Abstract
Hydrogen peroxide (H2O2) is involved in plant defense responses that follow mechanical damage, such as those that occur during herbivore or insect attacks, as well as pathogen attack. H2O2 accumulation is induced during wound healing processes as well as by treatment with the wound signal jasmonic acid. Plant polyamine oxidases (PAOs) are H2O2 producing enzymes supposedly involved in cell wall differentiation processes and defense responses. Maize (Zea mays) PAO (ZmPAO) is a developmentally regulated flavoprotein abundant in primary and secondary cell walls of several tissues. In this study, we investigated the effect of wounding on ZmPAO gene expression in the outer tissues of the maize mesocotyl and provide evidence that ZmPAO enzyme activity, protein, and mRNA levels increased in response to wounding as well as jasmonic acid treatment. Histochemically detected ZmPAO activity especially intensified in the epidermis and in the wound periderm, suggesting a tissue-specific involvement of ZmPAO in wound healing. The role played by ZmPAO-derived H2O2 production in peroxidase-mediated wall stiffening events was further investigated by exploiting the in vivo use of N-prenylagmatine (G3), a selective and powerful ZmPAO inhibitor, representing a reliable diagnostic tool in discriminating ZmPAO-mediated H2O2 production from that generated by peroxidase, oxalate oxidase, or by NADPH oxidase activity. Here, we demonstrate that G3 inhibits wound-induced H2O2 production and strongly reduces lignin and suberin polyphenolic domain deposition along the wound, while it is ineffective in inhibiting the deposition of suberin aliphatic domain. Moreover, ZmPAO ectopic expression in the cell wall of transgenic tobacco (Nicotiana tabacum) plants strongly enhanced lignosuberization along the wound periderm, providing evidence for a causal relationship between PAO and peroxidase-mediated events during wound healing.
Reactive oxygen species (ROS) are common components of plant developmental processes and defense responses. Their roles in developmental programmed cell death (PCD; for review, see Rogers, 2005), stomatal closure (Pei et al., 2000; Kwak et al., 2003), root gravitropism (Joo et al., 2001), and hair cell expansion (Foreman et al., 2003) are at present widely accepted. Rapid generation of ROS is also a basic component of a plant's response to pathogen challenges (for review, see Apel and Hirt, 2004) and wound stress (Orozco-Cárdenas et al., 2001), such as those which occur during physical injury and herbivore or insect attacks. ROS contribute to plant disease resistance either exerting a direct antimicrobical activity and/or by driving peroxidase-mediated oxidative cross-linking of cell wall components. Moreover, ROS induce hypersensitive response (HR) cell death in infected areas (Levine et al., 1994; Solomon et al., 1999; Delledonne et al., 2001) and behave as second messengers in signal transduction pathways leading to increased expression of defense genes (Orozco-Cárdenas et al., 2001; Jih et al., 2003; Takahashi et al., 2004; for review, see Apel and Hirt, 2004). The production of ROS in the cell wall is achieved by different enzymatic sources, some of which are membrane-bound NADPH oxidases (for review, see Torres and Dangl, 2005) and apoplastic oxalate oxidases (for review, see Lane, 2002), peroxidases (for review, see Bolwell et al., 2002), and amine oxidases (for review, see Cona et al., 2006a). All of these ROS delivering systems have been involved in plant defense responses, depending on tissue sources and disease identities. Up to date, NADPH oxidase, the plant homolog of the gp91phox subunit of the mammalian phagocyte NADPH oxidase, is believed to be the principal source of extracellular ROS, in both development and defense. Indeed, NADPH oxidase is involved in pathogen resistance in Arabidopsis (Arabidopsis thaliana; Torres et al., 2002; Love et al., 2005), tobacco (Nicotiana tabacum; Simon-Plas et al., 2002; Yoshioka et al., 2003), and potato (Solanum tuberosum; Kobayashi et al., 2006), wound healing in tomato (Solanum lycopersicum; Sagi et al., 2004), and ABA-induced antioxidant defense in maize (Zea mays) leaves (Jiang and Zhang, 2003). Moreover, this enzyme has recently been demonstrated to also have a role in suppressing the spread of cell death to the uninfected cells surrounding HR sites (Torres et al., 2005). Nevertheless, the specific contribution of each of the other ROS enzymatic sources to the apoplastic ROS production deserves attention and demands further analysis. In this connection an increasing amount of evidence indicates amine oxidases as players in extracellular hydrogen peroxide (H2O2) production during plant development and defense (Allan and Fluhr, 1997; Cona et al., 2006a). Amine oxidases are represented by an heterogeneous group of enzymes, including copper-containing amine oxidases (CuAO) and flavin-containing polyamine oxidases (PAO). Plant CuAO and PAO oxidize polyamines at the primary and secondary amino group, respectively, producing an aminoaldehyde, H2O2, as well as NH3 (CuAO) or 1,3-diaminopropane (Dap; PAO).
H2O2 deriving from polyamine oxidative catabolism has been correlated to cell wall stiffening events responsible for developmental cell wall maturation in chickpea (Cicer arietinum; Cona et al., 2006a), tobacco (Paschalidis and Roubelakis-Angelakis, 2005), and maize (Cona et al., 2003). Maize PAO (ZmPAO; formerly MPAO) has been involved in the light-induced inhibition of maize mesocotyl growth, since its expression level is induced by light and inhibited by auxin in the external mesocotyl tissues (Cona et al., 2003). Consistently, a progressive redistribution of ZmPAO from cytoplasm toward primary and secondary walls has been observed as a consequence of developmental or light-induced tissue differentiation (Cona et al., 2005). Moreover, a role has been suggested for amine oxidases in developmental PCD occurring in differentiating tracheary elements in maize (Cona et al., 2005) and Arabidopsis (Møller and McPherson, 1998).
CuAO have also been involved in extracellular H2O2 production during defense responses to pathogen attacks and wound healing (Cona et al., 2006a). In chickpea seedlings CuAO expression is strongly induced in response to wounding as well as after treatment with jasmonic acid (JA), a wound signal (Rea et al., 2002). Furthermore, the defense responses of resistant chickpea ‘Sultano’ infected with Ascochyta rabiei are severely impaired by CuAO inhibition (Rea et al., 2002). Treatment with methyl jasmonate, a plant regulator known to induce systemic protection from powdery mildew infection in field-grown barley (Hordeum vulgare; Walters et al., 2002), has been shown to enhance CuAO expression in tobacco (Biondi et al., 2001) and barley plants (Walters et al., 2002).
Analogously to what has been demonstrated in human cancer cells, which can undergo PCD through catabolism of intracellular polyamines (Ha et al., 1997), H2O2 derived from polyamine degradation has been shown to contribute to HR cell death in plants. Indeed, in tobacco plants infected with Tobacco mosaic virus (TMV), an increase in polyamine content as well as in its biosynthetic and catabolic enzymes has been observed (Yoda et al., 2003). Moreover, tobacco treatment with α-difluoromethyl-Orn or guazatine, respectively, inhibitors of Orn decarboxylase and PAO, reduced HR cell death caused by TMV infection (Yoda et al., 2003). In addition, the fungal cell wall-derived elicitor cryptogein induced HR cell death in wild-type tobacco cells, but it was ineffective in the transgenic BY-2 cells, in which tobacco PAO (NtPAO) activity was suppressed by RNAi silencing (Yoda et al., 2006). Furthermore, H2O2 derived from spermine (Spm) oxidation has been involved in the induction of HR- and defense-related gene expression in tobacco leaves infected by TMV, through a signal transduction pathway involving mitochondrial dysfunction and mitogen-activated protein kinase activation (Takahashi et al., 2003, 2004).
The aim of this study was to analyze the specific contribution of ZmPAO to the apoplastic ROS production during the wound healing response in the maize mesocotyl, exploiting the in vivo use of N-prenylagmatine (G3; Ki = 1.5 × 10−8 m), a powerful inhibitor of ZmPAO activity (Federico et al., 2001; Cona et al., 2004), whose selectivity toward ZmPAO has been recently addressed both in vitro and in vivo (Cona et al., 2006b). Indeed, the reliability of G3 as an inhibitor in discriminating ZmPAO-mediated H2O2 production in an in vivo multicomponent ROS production system such as that operating in the apoplast, was supported by its inability to affect peroxidase, oxalate oxidase, and NADPH oxidase enzyme activity. The involvement of ZmPAO-mediated H2O2 production in wound healing was further investigated by means of transgenic tobacco plants constitutively expressing high level of ZmPAO in the cell wall. Here, we suggest that extracellular ZmPAO activity is involved in defense response to wounding by delivering the H2O2 required for the peroxidase-mediated suberin polyphenolic domain and lignin synthesis.
RESULTS
Wounding Induces an Increase in ZmPAO Expression Level
To investigate the involvement of ZmPAO in the extracellular production of H2O2 during wound healing, we performed a time-course analysis of ZmPAO activity levels as well as ZmPAO protein and mRNA accumulation in response to wounding in the outer tissues (i.e. cortical and epidermal tissues) of the nongrowing zone of the maize mesocotyl. These tissues, in which ZmPAO gene expression was previously demonstrated to be up-regulated by light and down-regulated by auxin (Cona et al., 2003), represent a well-characterized and suitable system for studying ZmPAO regulation due to their low levels of basal ZmPAO enzyme activity in dark-grown seedlings as well as to their ability to rapidly respond to external stimuli, such as in the case of light. This study reports the local and systemic induction of extractable ZmPAO expression after mesocotyl wounding of dark-grown seedlings, already detectable in the mesocotyl outer tissues after 12 h from injury. To avoid photomorphogenic effects on ZmPAO activity (Cona et al., 2003), seedlings were kept in the dark for all the duration of the experiments and every technical operation was performed under photomorphogenically inactive green light. No differences in stem growth rate were observed between wounded and control plants at any time checked.
