tobacco leaf tissue (Fig. 4A). Transgenic tobacco plants containing the 35S∷ SpSR160/tBRI1 construct also did not show a response of the H+ flux to systemin (Fig. 4B). Nevertheless, the leaf tissues of both the wild-type and the transgenic plants responded to the application of 10 nm of flg22 (a kind gift of Dr. G. Felix) with a rapid increase of the H+ influx (25.5 ± 5.3 nmol m−2 s−1 [n = 4]) after 2 to 3 min and, consequently, an alkalinization of the assay medium after 3 to 4 min (Fig. 4C). The results presented here demonstrate that although tobacco cells are responsive to flg22, neither the wild-type nor the 35S∷SpSR160/tBRI1-containing tobacco plants respond to systemin, which indicates that systemin is not perceived or that the signal is not processed in the transgenic line that previously was shown to respond to systemin.
DISCUSSION
The MIFE Technique
Our experiments with systemin on tomato and other plant species demonstrate that MIFE is a sensitive and valuable technique suitable for the measurement of elicitor-induced changes in ion fluxes with a high spatial and temporal resolution, originating from almost intact tissue. To our knowledge, effects of systemin on ionic relations in intact tissue have not been reported before. Using intact tissue avoids the ambiguity about the physiological relevance of data obtained with cell cultures, and the MIFE technique enables the use of plant species for which cell cultures are unavailable.
The observed differences (e.g. oscillations and transient nature) between our results and the results with suspension cultures could have several explanations. In the traditional alkalinization experiments, the pH of the bulk medium is monitored, while we measure in the unstirred layer. The volume of the unstirred layer is much smaller than the volume of the bulk medium. The unstirred layer is directly adjacent to the tissue and two routes exist for ions to enter or leave the unstirred layer: exchange with either the tissue or the bulk medium. These two characteristics of the unstirred layer allow smaller changes in ion fluxes to be measured that reflect more closely the changes in the activity of the transporters. In the bulk solution, changes have to be much more massive and, therefore, will have a much lower temporal resolution, especially if one considers the transient nature of the fluxes. This also results in a higher temporal resolution of the MIFE system. Due to the proximity of the measuring electrode to the tissue, fluxes from a small number of cells are being monitored, and, therefore, the responses of very small groups of cells can be observed. Measuring in the bulk solution of a suspension culture, details can be averaged out due to stirring and the high number of cells, which all might react unsynchronized. The high resolution of the MIFE system is exemplified by the observation of oscillations in the fluxes. Oscillating H+ fluxes with comparable frequencies have been observed with other systems (Shabala et al., 1997; Shabala and Newman, 1997, 1998). This oscillatory behavior of H+ fluxes has been proposed to be the result of the oscillatory behavior of the electrogenic H+ ATPase in regulating the intracellular pH (Shabala et al., 1997).
Systemin Perception
The systemin needs to be perceived to trigger the defensive system of the plant. Peptide-like elicitors, especially those involved in the plant's defense against biotic threats, are extracellularly perceived by receptor-like kinases, such as FLS for flagellin (Gomez-Gomez and Boller, 2000), EFR for EF-Tu (Zipfel et al., 2006), SR160/tBRI1 for systemin (Scheer and Ryan, 1999, 2002; Scheer et al., 2003), and PEPR1 for atPEP1 (Yamaguchi et al., 2006).
Initially, the SR160 systemin receptor was isolated by cross-linking a photoreactive derivative of systemin to a 160-kD protein in microsomes. Two extra arguments supported the claim of this protein as the receptor of systemin. First, introduction of the SR160 gene results in sensitivity of tobacco suspension-culture cells to systemin (Scheer et al., 2003). Second, a tomato line with a nonsense mutation in the Cu3 gene, a gene highly homologous to the SR160/tBRI1gene, is impaired in the induction of defense gene products by systemin (Scheer et al., 2003). The mutation in the cu3 tomato line results in a partial receptor-like kinase without a transmembrane and kinase domain (Montoya et al., 2002) and, therefore, is highly unlikely to still be a functional receptor.