As shown in Figure 1A, the level of extractable ZmPAO activity expressed on a fresh weight basis increased in the mesocotyl outer tissues of wounded plants as compared to the respective unwounded (control) plants until the last time checked (72 h), when its level was 2-fold that of control plants. The greatest difference was detectable 24 h after wounding (Fig. 1A). Moreover, a systemic induction of extractable ZmPAO activity was observed in coleoptiles either at 24 or at 48 h after mesocotyl wounding (Fig. 2). Very similar results were obtained when extractable ZmPAO activity was expressed on total protein basis (data not shown). Experiments reported in Figures 1 and 2 were performed using 5-d-old seedlings grown on paper under loam (average stem length = 6.5 cm); however, similar results were obtained when 3-d-old seedlings germinated on paper were kept into a hydroponic culture containing a nutrient solution for 3 d before being wounded (data not shown; average stem length = 6.5 cm).
Figure 1.
Time-course analysis of ZmPAO expression during wound healing in the outer tissues of the nongrowing zone of the maize mesocotyl. Five-day-old seedlings were longitudinally wounded with a blade on the nongrowing zone of the mesocotyl. Two-centimeter-long segments were sampled, after eliminating 1 cm above the seed from unwounded (control) and wounded plants at the indicated time after wounding. Cortical plus epidermal tissues (outer tissues) were obtained by drawing out the stele, and used for determination of extractable ZmPAO activity, western-blot, and northern-blot analysis. A, Extractable ZmPAO activity levels (U: IUs; mean values ± se; n = 5) expressed on a fresh weight (FW) basis. P values indicate statistical significance of differences between ZmPAO activity levels in wounded with respect to control samples for each time. ns, Not significant; *, **, and ***, P values ≤ 0.05, 0.01, and 0.001, respectively. B, Western-blot and northern-blot analyses (samples as in A). Western immunoblotting of extractable ZmPAO fractions was performed after SDS-PAGE loaded on the basis of the total protein content (20 μg/well; top insert). Total RNA was fractionated by agarose/formaldehyde gel electrophoresis, blotted onto a nylon membrane, and hybridized with 32P-labeled maize PAO cDNA probe (middle insert). As a loading control, samples were also hybridized with the cDNA of the S13 ribosomal protein (bottom insert). C, Control; W, wounded; subscripted numbers indicate sampling time.
Figure 2.
Systemic induction of extractable ZmPAO activity in maize coleoptiles. Five-day-old seedlings were longitudinally wounded with a blade on the nongrowing zone of the mesocotyl and coleoptiles were sampled from unwounded (control) and wounded plants at the indicated time after wounding. Extractable ZmPAO activity levels (U: IUs; mean values ± se; n = 5) are expressed on a fresh weight (FW) basis. P values indicate statistical significance of differences between ZmPAO activity levels in wounded with respect to control samples for each time. ns, Not significant; *, **, and ***, P values ≤ 0.05, 0.01, and 0.001, respectively.
ZmPAO protein accumulation patterns in the outer tissues taken from wounded and control plants (Fig. 1B, top insert) resembled the relative ZmPAO activity values at each specific time (Fig. 1A). Indeed a ZmPAO protein band of higher intensity was already evident at 12 h after injury (Fig. 1B, top insert). Moreover higher ZmPAO protein levels were observed in the wounded with respect to control seedlings at every time checked (Fig. 1B, top insert) until 72 h from wounding (data not shown).
Northern-blot analysis of the total RNA purified from mesocotyl outer tissues at different time was performed using ZmPAO cDNA (EMBL DataBase accession number AJ002204) as a probe (Fig. 1B, middle insert; Tavladoraki et al., 1998) or maize rp-S13 (GenBank accession number AF067732) as loading control (Fig. 1B, bottom insert). As shown in Figure 1B (middle insert), the increase in ZmPAO mRNA accumulation preceded ZmPAO activity and protein level increases, since a much more intense hybridizing band was detectable in wounded samples 6 h after injury. A very strong increase in ZmPAO mRNA level was evident in wounded samples as compared to the respective control at 12 and 24 h and was still present at 48 h after injury.
No difference occurred after wounding in wall-bound to extractable ZmPAO activity ratio, since wall-bound ZmPAO activity increased to the same extent as the extractable enzyme activity at every time checked (data not shown). This result is consistent with previous studies reporting that no significant differences were observed in wall-bound to extractable ZmPAO activity ratio after light or auxin treatments (Cona et al., 2003).
Wound-Induced ZmPAO Activity Increase Is Evident in the Epidermis and Wound Periderm
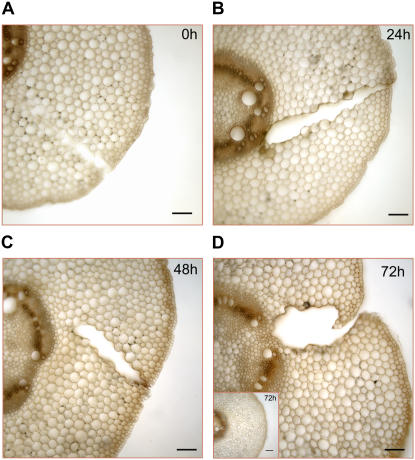
To investigate the tissue localization of the wound-induced ZmPAO activity increase, ZmPAO activity was histochemically detected by 3,3′-diaminobenzidine (DAB) staining in transverse sections of mesocotyl from wounded maize plants at 0, 24, 48, and 72 h after wounding (Fig. 3). The specificity of staining for PAO activity was confirmed by the evidence that sections from wounded plants, incubated in the staining solution lacking spermidine (Spd), were unstained (data not shown). Histochemical ZmPAO activity gradually intensified in the epidermis and in the wound periderm, displaying a strong staining 72 h after injury (Fig. 3D). Cortical parenchyma also exhibited a DAB staining increase from 0 to 72 h after wounding, although to a lower extent. As expected (Cona et al., 2005, 2006b), the high levels of ZmPAO activity in stelar tissues caused a strong DAB staining in all the samples, without any detectable difference among unwounded (control) and wounded plants at any time checked. Since no histochemical ZmPAO activity increase was detected in the mesocotyl epidermis and cortical parenchyma from unwounded (control) plants (Fig. 3D, inset), the ZmPAO activity increase observed in the corresponding tissues from wounded plants could reasonably be ascribed to wound healing response rather than to development.
Figure 3.
Histochemically detected ZmPAO activity in transversal mesocotyl sections from wounded maize seedlings. Hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the mesocotyl and from the corresponding zone from unwounded (control) plants were utilized for light microscopic investigation. ZmPAO activity was histochemically detected at 0 (A), 24 (B), 48 (C), and 72 (D) h after wounding using a peroxidase-coupled assay with DAB as the chromogenic artificial substrate. After washing in distilled water, sections were preincubated in sodium phosphate buffer 10 mm, pH 6.5, containing 60 μg/mL peroxidase and 0.04% DAB for 10 min and then incubated with 3 mm Spd. Reactions were blocked after 3 min by thoroughly washing sections in distilled water. Inset illustrates histochemical ZmPAO activity in section from control plants at 72 h after the onset of the experiment. Bars = 100 μm.
ZmPAO Expression Is Induced by JA Treatment
It is well known that JA accumulates locally and systemically in response to tissue damage such as that caused by feeding insect (for review, see León et al., 2001). To study the eventual role played by JA as a mediator in the signal transduction pathway leading to the wound-stimulated activation of ZmPAO gene expression, we analyzed the effect of this plant growth regulator on ZmPAO activity levels as well as on ZmPAO protein and mRNA accumulation. To this purpose, a dose-response and a time-course analysis were performed using dark-grown maize seedlings kept into a hydroponic culture containing a nutrient solution, in the presence or absence of JA at the indicated concentrations. Noteworthily, these experiments were carried out utilizing seedlings at earlier developmental stages as compared to those used for wounding, to supply the hormone in advance with respect to the developmental dependent increase of ZmPAO expression.
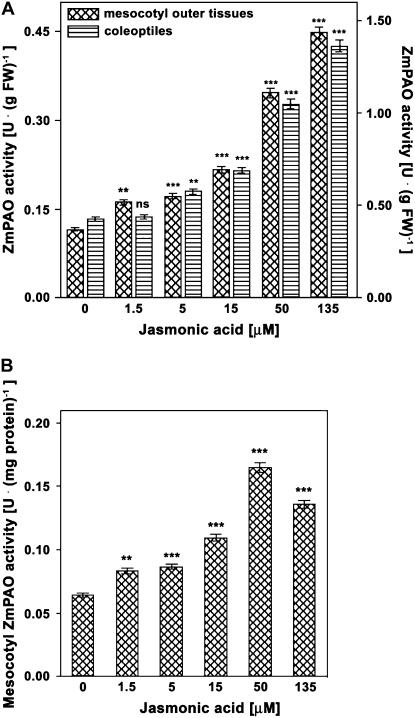
The dose-response curve (Fig. 4) relative to the effects of JA supply on extractable ZmPAO activity levels revealed that optimal JA concentrations averaged around 50 μm, for both mesocotyl outer tissues and coleoptiles. Indeed, despite the fact that levels of ZmPAO activity expressed on a fresh weight basis gradually increased in all treated plants as compared to JA-untreated (control) plants, including the 135 μm JA-treated plants (Fig. 4A), the level of ZmPAO activity expressed on total protein basis decreased in the latter plants as compared to 50 μm JA-treated plants both in mesocotyls (Fig. 4B) and in coleoptiles (data not shown), probably due to a nonspecific effect on total protein content.
Figure 4.
Dose-response curve of JA treatment on ZmPAO activity levels in coleoptiles and outer tissues of the nongrowing zone of the maize mesocotyl. Three-day-old seedlings were transferred into an aerated hydroponic culture supplied with a nutrient solution, in the presence or absence of JA at the indicated concentrations. Coleoptiles and mesocotyl segments were sampled after eliminating 0.3 cm above the seed and 0.3 cm below the node from JA-untreated (control) and JA-treated plants 48 h after the onset of the treatments. Mesocotyl cortical plus epidermal tissues (outer tissues) were obtained by drawing out the stele. Coleoptiles and mesocotyl outer tissues were used for determination of extractable ZmPAO activity (U: IUs; mean values ± se; n = 5). P values indicate statistical significance of differences between ZmPAO activity levels in JA-treated with respect to control samples for each concentration tested. ns, Not significant; *, **, and ***, P values ≤ 0.05, 0.01, and 0.001, respectively. A, Extractable ZmPAO activity levels expressed on a fresh weight (FW) basis in the outer tissues of the nongrowing zone of the maize mesocotyl (left y axis) and in coleoptiles (right y axis). B, Extractable ZmPAO activity levels expressed on total protein basis in the outer tissues of the nongrowing zone of the maize mesocotyl.
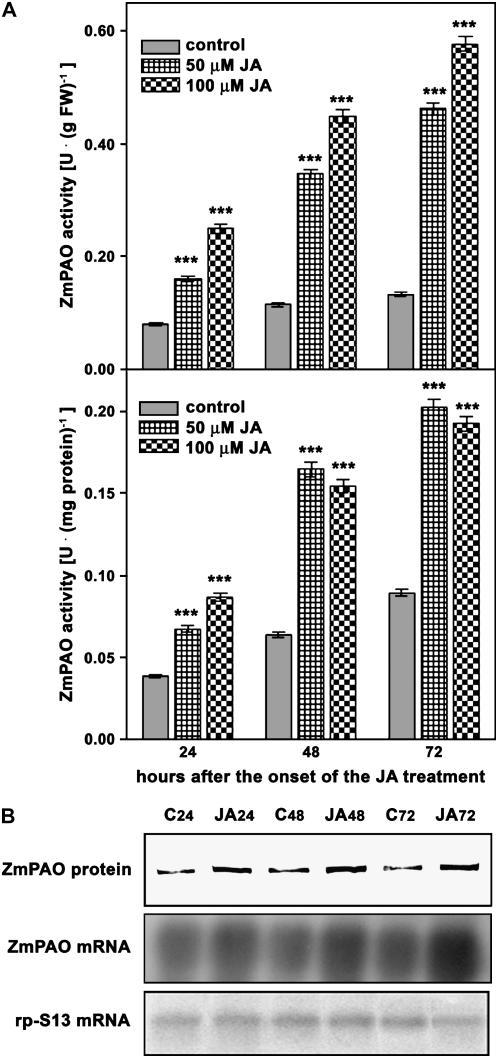
Concerning the time-course analysis (Fig. 5), experiments were focused on the effect of JA supply on ZmPAO gene expression in mesocotyl outer tissues, since it represents the model system as regards the analysis of ZmPAO expression regulation (Cona et al., 2003). Analogously to what was observed after wounding, extractable ZmPAO activity expressed on a fresh weight basis increased in both 50 and 100 μm JA-treated plants compared to control plants until the last time checked (72 h; Fig. 5A, top insert). However, ZmPAO activity expressed on total protein basis exhibited a lower increase in 100 μm JA-treated plants with respect to the corresponding 50 μm JA-treated plants at either 48 or 72 h from treatment onset (Fig. 5A, bottom insert), further confirming the occurrence of a nonspecific JA effect on total protein content for prolonged JA treatments at concentration higher than 50 μm. Noteworthily, spraying plants with aqueous solution containing JA or methyl-JA resulted in a lower increase of ZmPAO expression likely due to a bad penetration of the chemicals.
Figure 5.
Time-course analyses of ZmPAO expression after JA treatment in the outer tissues of the nongrowing zone of the maize mesocotyl. Three-day-old seedlings were transferred into an aerated hydroponic culture supplied with a nutrient solution in the presence or absence of 50 or 100 μm JA. Mesocotyl segments were sampled, after eliminating 0.3 cm above the seed and 0.3 cm below the node, from JA-untreated (control) and JA-treated plants at the indicated time after the onset of the treatments. Cortical plus epidermal tissues (outer tissues) were obtained by drawing out the stele and used for determination of extractable ZmPAO activity, western-blot, and northern-blot analysis. A, Extractable ZmPAO activity levels (U: IUs; mean values ± se; n = 5) expressed on a fresh weight (FW) basis (top insert) or total protein content (bottom insert). P values indicate statistical significance of differences between ZmPAO activity levels in JA-treated with respect to control samples for each time. ns, Not significant; *, **, and ***, P values ≤ 0.05, 0.01, and 0.001, respectively. B, western-blot and northern-blot analysis (samples as in A). Western immunoblotting of extractable ZmPAO fractions was performed after SDS-PAGE loaded on the basis of the total protein content (20 μg/well; top insert). Total RNA was fractionated by agarose/formaldehyde gel electrophoresis, blotted onto a nylon membrane, and hybridized with 32P-labeled maize PAO cDNA probe (middle insert). As a loading control, samples were also hybridized with the cDNA of the S13 ribosomal protein (bottom insert). C, Control; JA, JA-treated samples; subscripted numbers indicate sampling time.
Western-blot and northern-blot analysis of JA-treated and control samples showed a ZmPAO protein and mRNA accumulation pattern similar to that exhibited in the corresponding ZmPAO activity values (Fig. 5B, top insert). Indeed, western-blot analysis of 50 μm JA-treated seedlings revealed that the intensity of ZmPAO protein bands were higher at each time checked (Fig. 5B, top insert). Moreover, northern-blot analysis showed more intense hybridization bands in 50 μm JA-treated samples at every time checked, with the highest response at 72 h after treatment onset (Fig. 5B, middle insert).
Salicylic Acid Treatment Enhances JA Effect on ZmPAO Activity
It has been suggested that salicylic acid (SA)- and JA-signaling pathways are antagonistic to each other and SA has been reported to suppress expression of wound- and JA-induced genes (for review, see Pieterse and Van Loon, 2004). Such antagonism has been linked either to SA-mediated suppression of JA biosynthesis (Peña-Cortés et al., 1993), or more recently, to a cytosolic function of the SA-activated NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 protein, a key regulator of systemic acquired resistance, whose site of action in inhibiting JA signaling is still unknown (for review, see Pieterse and Van Loon, 2004). However, emerging evidence reveals a more complex perspective by which the two mediators affect plant gene expression antagonistically or synergistically, depending on their relative concentrations or on the duration of coaccumulation (Mur et al., 2006). Taking these considerations into account, we analyzed the effect of SA or JA/SA treatments on extractable ZmPAO activity in the mesocotyl outer tissues from maize seedlings kept into a hydroponic culture supplied with SA in a concentration ranging between 0.1 and 5 mm for dose-response studies and 1 mm for time-course analysis. Moreover, plants were simultaneously supplied with 50 μm JA/1 mm SA or 100 μm JA/1 mm SA, to analyze eventual interactions of these plant growth regulators in affecting ZmPAO activity.
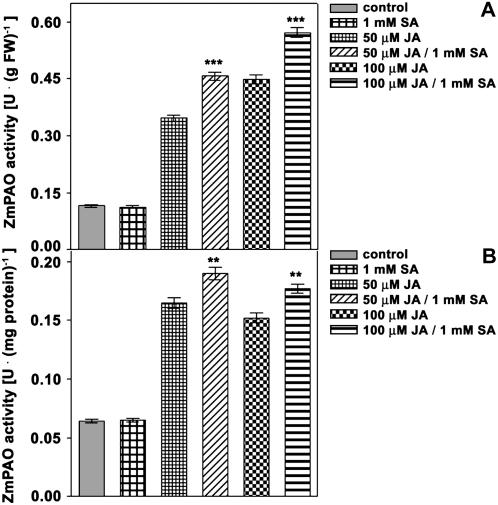
ZmPAO activity was not altered in SA-treated versus SA-untreated plants up to 1 mm SA (data not shown) that represents the highest nontoxic concentration in our experimental system. Otherwise, SA and JA, both 50 and 100 μm, displayed a synergistic effect on ZmPAO activity. In fact, ZmPAO activity in 50 μm JA/1 mm SA- or in 100 μm JA/1 mm SA-treated plants exhibited higher levels after 48 h from the onset of the treatments as compared, respectively, to 50 μm JA- or 100 μm JA-treated plants. Indeed, differences were statistically significant when ZmPAO was expressed either on fresh weight basis or total protein content (Fig. 6, A and B).
Figure 6.