Our data indicate that the kinetical and pharmacological characteristics of the perception of systemin and the subsequent signal transduction by cu3 tomato plant are identical to those of the wild-type plant. Time dependence of the effect was similar, as was the lag time and the presence of oscillations. The receptor system in both wild-type and cu3 leaves presumably has a high affinity, as 0.1 nm systemin already triggers a considerable response. Peptide-protein interactions are involved as was suggested by the experiments with suramin (Stratmann et al., 2000). Phosphorylation is involved because of the inhibitory effect of the broad range kinase inhibitor K252a (Schaller and Oecking, 1999), and Ca2+ is an intermediate because the systemin response is inhibited by the presence of La3+ (Schaller, 1999; Schaller and Oecking, 1999). Hence, in situ cu3 tomato leaves are able to perceive systemin in a similar way as wild-type leaves and are at least able to effectuate this signal into changes of ion fluxes, comparable with these from wild type. Thus, cu3 plants still contain a functional systemin receptor with characteristics identical to the one present in wild-type plants. The possibility that a developmentally regulated, additional perception mechanism only present in the youngest leaves or cell cultures (Scheer et al., 2003; Holton et al., 2007) is responsible for this discrepancy can be excluded, because the first true leaves from plants with at least three to five leaves were consistently used in our experiments. Our findings mean that cu3 and wild type respond identically to systemin and, hence, that the Cu3 receptor is not the systemin receptor.
Additional indications for the SR160/tBRI1 receptor not being the systemin receptor are obtained from our MIFE studies with intact tissue of the transgenic tobacco line, which harbors the SR160/tBRI1 gene of tomato (Scheer et al., 2003). In these transgenic plants, the presence of SR160/tBRI1 transcript could be confirmed, and they show a phenotype that is consistent with a disturbed brassinosteroid signal transduction. Hence, these plants produce a functional brassinosteroid receptor. However, we were unable to detect flux changes provoked by systemin in the transgenic line. The absence of a systemin-induced alkalinization in the SR160/tBRI1 transgene-containing tobacco plants indicates that the plants do not display a functional systemin receptor at their plasma membrane. Tobacco is responsive to several other elicitors (Felix et al., 1999) with changes in ion fluxes, as is evident from our experiments with flg22 in both wild-type and the transgenic line tobacco line. However, it is possible that production of a functional SR160/tBRI1 receptor is restricted to certain tissues or developmental stages that we have not tested.
The Reduced Defense Gene Expression in the cu3 Mutant Tomato Plant
The ability of the cu3 mutant tomato plant to perceive systemin necessitates an alternative explanation for the reduced, but not completely abolished, induction of defense genes in this mutant (Scheer et al., 2003). First, in the experiments demonstrating the reduced induction, systemin is applied through the cut stem of 2-week-old seedlings. The statures of wild-type and mutant plants differ greatly; while the wild-type plant shows a normal open structure, the mutants are dwarf like and display a compact structure. Transpiration by the cu3 mutant is reduced compared to wild type, and, hence, systemin uptake and its distribution throughout the cu3 plant differs from wild type (Holton et al., 2007), resulting in the reduction of the expression level of defense genes and the typical expression pattern observed in cu3 plants (Scheer et al., 2003).
Second, early systemin-induced ion flux changes could depend on a receptor different from the one necessary to evoke changes in gene expression. The Cu3 receptor, then, is the one involved in regulation of defense gene expression and not involved in regulation of ion fluxes. However, ion flux modulations are essential components for the induction of defense genes (Schaller and Oecking, 1999). Moreover, in an analogous system, the receptor of flagellin, FLS2, functions as the sole receptor in the induction of both early responses (e.g. hydrogen peroxide production and ion flux changes) and late responses (e.g. callose deposition; Gomez-Gomez and Boller, 2000).
Third, interaction of brassinosteroids with defense responses might result in a reduced systemin-induced defense gene expression of the cu3 plants. Scheer et al. (2003) demonstrated a competitive inhibition of brassinosteroid on the induction of proteinase inhibitors in wild-type tomato plants, which they explain as a result of systemin and brassinosteroid sharing the receptor. It has been demonstrated that cu3 mutant plants contain an approximately 27-fold higher level of endogenous brassinosteroid (Montoya et al., 2002) but also that plants contain more functional brassinosteroid receptor proteins (Cano-Delgado et al., 2004; Sun et al., 2004; Zhou et al., 2004). The interaction between these additional brassinosteroid receptors and the highly increased level of brassinosteroid could effectuate the observed inhibition of defense gene expression in the cu3 mutant. Such an interaction between systemin and brassinosteroid perception could be effectuated via ethylene (Holton et al., 2007) or could result from an interaction between the systemin receptor and BAK1 in a manner similar to the recently demonstrated interaction of SERK3/BAK1 with FLS2 and EFR signaling (Chinchilla et al., 2007; He et al., 2007; Heese et al., 2007; Kemmerling et al., 2007).
Fourth, experiments have been presented that suggest stimulating effects of brassinosteroids on plant defense responses (Krishna, 2003; Nakashita et al., 2003; Mussig et al., 2006). Absence of the major brassinosteroid receptor in tomato and, thus, absence of a possible positive effect of brassinosteroids on defense responses could, therefore, result in the reduced induction of proteinase inhibitor genes as observed in the cu3 mutant tomato plant (Scheer et al., 2003). Recently, Holton et al. (2007) observed necrotic patches in cu3 mutant plants complemented with the 35S:tBRI1, which might indicate an interaction between brassinosteroid signaling and plant defense responses.
The phenotype of the cu3 mutant plant clearly points to the Cu3 protein as being the major brassinosteroid receptor in tomato, a notion that is further supported by the phenotype of the tobacco plants that contain the SR160/tBRI1 transgene. The data presented in Holton et al. (2007) and our data cast serious doubts on the claim that this protein is also a systemin receptor. Together, these observations suggest that a receptor other than the SR160/tBRI is the principal systemin receptor. Additional biochemical studies on systemin binding in and the isolation of the systemin receptor of cu3 plants should help to elucidate this enigma.
MATERIALS AND METHODS
Plant Material
Plants were grown on soil in a greenhouse. Light conditions were maintained at 14 h light/10 h dark with supplementary light when necessary. Temperature conditions were set at 22°C during the light period and 16°C during the dark period. Solanum jasminoides was grown in the garden of the spouse of one of the authors. For cu3 plants, the first true leaves from plants with at least three to five leaves were used, because the younger leaves of cu3 plants were difficult to prepare for the flux measurements.
Leaves were cut from the plants and transported in a humid container. The abaxial epidermis was removed with a forceps, and pieces of approximately 4 to 7 mm2 were cut from the leaf. The pieces were placed floating on experimental solution (0.2 mm CaCl2, 0.1 mm KCl, 0.1 mm MgCl2, with or without 0.5 mm MES-1,3-Bis(Tris[hydroxymethyl]methylamino) propane, pH 6.0) with the abaxial side down. Buffering of the medium resulted in a more uniform pH of the medium during the start of the experiment. Because the pH of the medium determines the size of the influx of protons (compare Figs. 1 with 2), more comparable fluxes were obtained by buffering the medium. An inverse linear relationship between the logarithm of the flux size and the pH of the medium was observed (Supplemental Fig. S1).
Seeds of Solanum nigrum were kindly provided by Dr. I. Baldwin.