Effects of JA, SA, or JA/SA treatment on ZmPAO activity levels in the outer tissues of the nongrowing zone of the maize mesocotyl. Three-day-old seedlings were transferred into an aerated hydroponic culture supplied with a nutrient solution, in the presence or absence of 50 or 100 μm JA, 1 mm SA, or JA/SA. Mesocotyl segments were sampled, after eliminating 0.3 cm above the seed and 0.3 cm below the node from hormone-untreated (control) and hormone-treated plants after 48 h from the onset of the treatments. Cortical plus epidermal tissues (outer tissues) were obtained by drawing out the stele, and used for extractable ZmPAO enzyme activity analysis at the indicated time after the onset of the treatments (U: IUs; mean values ± se; n = 5). P values indicate statistical significance of differences between ZmPAO activity levels in JA-treated with respect to the respective JA/SA-treated samples for each time. ns, Not significant; *, **, and ***, P values ≤ 0.05, 0.01, and 0.001, respectively. A, Extractable ZmPAO activity levels expressed on a fresh weight (FW) basis. B, Extractable ZmPAO activity levels expressed on total protein content.
Wound-Induced H2O2 Accumulation Is Inhibited by G3, a Specific ZmPAO Inhibitor
G3 is a specific, powerful inhibitor of ZmPAO activity with a Ki of 1.5 × 10−8 m (Federico et al., 2001). The selectivity of G3 toward ZmPAO has been demonstrated by the in vitro and in vivo analysis of its inhibitory activity on the other putative ROS-generating enzyme systems in the apoplast (Cona et al., 2006b). In detail, G3 at a concentration of 1 × 10−4 m did not inhibit both barley oxalate oxidase and horseradish (Armoracia lapathifolia) peroxidase enzyme activity in vitro. Moreover at the same concentration the inhibitor did not inhibit the NADPH oxidase-mediated O2− production in vivo (Cona et al., 2006b). On the basis of these results it has been established that G3, even at a concentration 10-fold higher than that used in this work, represents a selective diagnostic tool in discriminating ZmPAO-mediated H2O2 production in vivo from that generated by peroxidase, oxalate oxidase, or NADPH oxidase activity (Cona et al., 2006b). To analyze PAO-specific contribution to wound-induced H2O2 production, the accumulation of this compound was evaluated in maize mesocotyl from wounded seedlings after 8 h incubation in DAB staining solution in the presence or absence of G3. As shown in Figure 7, H2O2 accumulation was totally inhibited by G3 in wounded mesocotyls either in longitudinally cut seedlings (Fig. 7, A and B) or after removal of an epidermal strip (Fig. 7, C and D). Unwounded mesocotyls did not show a DAB-staining detectable production of H2O2 in the epidermal tissues 8 h after injury (data not shown), as also confirmed by the absence of DAB staining in the epidermal areas far from the wound border in wounded mesocotyls (Fig. 7, A and C).
Figure 7.
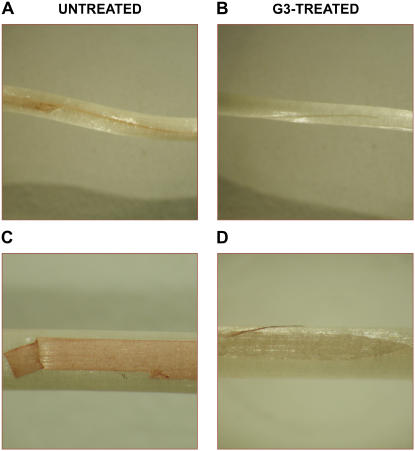
Inhibition of H2O2 accumulation by G3 in wounded maize mesocotyl. Etiolated seedlings were longitudinally wounded on the mesocotyl with a razor blade (A and B). Alternatively, an epidermal strip was removed (C and D). After root deprivation, wounded seedlings were preincubated for 30 min in the presence or absence of 1 × 10−5 m G3 (in 10 mm NaH2PO4 solution containing 60 μg/mL peroxidase) prior to be supplied with 1 mg mL−1 DAB for 8 h. Afterward, reactions were stopped by thoroughly washing in distilled water and H2O2 was directly visualized. Photograph magnification: A and B, ×2.0; C and D, ×3.4.
G3 Strongly Reduces Lignin and Suberin Polyphenolic Domain Levels Along the Wound Periderm
Plants respond to wounding by differentiating a lignosuberized layer that constitutes the wound periderm. Besides fortifying cell wall structure, lignin and suberin form important defense barriers against dehydration and pathogens. Lignin is a polyphenolic polymer whose composition is highly variable, differing even among different cell types of a single tissue (Joseleau and Ruel, 1997), while suberin contains both polyphenolic and aliphatic domains, the latter consisting of esterified fatty acids.
To explore the role played by ZmPAO in lignin and suberin deposition along wound lesion, we analyzed the effect of G3 supply on the spatiotemporal accumulation of ester-linked phenolics, lignin and suberin, during wound healing, by the means of a combined approach of UV-light-induced autofluorescence and specific staining. To this scope, hand-cut cross sections from wounded mesocotyls of G3-treated and G3-untreated maize plants were examined for either autofluorescence coupled with ammonium hydroxide treatment or Sudan IV staining observed under light microscopy at 0, 24, 48, and 72 h after wounding.
Cross sections of wounded mesocotyls from G3-untreated seedlings showed UV-induced blue autofluorescence in the phloem as well as in the cortical and pith parenchyma cells (Fig. 8, A, C, and E) that turned to blue green upon treatment of the sections with ammonium hydroxide (Fig. 9, A, C, and E). This is indicative of the presence of ester-linked phenolics within cell walls (Harris and Hartley, 1976). Lignified tissues, such as endodermis, xylem, xylem parenchyma, and innermost cell layers underneath wound periderm displayed a UV-induced whitish-blue or blue autofluorescence (Fig. 8, A, C, and E) that was unchanged upon ammonium hydroxide treatment (Fig. 9, A, C, and E) according to what previously was described for lignified tissues (Harris and Hartley, 1976). In particular, this phenomenon was already evident after 24 h in wound periderm (Fig. 9A) and later in the other tissues (Fig. 9, C and E). Moreover, a strong UV-induced yellow autofluorescence, already described in wound-healed periderms (Sherf et al., 1993), was evident in the maize mesocotyl wound periderm (Fig. 8, A, C, and E) owing to the presence of polymerized phenolics associated to lignin and suberin polyphenolic domain (Sherf et al., 1993). Interestingly, these cells turned into blue autofluorescence upon ammonium hydroxide treatment (Fig. 9, A, C, and E). The occurrence of suberin or other aliphatic-domain containing molecules, such as waxes or cutin, in the cell walls of wound periderm was confirmed by staining with the lipophilic dye Sudan IV (Fig. 10, A, C, and E).
Figure 8.
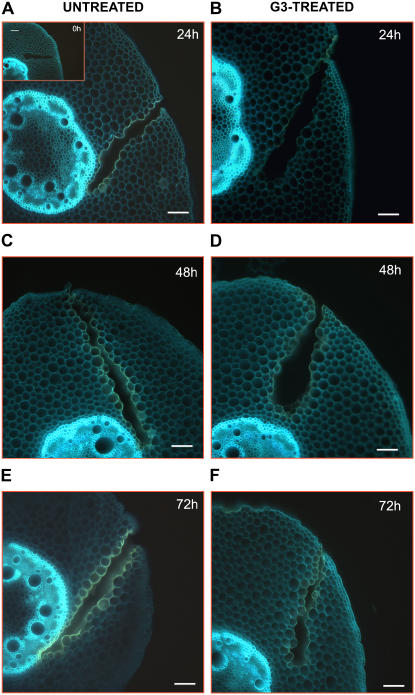
UV-induced autofluorescence microscopic analysis in wound-healing maize mesocotyls. Histochemical analysis was performed in hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the mesocotyl of G3-untreated and G3-treated plants, at 0 (inset), 24 (A and B), 48 (C and D), and 72 (E and F) h after wounding. Sections were directly mounted in buffer on slides and observed for autofluorescence under UV light. Bars = 100 μm.
Figure 9.
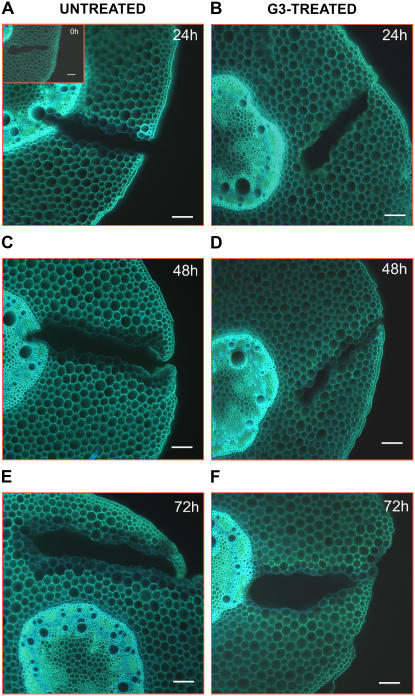
UV-induced autofluorescence microscopic analysis after ammonium hydroxide treatment in wound-healing maize mesocotyls. Histochemical analysis was performed in hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the mesocotyl of G3-untreated and G3-treated plants, at 0 (inset), 24 (A and B), 48 (C and D), and 72 (E and F) h after wounding. Sections were incubated for 1 min in 10 mm NH4OH, pH 10, prior to observation under UV light. Bars = 100 μm.
Figure 10.
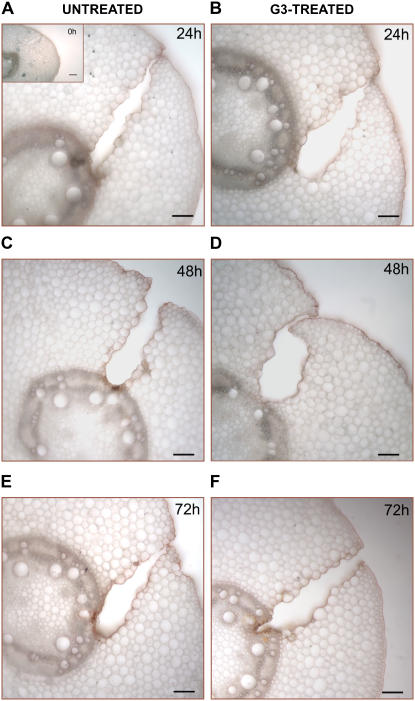
Light microscopy detection of suberin aliphatic domain in wound-healing maize mesocotyls. Histochemical analysis was performed in hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the mesocotyl of G3-untreated and G3-treated plants, at 0 (inset), 24 (A and B), 48 (C and D), and 72 (E and F) h after wounding. Sections were preincubated for 10 min in 50% ethanol and then stained for 20 min in a filtered saturated solution of Sudan IV in 70% ethanol. After washing in 50% ethanol (1 min), sections were observed under light microscopy. Bars = 100 μm.
As shown in Figure 8, G3 treatment strongly inhibited deposition of poliphenolic associated to lignin and suberin polyphenolic domain occurring at the wound site of G3-untreated plants from 0 h up to 72 h after injury. Indeed the increase in yellow autofluorescence observable along the lesions of G3-untreated plants (Fig. 8, A, C, and E) was greatly impaired by the G3-mediated ZmPAO inhibition (Fig. 8, B, D, and F).
The autofluorescence level, after ammonium hydroxide treatment of cross section, was not affected by G3 treatment in phloem as well as in cortical and pith parenchyma, indicating that the level of ester-linked phenolics was unchanged. Nevertheless, G3 treatment inhibited lignin deposition in the innermost cell layers underneath wound periderm (Fig. 9). In fact, the intensity and the tissue extension of UV-induced blue autofluorescence, after ammonium hydroxide treatment, evident along the wound of G3-untreated plants (Fig. 9, A, C, and E) was strongly reduced after G3 treatment (Fig. 9, B, D, and F).
Conversely, G3 supply failed to inhibit suberin aliphatic-domain deposition (Fig. 10) as demonstrated by the absence of any detectable differences in Sudan IV staining intensity along the periderm of sections from G3-untreated (Fig. 10, A, C, and E) and G3-treated plants (Fig. 10, B, D, and F).
Polyamine Levels Are Not Altered during Wound Healing
In plants, polyamine levels are altered by biotic and abiotic stresses (for review, see Walters, 2003; Alcázar et al., 2006) and changes in the levels of putrescine (Put), Spd, or Spm occur in plants interacting with fungal or viral pathogens (Yoda et al., 2003; for review, see Walters, 2003) as well as after wounding (Perez-Amador et al., 2002). To analyze eventual alterations of polyamine levels consequent to wounding, we examined the levels of free and soluble-conjugated polyamines in the outer tissues of wounded and unwounded (control) maize plants at 0, 24, 48, and 72 h after injury. No detectable variations were determined (Supplemental Table S1) in the levels of free and soluble-conjugated Dap, Put, Spd, and Spm in wounded versus control plants at each time checked. In particular, soluble-conjugated polyamines were absent in both control and wounded plants. Moreover, in agreement with previous reports demonstrating the absence of detectable polyamines in the apoplast of healthy leaves (Yoda et al., 2003), no free polyamines were revealed in the intercellular fluids extracted from control mesocotyls. Nevertheless, no free polyamine accumulation occurred in the intercellular fluids extracted from the mesocotyls of wounded plants.
ZmPAO Expression in Transgenic Tobacco Plants Enhances Lignin and Suberin Polyphenolic Domain Deposition during Wound Healing
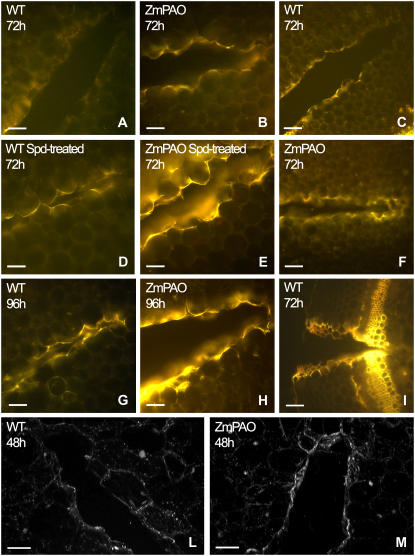
To verify the involvement of ZmPAO in the formation of lignosuberized depositions along wound periderm, we exploited the availability of transgenic tobacco plants constitutively expressing ZmPAO in the cell wall (Rea et al., 2004). Wild-type and ZmPAO transgenic tobacco plants were blade wounded on the second internode (numbered from the apex) and examined for blue- (Fig. 11) or UV-light-induced (Fig. 12) autofluorescence coupled with ammonium hydroxide treatment. The spatiotemporal analysis of lignin and suberin polyphenolic domain deposition along the wound periderm cell walls indicated that ZmPAO overexpression in this compartment greatly accelerates the phenomenon (Figs. 11 and 12, A and B). Moreover, treatment of wounded plants with Spd, the preferential ZmPAO substrate, enhanced lignosuberized deposition both in wild-type and transgenic tobacco plants (Fig. 11, D and E).
Figure 11.
Blue-induced autofluorescence and laser scanning confocal microscopy analysis in wound-healing tobacco plants overexpressing ZmPAO in the cell wall. Histochemical analysis under fluorescence microscopy was performed in hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the second internode (numbering from the shoot apex) of Spd-untreated (A–C and F–I) and Spd-treated (D–E) wild-type (WT) as well as ZmPAO transgenic tobacco plants overexpressing ZmPAO in the cell wall (ZmPAO), at 72 and 96 h after wounding. Sections were directly mounted on slides and observed for autofluorescence under blue light (A–I). A, B, D, E, and G to I, bar = 100 μm; C and F, bar = 200 μm. Confocal microscopy analysis was performed on hand-cut cross sections from wild-type and ZmPAO transgenic tobacco plants at 48 h after wounding. L and M sections show a three-dimensional reconstruction of autofluorescence images after blue excitation. L and M, bar = 100 μm.
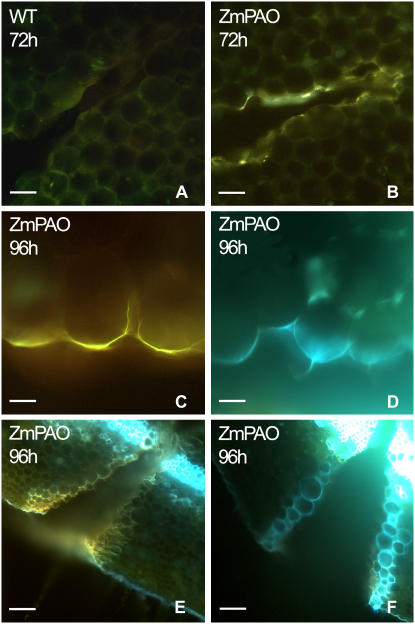
Figure 12.
UV-induced autofluorescence microscopic analysis after ammonium hydroxide treatment in wound-healing tobacco plants overexpressing ZmPAO in the cell wall. Histochemical analysis was performed in hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the second internode (numbering from the shoot apex) of Spd-untreated wild type (WT) as well as tobacco plants overexpressing ZmPAO in the cell wall (ZmPAO), at 72 and 96 h after wounding. Some sections were directly mounted on slides and observed for autofluorescence under UV (A–C and E) light. Other sections were incubated for 1 min in 10 mm NH4OH, pH 10, prior to observation under UV light (D and F). A, B, E, and F, bar = 100 μm; C and D, bar = 50 μm.
In detail, the microscopic analysis of cross sections from wild-type tobacco plants indicated that lignosuberization events occurred early on the external border and late on the innermost part of the wound lesion. Accordingly, after 72 h from wounding a remarkable and similarly intense yellow autofluorescence was already detected under blue light-induced fluorescence microscopy on the external margin of the wound of both wild-type (Fig. 11I) and ZmPAO transgenic plants (data not shown), while the middle and inner boundary cells of the wound periderm displayed a very different lignosuberization level in these two sets of plants for each time examined (Fig. 11, A–C and F–H). Indeed, a very pale yellow autofluorescence was detected only on the outer margins of the wound periderm in wild-type plants at 48 h after injury (data not shown), whose intensity and extension toward the boundary cells of the middle part of the wound increased after 72 h (Fig. 11, A and C) until becoming evident in the innermost terminal part of the wound lesion at 96 h (Fig. 11G). Conversely, in ZmPAO transgenic tobacco plants at 72 h from injury a stronger yellow autofluorescence as compared to the corresponding wild-type plants was revealed on the boundary cells of both the middle part and the innermost terminal part of the wound (Fig. 11, B and F). Moreover, in the same plants at 96 h from wounding, a very strong yellow autofluorescence was present in the whole lesion (Fig. 11H), especially in the innermost part of the wound, indicating that an acceleration of wound healing processes occurred as a consequence of ZmPAO ectopic expression. The presence of lignin in the cell walls of wound periderm was also suggested by the emission of blue autofluorescence under UV light upon ammonium hydroxide treatment both in wild-type (data not shown) and transgenic (Fig. 12, D and F) tobacco plants (Harris and Hartley, 1976), as compared to respective wild-type (data not shown) and transgenic (Fig. 12, C and E) untreated plants.