Ion Flux Experiments
Net fluxes of H+ and K+ from leaves were measured using H+- and K+-selective microelectrodes with the MIFE technique (Shabala et al., 1997; Newman, 2001; Vreeburg et al., 2005). Microelectrodes were pulled from borosilicate glass capillaries (GC150-10; Harvard Apparatus) and silanized with tributylchlorosilane (Fluka 90974). The H+-selective electrodes were back filled with 15 mm of NaCl plus 40 mm of KH2PO4 and front filled with Hydrogen Ionophore II (Cocktail A; Fluka 95297). The average response of the H+ electrodes was 56.7 ± 1.0 mV per decade (i.e. per pH unit; pH range 5.1–7.8). The K+-selective electrodes were back filled with 200 mm of KCl and front filled with Potassium Ionophore I (Cocktail A; Fluka 60031). The average response of the K+ electrodes was 42.8 ± 3.3 mV per decade (i.e. per pK unit; K+ concentrations for calibration were 0.1, 1, and 10 mm). This small response voltage was always observed and is considered to be the result of a small leakage of ions in the tip of the electrode. Apparently, the sealing between the silanized glass and the ionophore mix was not optimal. To avoid potassium ion leakage from the reference electrode, the reference electrode was placed in a compartment different from the measuring chamber. The two compartments were electrically connected via a salt bridge, which consisted of 300 mm (NH4)2SO4 in 2% (w/v) agar.
Leaf material was immobilized on a glass capillary using grease (consisting of 49% petroleum jelly, 34% bee wax, and 17% lanoline) with the abaxial epidermisless side exposed to the solution and placed in a measuring chamber with a transparent bottom. The chamber was filled with 1 mL of experimental solution, submerging the leaf material. The whole chamber was placed on a Nikon TMS inverted microscope. The ion-selective microelectrodes were mounted at an angle between 30° and 40° with the horizontal in a holder (MMT-5; Narishige) on a three-way piezo-controlled micromanipulator (PCT; Luigs and Neumann) driven by a computer-controlled motor (MO61-CE08; Superior Electric). The electrodes were positioned 10 μm from the surface of the tissue. During measurements, the distances between the tissue and the electrodes were changed from 10 to 50 μm at a frequency of 0.1 Hz. The chemical activities of H+ and K+ in solution were continuously recorded at the two distances from the tissue, and from these data, net H+ and K+ fluxes were calculated according to Newman (2001). Whereas positioning the material in the MIFE apparatus was performed under light conditions (150 μmol m−2 s−1), the measurements were performed in the dark at ambient room temperature.
Stock solutions of 100 μm systemin (water) or its Ala-17 derivative (water), 0.5 m LaCl3 (water), 0.1 m suramin (water), and 200 μm K252a (dimethyl sulfoxide) were made. When the effect of K252a was tested, controls with the same dimethyl sulfoxide concentrations were performed.
Control experiments demonstrated that apparent flux and ion concentration changes can be introduced by the disturbance caused by the addition of the solutes during the first 90 s (Supplemental Fig. S2). Hence, the data within this interval after the addition was accordingly discarded.
Tissue Vitality Control
After the experiments, the vitality of the tissue was checked by monitoring the light/dark response of the tissue (Supplemental Fig. S3). During the light period, photosynthesis in vital tissue results in a removal of HCO3− from the bulk solution and, consequently, an alkalinization, whereas during the dark period, respiration produces CO2 and, as a result, the bulk medium acidifies (a change in H+ flux of at least 20 nmol m−2 s−1 at a pH of approximately 6.0 was considered to indicate full vitality).
Semiquantitative Reverse Transcription-PCR
Semiquantitative reverse transcription-PCR was performed to estimate the transcript levels of SpBRI1 in wild-type tobacco (Nicotiana tabacum) plants. Seeds from line 2 of transgenic 35S∷SR160/tBRI1 tobacco plants were obtained from C.A. Ryan (Scheer et al., 2003) and were germinated on Murashige and Skoog medium containing kanamycin (50 mg/L). At the two-leaf stage, they were transferred to soil and grown under standard conditions in a growth chamber (27°C, 500 μmol m−2 s−1, 16-h-light:8-h-dark regime). Leaves were extracted for mRNA analysis 1 month after the transfer to soil (approximately 20 cm tall).