A more sensitive investigation performed under confocal laser scanning microscopy allowed to detect healing progressing inside the wound cavity at earlier developmental stages as compared to the blue- or UV-induced fluorescence microscopic analysis (Fig. 11, L and M). The three-dimensional images of cross sections from wild-type and transgenic tobacco plants revealed a remarkable blue-induced autofluorescence on the innermost terminal part of the wound of transgenic plants (Fig. 11M) already 48 h after wounding, that was not present in wild-type plants at the same time (Fig. 11L), confirming the role played by ectopically expressed ZmPAO in accelerating wound healing progression.
Spd-treated wild-type plants (Fig. 11D) showed a stronger yellow autofluorescence under blue-induced fluorescence microscopy as compared to the Spd-untreated plants 72 h after wounding (Fig. 11A). As shown in Figure 11D, autofluorescence was present also in the inside end of the lesion, suggesting that an endogenous amine oxidase could be involved in wound healing processes in tobacco plants. As expected, Spd supply in ZmPAO transgenic plants (Fig. 11E) resulted in even stronger yellow autofluorescence as compared to Spd-untreated transgenic plants (Fig. 11B).
DISCUSSION
To counteract chewing insects or larger herbivores as well as invasion by microbial pathogens, plants make use of preexisting physical barriers and inducible defense mechanisms, which largely depend on the transcriptional activation of specific genes, directed to the healing of the damaged tissues and to prevent further damage. In this connection, owing to the ease of microbe penetration allowed for the physical injury consequent to either environmental stresses or animal feeding, plants respond to wounding by enhancing defense capacity against microbial pathogens. Therefore, defense pathways activated in response to physical injury or pathogen attack cross communicate each other, leading to the activation of distinct or partially overlapping sets of genes. A common feature of these defense strategies is a transient production of ROS, whose chemical identity is spatially and temporally regulated. In this regard, it is known that superoxide anion is produced in the damaged tissues of wounded plants only a few minutes after injury, while H2O2 is produced both locally and systemically, reaching a peak after 4 to 6 h (for review, see León et al., 2001).
Here, it is suggested that ZmPAO represents the main source of apoplastic H2O2 production during wound healing. Indeed, the increase in ZmPAO enzyme activity, protein, and mRNA levels in mesocotyls from wounded plants (Fig. 1) clearly demonstrates the occurrence of a positive regulation of ZmPAO expression in damaged tissues. Noteworthily, the temporal features of the increase of ZmPAO expression suggest that ZmPAO is involved in the late phase of the oxidative burst. Indeed, the increase in ZmPAO mRNA accumulation that precedes the rise in ZmPAO enzyme activity and protein levels is strongly evident 6 h after wounding and persists for several hours, up to 48 h. This result is in keeping with previous studies, suggesting that H2O2 produced by polyamine degradation contributes to the second phase of ROS production during TMV-induced HR in tobacco plants (Yoda et al., 2003; Takahashi et al., 2004) as well as in tobacco cultured cells elicited with cryptogein (Yoda et al., 2006). Further support for the hypothesis of a ZmPAO role in the late phase of the oxidative burst arises from previous studies that demonstrated that in maize mesocotyls elicited with the phosphatase inhibitor cantharidin, ZmPAO-mediated H2O2 accumulation became evident 3 h after elicitation (Cona et al., 2006b). The systemic induction of ZmPAO activity in response to wounding (Fig. 2) indicated by the increase of ZmPAO activity in coleoptiles of mesocotyl-wounded plants is consistent with the evidence that H2O2 is systemically produced after wounding (for review, see León et al., 2001), which is in line with the previously demonstrated systemic induction of copper amine oxidase in Cicer aretinum (Rea et al., 2002). The involvement of ZmPAO activity in wound healing processes is also supported by the tissue specificity of its increase (Fig. 3), especially occurring in the damaged tissues of wounded plants. In fact the histochemical visualization of ZmPAO enzyme activity revealed the occurrence of a gradual intensification of DAB staining from 0 up to 72 h after wounding in the epidermis and in the wound periderm, while ZmPAO histochemical staining in the stele of unwounded and wounded plants was similar.
Noteworthy is the wound-induced increase of ZmPAO activity; besides confirming the ability of epidermal ZmPAO to be rapidly regulated by various external stimuli, it presents interesting analogies with either the light-induced increase of ZmPAO gene expression or the ZmPAO-mediated H2O2 production upon cantharidin treatments, both also specifically occurring in the outer tissues of the maize mesocotyls. Indole-3-acetic acid, whose levels are known to decline in maize mesocotyl epidermis upon light exposure (Barker-Bridgers et al., 1998), has been reported to exert a negative effect on the light-induced increase of ZmPAO gene expression in the same tissues (Cona et al., 2003). Likewise, endogenous levels of indole-3-acetic acid have been shown to decrease in wounded tissues, the recovery of initial levels of active auxins representing a mechanism to limit the duration of the wound response through the inhibition of wound-induced gene expression (for review, see León et al., 2001). Therefore, it is reasonable to presume that auxin represents a common means of ZmPAO gene expression regulation during both the wound and the deetiolation response. Another interesting consideration is that reversible protein phosphorylation regulates JA-dependent and -independent wound signaling pathways (for review, see León et al., 2001). In this connection wound-responsive genes are known to be activated by protein phosphatase inhibitors in tomato cell culture (for review, see León et al., 2001); likewise, ZmPAO activity levels increase upon plant treatments with the phosphatase inhibitor cantharidin (Cona et al., 2006b).
With the aim of analyzing the molecular signals responsible for the control of ZmPAO expression upon mechanical injury, we tested the effect of plant treatments with JA and SA, well known mediators of wound and pathogen signaling, respectively. In line with the hypothesis of ZmPAO involvement in wound healing processes, JA treatments resulted in an increase of ZmPAO expression, either in coleoptiles or in mesocotyl outer tissues (Figs. 4 and 5). Conversely, SA treatments had no effect on ZmPAO activity (data not shown), confirming the specificity of the role played by JA in the activation of the wound-responsive ZmPAO gene. Worth noting is that JA-mediated increase of ZmPAO gene expression could be ascribed to the presence of a G-box motif in its promoter region (Cervelli et al., 2000), since this light-responsive motif has been identified as an essential element for JA responsiveness of wound-inducible genes (Kim et al., 1992). However, ZmPAO activity levels in mesocotyl outer tissues of plants treated with both JA and SA were higher when compared to those observed when applying JA alone (Fig. 6). This result reflects the complexity of the relations between JA and SA signaling pathway, whose interaction can be synergistic or antagonistic, depending on their relative concentrations or on the duration of coaccumulation (Mur et al., 2006). The increase of ZmPAO activity described in JA-treated plants suggests the hypothesis that the accumulation of H2O2 observed in plants upon JA treatments (Mur et al., 2006) could be produced via the PAO-mediated oxidation of polyamines, whose synthesis is activated by JA as well (Walters, 2003).
The availability of a specific and selective inhibitor of ZmPAO activity, such as G3 (Ki = 1.5 × 10−8 m; Federico et al., 2001), which is able to discriminate in vivo between ZmPAO-mediated H2O2 production and that derived by the other apoplastic sources of H2O2 (Cona et al., 2006b), allowed us to analyze the specific contribution of polyamine-derived H2O2 production to the peroxidase-mediated oxidative cross-linking of cell wall components occurring during wound healing. Effectively, this inhibitor totally inhibited the extracellular H2O2 production normally occurring in wounded mesocotyl (Fig. 7). It is well known that H2O2 level could represent a limiting factor in the peroxidase-catalyzed reactions leading to cross-linking of lignin and suberin polyphenolic domain (Razem and Bernards, 2002). Accordingly, G3 treatment decreases the level of polymerized phenols in the wound periderm (Fig. 8 and 9), while being ineffective in the deposition of aliphatic suberin domain (Fig. 10), which does not require H2O2-dependent cross-linking reaction. As the cicatrization periderm autofluorescence of wounded G3-treated samples is lower as compared to that of wounded untreated ones, it could be hypothesized that nonpolymerized phenols that should have been incorporated into lignin and suberin polyphenolic domain are uptaken by the cell and remetabolized.
The absence of variation in polyamine content after wounding in mesocotyl outer tissues (Supplemental Table S1) could be ascribed to the occurrence of a strictly balanced rate of polyamine biosynthesis and degradation, which results in no detectable polyamine accumulation. On the other hand, this phenomenon could arise also from variations occurring only in tissues directly involved in wound healing processes, such as the epidermis and wound periderm, and therefore not measurable in a whole tissue extract.
A stronger causal relationship between ZmPAO activity and lignosuberized depositions along wound lesions has been established by the analysis of transgenic tobacco plants constitutively expressing ZmPAO in the cell wall. As previously reported (Rea et al., 2004), these plants, despite the high expression levels of the transgene in the cell wall, did not show any changes in the amount of free polyamines as well as in the cell wall lignification levels with respect to those of the wild-type plants. Furthermore, high levels of H2O2 were produced only in the presence of exogenously supplied enzyme substrate, suggesting the occurrence of a different compartmentalization of the recombinant protein and the polyamine bulk amount (Rea et al., 2004). In this connection, the absence of detectable polyamines in the cell wall of healthy tobacco leaves (Yoda et al., 2003) supported the idea that apoplastic polyamine levels represent the limiting factor for the ZmPAO-mediated H2O2 production in transgenic plants under physiological conditions. However, polyamines have been reported to accumulate in the intercellular spaces of tobacco leaves upon TMV infection, reaching 30 nmol per gram fresh weight after 50 h (Yoda et al., 2003). On this basis, it has been proposed that an increase in apoplastic polyamine levels is a required event in the NtPAO-mediated triggering of HR onset (Yoda et al., 2003) and could be a general response to various stresses. Thus, the stronger autofluorescence observable during wound healing in tobacco plants expressing ZmPAO in the cell wall with respect to wild-type plants (Figs. 11 and 12) could be ascribable to an enhanced H2O2 production, and consequent higher cell wall lignosuberization, triggered by degradation of increased amount of apoplastic polyamines. Indeed it has been demonstrated that cell wall H2O2 level is a limiting factor in lignin synthesis (Müsel et al., 1997). Otherwise, the higher autofluorescence levels revealed along the periderm upon Spd supply in wild-type (Fig. 11D) plants with respect to the corresponding Spd-untreated plants (Fig. 11A) could suggest the involvement of an endogenous amine oxidase in lignosuberization events, likely the cell wall localized NtPAO previously reported to be implicated in defense responses against pathogens (Yoda et al., 2003, 2006).
Overall these results suggest the involvement of ZmPAO in wound healing processes, as a main provider of apoplastic H2O2 needed for cell wall strengthening and lignosuberization events.
MATERIALS AND METHODS
Chemicals
N-prenylagmatine [G3; N-(4-aminobutyl)-N'-(3-methyl-2-butenyl)guanidine] was prepared as previously described (Corelli et al., 2002). Horseradish (Armoracia lapathifolia) peroxidase, DAB, 4-aminoantipyrine (AAP), 3,5-dichloro-2-hydroxybenzenesulfonic acid (DCHBS), Spd, Spm, Dap, Put, 1,6-diaminohexane, JA, SA, and Sudan IV were from Sigma. TRIzol reagent was from Invitrogen.
Plant Materials and Treatments
Maize (Zea mays ‘Corona’; from Monsanto Agricoltura) seeds were soaked for 12 h in running water and germinated on paper under 2 cm of loam, at 22°C in a growth chamber in the dark. Five-day-old seedlings (average stem length = 6.5 cm; average mesocotyl length = 4 cm) were longitudinally wounded (wound length = 2 cm) on the nongrowing zone of the mesocotyl with a blade, precisely from 1 cm above the seed up to 1 cm below the node. After injury some seedlings were treated with 1 × 10−5 m G3 in aqueous solution, by brushing 50 μL of the inhibitor along the wound at 0, 24, and 48 h after wounding. In detail, the inhibitor was first applied with a pipette over the wound surface and then brushed to avoid dripping. G3-untreated plants were supplied with 50 μL of distilled water. Five-, 6-, 7- and 8-d-old plants were collected at 0, 24, 48, and 72 h after wounding, respectively.
Alternatively, after soaking, maize seeds were germinated on paper at 22°C in a growth chamber in the dark for 3 d (average stem length = 1 cm) and then transferred into an aerated hydroponic culture supplied with a nutrient solution. Nutrient solution was prepared as follows: 4 mm KNO3, 1 mm Na2HPO4, 1 mm MgSO4, 0.5 mm CaCl2, 1 mg L−1 Fe-citrate, pH value was adjusted to 6 with NaOH. Hormone treatments were performed by adding JA and SA at the appropriate concentration into the nutrient solution. Nutrient solution alone (control plants) or nutrient solution plus JA or SA or JA/SA were replaced every day. Four-, 5-, and 6-d-old plants were harvested after 24, 48, and 72 h from the onset of hormone treatments, respectively.
Coleoptiles from unwounded (control) and wounded maize plants were collected at 0, 24, and 48 h after wounding, as well as coleoptiles from untreated (control) and JA-treated plants were collected at 48 h from the onset of JA treatments and then used for extractable ZmPAO activity analysis. Two-centimeter-long mesocotyl segments were excised after eliminating 1 cm above the seed from control and wounded maize plants at the indicated times after wounding. Mesocotyl segments of variable length depending on plant age were excised after eliminating 0.3 cm above the seed and 0.3 cm below the node from control and hormone-treated maize plants at the indicated times after the onset of the treatments. Cortical plus epidermal tissues (outer tissues) from the mesocotyl were obtained by drawing out the stele and used for determination of enzyme activity, western-blot, northern-blot, and polyamine level analysis.
Maize plants were kept in the dark until the end of the treatments. All technical operations were performed under photomorphogenically inactive green light.
Plants of tobacco (Nicotiana tabacum ‘Petit Havana SR1’) constitutively expressing ZmPAO in the cell wall (Rea et al., 2004) were grown in a growth chamber with an irradiance of approximately 150 μE m−2 s−1, a mean temperature of 24°C, and a 16-h day length. Wild-type and transgenic 8-week-old plants were selected for homogeneity of growth and wounded (wound length = 1.5 cm) with a blade on the second internode (numbered from shoot apex). After injury, some plants were treated with 1 × 10−3 m Spd in 0.01 m sodium phosphate buffer, pH 6.5, by brushing 50 μL of the polyamine solution along the wound at 0, 24, 48, and 72 h after wounding, as previously described for G3 treatment of wounded maize plants. Spd-untreated plants were supplied with 50 μL of 0.01 m sodium phosphate buffer, pH 6.5.
Preparation of Extractable and Wall-Bound ZmPAO Fractions from Maize Seedlings
Plant material was ground with mortar and pestle at 4°C in 0.2 m sodium phosphate buffer, pH 6.5 (tissue to buffer ratio 1:3, w/v). Homogenates were centrifuged at 12,000g for 10 min at 4°C. Supernatants were used for the determination of protein concentration, extractable ZmPAO activity, and western-blot analysis. For determination of wall-bound ZmPAO activity, pellets obtained after centrifugation of crude homogenates were resuspended in the appropriate volume of 0.2 m sodium phosphate buffer, pH 6.5, containing 0.01% Triton X-100 and then filtered onto Miracloth. This step was repeated three times to remove traces of extractable enzyme. The washed pellets were resuspended in 0.2 m sodium phosphate buffer, pH 6.5 [1 mL (g fresh weight)−1], and the suspension used for the polarographic determination of wall-bound ZmPAO activity as described in the following paragraph.
ZmPAO Enzyme Assays, Protein Determination, and Western-Blot Analysis
Extractable ZmPAO enzyme activity was measured spectrophotometrically by following the formation of a pink adduct (ɛ515 nm = 2.6 × 104 m−1 cm−1) as a result of the H2O2-dependent oxidation of AAP catalyzed by horseradish peroxidase and the subsequent condensation of the oxidized AAP with DCHBS (Artiss and Entwistle, 1981). Assays were carried out in 0.2 m sodium phosphate buffer pH 6.5, containing 60 μg horseradish peroxidase, 0.1 mm AAP, and 1 mm DCHBS with 2 mm Spd as a substrate in 1 mL total volume. Wall-bound as well as extractable ZmPAO activity were determined in 0.2 m sodium phosphate buffer pH 6.5 and with 2 mm Spd as a substrate, by measuring oxygen consumption in an oxygraph (Hansatech) equipped with a Clark electrode, as described by Augeri et al. (1990).
Enzyme activities were expressed in International Units (U; 1 unit is the amount of enzyme that catalyzes the oxidation of 1 μmol substrate per min). The reported data are the average of values obtained in five different experiments, each performed with two technical replicates. All data were obtained at 25°C.
Protein content was evaluated by the method of Bradford (1976). Western-blot analyses were performed after protein deglycosylation (Woodward et al., 1985) to eliminate cross-reactivity due to glycan moieties, using a rabbit polyclonal anti-MPAO antiserum (Federico et al., 1988). Analyses were performed on 20 μg of total soluble proteins of each extract. Experiments were performed independently at least five times, yielding reproducible results. Single representative experiments are shown in the figures.
RNA-Blot Analysis
RNA-blot analysis was performed on maize mesocotyl outer tissues from wounded, JA-treated, and control plants at different times from the onset of the treatment.
Total RNA was isolated using the TRIzol reagent, following the manufacturer's instructions. Blotting and hybridization procedures were performed as reported by Sambrook et al. (1989). Twenty micrograms of total RNA for each sample were fractionated on a 1.2% agarose/formaldehyde gel. Hybridization was carried out using 32P-labeled ZmPAO cDNA as probe (EMBL DataBase accession number AJ002204). As a control, samples were also hybridized with an internal portion of the cDNA specific for the ribosomal protein S13 (rp-S13, GenBank accession number AF067732). Experiments were performed independently at least three times, yielding reproducible results. Single representative experiments are shown in the figures.
In Vivo Detection of H2O2
H2O2 was directly visualized in maize mesocotyls by DAB staining. Etiolated maize seedlings (average stem length = 6 cm) were longitudinally wounded on the mesocotyl with a razor blade. Alternatively, an epidermal strip was removed. Afterward wounded seedlings were deprived of the roots and then incubated for 30 min in 10 mm NaH2PO4 containing 60 μg mL−1 peroxidase in the presence or absence of 1 × 10−5 m G3, prior to be supplied with 1 mg mL−1 DAB for 8 h. Reactions were stopped by thoroughly washing in distilled water. Experiments were performed independently three times and a minimum of three plants for each treatment were observed, yielding reproducible results. Single representative experiments are shown in the figures.