Total RNA from tobacco leaves was isolated according to Chomczynski et al. (1997) using TRIZOL reagent (Invitrogen). First-strand cDNA synthesis on 6 μg of total RNA was done using the Super Script cDNA synthesis kit (Invitrogen). Two microliters from the total 20-μL volume of cDNA was used for PCR amplification using TripleMaster polymerase (Eppendorf) in a 50-μL reaction volume according to the following program: 95°C for 1 min, then 34 cycles consisting of 95°C for 15 s; 56°C for 30 s, and 72°C for 45 s. During the PCR, 5-μL samples were taken at cycle numbers 22, 24, 26, 28, 30, 32, and 34. The primers used for the SpSR160 detection were: SR160cdsf, 5′-ATGAAAGCTCACAAAACTGT-3′ and SR160r, 5′-CAAATTAGAAAGGGGAAGCAAA-3′. These primers are specific for the tomato (Solanum lycopersicum) ortholog and do not amplify tobacco BRI1 (Fig. 7). As loading controls, ACTIN was amplified using the following primers that amplify tomato and tobacco actin: ActinF, 5′-ATGACTCAAATCATGTTTGAGACCTTC-3′ and ActinR, 5′-ACCTTAATCTTCATGCTGCTTGGAGC-3′.
Systemin and Ala-17 Derivatives
Systemin and its Ala-17 derivative were kindly donated to us by Dr. C.A. Ryan.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The inverse relationship between the pH at the moment of the application of systemin and the logarithm of the amplitude of the first oscillation of the systemin-induced proton-flux change in wild-type and cu3 S. pimpinellifolium plants.
Supplemental Figure S2. The effect of adding a solution in an experiment without plant tissue.
Supplemental Figure S3. Typical light-dark response effects of vital stripped cu3 S. pimpinellifolium leaf tissue.
Supplementary Material
Acknowledgments
We thank Marijke Korstenbroek for generously providing the S. jasminoides tissue, Dr. I. Baldwin for providing seeds of S. nigrum, and Dr. C.A. Ryan for donating the systemin and its Ala-17 analog. We appreciate the constructive discussions with the late Dr. C.A. Ryan and members of his group.
This work was supported by the U.S. National Science Foundation (award no. 0418890 to J.W.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Frank C. Lanfermeijer (f.c.lanfermeijer@rug.nl).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bergey DR, Ryan A (1999) Wound- and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol Biol 40 815–823 [DOI] [PubMed] [Google Scholar]
- Cano-Delgado A, Yin Y, Yu C, Vafeados D, Mora-Garcia S, Cheng JC, Nam KH, Li J, Chory J (2004) BRL1 and BRL3 are novel brassinosteroid receptors that function in vascular differentiation in Arabidopsis. Development 131 5341–5351 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JDG, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448 497–500 [DOI] [PubMed] [Google Scholar]
- Choe S (2006) Brassinosteroid biosynthesis and inactivation. Physiol Plant 126 539–548 [Google Scholar]
- Chomczynski P, Mackey K, Drews R, Wilfinger W (1997) DNAzol: a reagent for the rapid isolation of genomic DNA. Biotechniques 22 550–553 [DOI] [PubMed] [Google Scholar]
- Constabel CP, Yip L, Ryan CA (1998) Prosystemin from potato, black nightshade, and bell pepper: primary structure and biological activity of predicted systemin polypeptides. Plant Mol Biol 36 55–62 [DOI] [PubMed] [Google Scholar]
- Farmer EE, Almeras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6 372–378 [DOI] [PubMed] [Google Scholar]
- Felix G, Boller T (1995) Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianum cells. Plant J 7 381–389 [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18 265–276 [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5 1003–1011 [DOI] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J (2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17 1109–1115 [DOI] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, Schroeder JI, Peck SC, Rathjen JP (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton N, Cano-Delgado A, Harrison K, Montoya T, Chory J, Bishop GJ (2007) Tomato BRASSINOSTEROID INSENSITIVE1 is required for systemin-induced root elongation in Solanum pimpinellifolium but is not essential for wound signaling. Plant Cell 19 1709–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffaker A, Pearce G, Ryan CA (2006) An endogenous peptide signal in Arabidopsis activates components of the innate immune response. Proc Natl Acad Sci USA 103 10098–10103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandoth PK, Ranf S, Pancholi SS, Jayanty S, Walla MD, Miller W, Howe GA, Lincoln DE, Stratmann JW (2007) Tomato MAPKs LeMPK1, LeMPK2, and LeMPK3 function in the systemin-mediated defense response against herbivorous insects. Proc Natl Acad Sci USA 104 12205–12210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerling B, Schwedt A, Rodriguez P, Mazzotta S, Frank M, Qamar SA, Mengiste T, Betsuyaku S, Parker JE, Mussig C, et al (2007) The BRI1-associated kinase 1, BAK1, has a brassinolide-independent role in plant cell-death control. Curr Biol 17 1116–1122 [DOI] [PubMed] [Google Scholar]
- Krishna P (2003) Brassinosteroid-mediated stress responses. J Plant Growth Regul 22 289–297 [DOI] [PubMed] [Google Scholar]
- Lee GI, Howe GA (2003) The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J 33 567–576 [DOI] [PubMed] [Google Scholar]
- Li L, Li C, Lee GI, Howe GA (2002) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA 99 6416–6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo O, Solano R (2005) Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol 8 532–540 [DOI] [PubMed] [Google Scholar]
- McGurl B, Pearce G, Orozco-Cardenas M, Ryan CA (1992) Structure, expression, and antisense inhibition of the systemin precursor gene. Science 255 1570–1573 [DOI] [PubMed] [Google Scholar]
- Meindl T, Boller T, Felix G (1998) The plant wound hormone systemin binds with the N-terminal part to its receptor but needs the C-terminal part to activate it. Plant Cell 10 1561–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya T, Nomura T, Farrar K, Kaneta T, Yokota T, Bishop GJ (2002) Cloning the tomato Curl3 gene highlights the putative dual role of the leucine-rich repeat receptor kinase tBRI1/SR160 in plant steroid hormone and peptide hormone signaling. Plant Cell 14 3163–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyen C, Hammond-Kosack KE, Jones J, Knight MR, Johannes E (1998) Systemin triggers an increase of cytoplasmic calcium in tomato mesophyll cells: Ca2+ mobilization from intra- and extracellular compartments. Plant Cell Environ 21 1101–1111 [Google Scholar]
- Moyen C, Johannes E (1996) Systemin transiently depolarizes the tomato mesophyll cell membrane and antagonizes fusicoccin-induced extracellular acidification of mesophyll tissue. Plant Cell Environ 19 464–470 [Google Scholar]
- Mussig C, Lisso J, Coll-Garcia D, Altmann T (2006) Molecular analysis of brassinosteroid action. Plant Biol 8 291–296 [DOI] [PubMed] [Google Scholar]
- Nakashita H, Yasuda M, Nitta T, Asami T, Fujioka S, Arai Y, Sekimata K, Takatsuto S, Yamaguchi I, Yoshida S (2003) Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant J 33 887–898 [DOI] [PubMed] [Google Scholar]
- Narvaez-Vasquez J, Florin-Christensen J, Ryan CA (1999) Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 11 2249–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez-Vasquez J, Orozco-Cardenas ML, Ryan CA (1994) A sulfhydryl reagent modulates systemic signaling for wound-induced and systemin-induced proteinase inhibitor synthesis. Plant Physiol 105 725–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman IA (2001) Ion transport in roots: measurement of fluxes using ion-selective microelectrodes to characterize transporter function. Plant Cell Environ 24 1–14 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc Natl Acad Sci USA 96 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, Narvaez-Vasquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13 179–191 [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Johnson S, Ryan CA (1993) Structure-activity of deleted and substituted systemin, an 18-amino acid polypeptide inducer of plant defensive genes. J Biol Chem 268 212–216 [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253 895–898 [DOI] [PubMed] [Google Scholar]
- Ryan CA (2000) The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta 1477 112–121 [DOI] [PubMed] [Google Scholar]
- Ryan CA, Moura DS (2002) Systemic wound signaling in plants: a new perception. Proc Natl Acad Sci USA 99 6519–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA, Pearce G (2003) Systemins: a functionally defined family of peptide signals that regulate defensive genes in Solanaceae species. Proc Natl Acad Sci USA 100 14577–14580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller A (1999) Oligopeptide signalling and the action of systemin. Plant Mol Biol 40 763–769 [DOI] [PubMed] [Google Scholar]
- Schaller A, Oecking C (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JM, Pearce G, Ryan CA (2003) Generation of systemin signaling in tobacco by transformation with the tomato systemin receptor kinase gene. Proc Natl Acad Sci USA 100 10114–10117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JM, Ryan CA (1999) A 160-kD systemin receptor on the surface of Lycopersicon peruvianum suspension-cultured cells. Plant Cell 11 1525–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JM, Ryan CA (2002) The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc Natl Acad Sci USA 99 9585–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Howe GA (2005) Systemic signaling in the wound response. Curr Opin Plant Biol 8 369–377 [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA (1997) Proton and calcium flux oscillations in the elongation region correlate with root nutation. Physiol Plant 100 917–926 [PubMed] [Google Scholar]
- Shabala SN, Newman IA (1998) Osmotic sensitivity of Ca2+ and H+ transporters in corn roots: effect on fluxes and their oscillations in the elongation region. J Membr Biol 161 45–54 [DOI] [PubMed] [Google Scholar]
- Shabala SN, Newman IA, Morris J (1997) Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiol 113 111–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Ryan CA (1997) Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc Natl Acad Sci USA 94 11085–11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Scheer J, Ryan CA (2000) Suramin inhibits initiation of defense signaling by systemin, chitosan, and a beta-glucan elicitor in suspension-cultured Lycopersicon peruvianum cells. Proc Natl Acad Sci USA 97 8862–8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Fokar M, Asami T, Yoshida S, Allen RD (2004) Characterization of the brassinosteroid insensitive 1 genes of cotton. Plant Mol Biol 54 221–232 [DOI] [PubMed] [Google Scholar]
- Szekeres M (2003) Brassinosteroid and systemin: two hormones perceived by the same receptor. Trends Plant Sci 8 102–104 [DOI] [PubMed] [Google Scholar]
- Tegg RS, Melian L, Wilson CR, Shabala S (2005) Plant cell growth and ion flux responses to the streptomycete phytotoxin thaxtomin A: calcium and hydrogen flux patterns revealed by the non-invasive MIFE technique. Plant Cell Physiol 46 638–648 [DOI] [PubMed] [Google Scholar]
- Vreeburg RAM, Benschop JJ, Peeters AJM, Colmer TD, Ammerlaan AHM, Staal M, Elzenga JTM, Staals RHJ, Darley CP, Queen-Mason SJ, et al (2005) Ethylene regulates fast apoplastic acidification and expansin A transcription during submergence-induced petiole elongation in Rumex palustris. Plant J 43 597–610 [DOI] [PubMed] [Google Scholar]
- Wang ZY, He JX (2004) Brassinosteroid signal transduction: choices of signals and receptors. Trends Plant Sci 9 91–96 [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Pearce G, Ryan CA (2006) The cell surface leucine-rich repeat receptor for AtPep1, an endogenous peptide elicitor in Arabidopsis, is functional in transgenic tobacco cells. Proc Natl Acad Sci USA 103 10104–10109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou A, Wang H, Walker JC, Li J (2004) BRL1, a leucine-rich repeat receptor-like protein kinase, is functionally redundant with BRI1 in regulating Arabidopsis brassinosteroid signaling. Plant J 40 399–409 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JDG, Boller T, Felix G (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125 749–760 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.