Histochemical Methods
Hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of the maize mesocotyl at 0, 24, 48, and 72 h after wounding were utilized for light and fluorescence microscopic investigation of ZmPAO histochemistry as well as cell wall-associated ester-linked phenolics, lignin and suberin aliphatic and polyphenolic domain. Moreover, hand-cut cross sections (approximately 100 μm thick) obtained from the wounded zone of tobacco second internodes at 48, 72, and 96 h after wounding were used for fluorescence and confocal laser scanning microscopic investigation of lignosuberized deposition. To this purpose, some sections were mounted on glass slides and observed in an Axioplan 2 Zeiss microscope equipped with a video camera (Delta Sistemi). Digitized images were acquired by an IAS 2000 software (Delta Sistemi). Other sections were analyzed under Leica TCS-SP5 confocal microscope supplied with the Leica Application Suite Advanced Fluorescence software. Experiments were performed independently five times and a minimum of three plants for each time and each treatment were observed, yielding reproducible results. A minimum of 10 sections for mesocotyl or internode was analyzed. The reported micrographs are representative of single experiments.
Histochemical Visualization of ZmPAO Activity
ZmPAO activity was histochemically detected in sections obtained from the wounded zone of the maize mesocotyl and from the corresponding mesocotyl zone from unwounded (control) plants at 0, 24, 48, and 72 h after wounding, using a peroxidase-coupled assay with the artificial substrate DAB as the chromogen. After washing in distilled water, sections were preincubated in sodium phosphate buffer 10 mm, pH 6.5, containing 60 μg mL−1 peroxidase and 0.04% DAB for 10 min and then incubated with 3 mm Spd. Reactions were blocked after 3 min by thoroughly washing sections in distilled water. Sections were mounted in 25% glycerol prior to being observed under light microscopy. Sections from wounded plants incubated in the staining solutions lacking Spd were negative.
Autofluorescence Analysis Coupled with Ammonium Hydroxide Treatment and Light Microscopic Sudan IV Staining
Ester-linked phenolics and lignosuberized deposition were histochemically detected under fluorescence microscopy in sections obtained from the wounded zone of the maize mesocotyl of G3-untreated and G3-treated plants, at 0, 24, 48, and 72 h after wounding, as well as in sections obtained from wounded internodes of Spd-untreated and Spd-treated wild-type and ZmPAO-transgenic tobacco plants at 48, 72, and 96 h after wounding. Sections were directly mounted on slides in 25% glycerol and observed for autofluorescence under UV light (McLusky et al., 1999) or blue light (Daniel and Guest, 2006) or were incubated for 1 min in 10 mm NH4OH, pH 10 (Harris and Hartley, 1976), prior to observation under UV light. Lignosuberized depositions were histochemically detected under confocal laser scanning microscope in sections obtained from wounded internodes of wild-type and ZmPAO transgenic tobacco plants 48 h after injury. Autofluorescence of lignosuberized deposition was induced using an argon laser emitting at wavelength of 478 nm according to Xu et al. (2006) and detected with a selected emission band ranging from 503 to 611 nm. For suberin aliphatic domain visualization sections from maize mesocotyl were preincubated for 10 min in 50% ethanol and then stained for 20 min in a filtered saturated solution of Sudan IV in 70% ethanol. After washing in 50% ethanol (1 min), sections were mounted in 25% glycerol prior to be observed under light microscopy (Krishnamurthy, 1999).
Polyamine Quantification
The levels of free and soluble-conjugated polyamines were determined in the outer tissues obtained from the wounded zone of the maize mesocotyl and from the corresponding mesocotyl zone of unwounded (control) plants, at 0, 24, 48, and 72 h after wounding. Polyamines were extracted overnight from mesocotyl outer tissues in 5% (w/v) perchloric acid (PCA) containing 0.12 mm 1,6-diaminohexane as an internal standard (tissue to 5% PCA ratio 1:3 [w/v]). The extracts were centrifuged at 20,000g for 15 min and then PCA-soluble free polyamines were analyzed in one-half of the supernatant. The remaining supernatant was acid hydrolyzed in 6 m HCl for 18 h at 110°C to obtain PCA-soluble conjugates of polyamines (Slocum et al., 1989). Free polyamines were determined also in intercellular fluid extracted from mesocotyl of wounded and control plants as follows. After washing in distilled water, mesocotyls were submerged in 20 mm Tris-HCl buffer, pH 8, containing 0.1 m NaCl and 1 × 10−5 m G3, under vacuum. Intercellular fluids were recovered by centrifugation at 1,500g for 10 min and immediately mixed with PCA to a final concentration of 5% (v/v). A reference solution containing Dap, Put, 1,6-diaminohexane, Spd, and Spm was also prepared and treated as above, to establish retention times and signal intensities for each compound during HPLC analysis.
Polyamines were quantified after derivatization with dansyl chloride according to Smith and Davies (1985) with minor modifications. Dansylated polyamines were separated by HPLC (Spectra System P2000; Thermo Separation Product) on a reverse-phase C18 column (Sperisorb S5 ODS2, 5-μm particle diameter, 4.6 × 250 mm) using a discontinued methanol to water gradient (40%–60% methanol in 2 min, 60%–95% methanol in 20 min, 95%–100% in 2.5 min, 100% for 1.5 min, 100%–40% in 6 min at a flow rate of 1.5 mL min−1). Eluted peaks were detected by a spectrofluorometer (Spectra System FL 3000; excitation 365 nm, emission 510 nm), recorded, and integrated by an attached computer using Thermo Finnigan Chrom-Card 32-bit software. The retention times are as follows: Dap, 16.1 min; Put, 16.6 min; 1,6-diaminohexane, 18.08 min; Spd, 21.3 min; and Spm, 24.4 min.
The reported data are the average of values obtained in three different experiments, each performed with two technical replicates.
Statistics
All statistical tests were performed using ANOVA using GraphPad Prism. Statistical significance of differences was evaluated by P level.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AJ002204 and AF067732.
Supplemental Data
The following material is available in the online version of this article.
Supplemental Table S1. Levels of free polyamines determined in maize mesocotyl outer tissues from wounded and unwounded (control) plants at 0, 24, 48, and 72 h after wounding.
Supplementary Material
Acknowledgments
We wish to thank Paraskevi Tavladoraki (Biology Department, University Roma Tre, Italy) for critical reading of the manuscript.
This work was supported by the Italian Ministry for University and Research (PRIN 2005, project contract 2005052297_002 to R.A.).
This work is dedicated to the memory of Daniele Liberatori.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alessandra Cona (cona@uniroma3.it).
The online version of this article contains Web-only data.
References
- Alcázar R, Marco F, Cuevas JC, Patron M, Ferrando A, Carrasco P, Tiburcio AF, Altabella T (2006) Involvement of polyamines in plant response to abiotic stress. Biotechnol Lett 28 1867–1876 [DOI] [PubMed] [Google Scholar]
- Allan AC, Fluhr R (1997) Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell 9 1559–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress and signal transduction. Annu Rev Plant Biol 55 373–399 [DOI] [PubMed] [Google Scholar]
- Artiss JD, Entwistle WM (1981) The application of a sensitive uricase-peroxidase couple reaction to a centrifugal fast analyser for the determination of uric acid. Clin Chim Acta 116 301–309 [DOI] [PubMed] [Google Scholar]
- Augeri M, Angelini R, Federico R (1990) Sub-cellular localization and tissue distribution of polyamine oxidase in maize (Zea mays L.) seedlings. J Plant Physiol 136 690–695 [Google Scholar]
- Barker-Bridgers M, Ribnicky DM, Cohen JD, Jones AM (1998) Red-light-regulated growth: changes in the abundance of indole acetic acid in the maize (Zea Mays L.) mesocotyl. Planta 204 207–211 [Google Scholar]
- Biondi S, Scaramagli S, Capitani F, Altamura MM, Torrigiani P (2001) Methyl jasmonate up regulates biosynthetic gene expression, oxidation and conjugation of polyamines, and inhibits shoot formation in tobacco thin layers. J Exp Bot 52 231–242 [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F (2002) The apoplastic oxidative burst in response to biotic stress in plants: a three-component system. J Exp Bot 53 1367–1376 [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Cervelli M, Tavladoraki P, Di Agostino S, Angelini R, Federico R, Mariottini P (2000) Isolation and characterization of three polyamine oxidase genes from Zea mays. Plant Physiol Biochem 38 667–677 [Google Scholar]
- Cona A, Cenci F, Cervelli M, Federico R, Mariottini P, Moreno S, Angelini R (2003) Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol 131 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cona A, Manetti F, Leone R, Corelli F, Tavladoraki P, Polticelli F, Botta M (2004) Molecular basis for the binding of competitive inhibitors of maize polyamine oxidase. Biochemistry 43 3426–3435 [DOI] [PubMed] [Google Scholar]
- Cona A, Moreno S, Cenci F, Federico R, Angelini R (2005) Cellular re-distribution of flavin-containing polyamine oxidase in differentiating root and mesocotyl of Zea mays L. seedlings. Planta 221 265–276 [DOI] [PubMed] [Google Scholar]
- Cona A, Rea G, Angelini R, Federico R, Tavladoraki P (2006. a) Functions of amine oxidases in plant development and defense. Trends Plant Sci 11 80–88 [DOI] [PubMed] [Google Scholar]
- Cona A, Rea G, Botta M, Corelli F, Federico R, Angelini R (2006. b) Flavin-containing polyamine oxidase is a hydrogen peroxide source in the oxidative response to the protein phosphatase inhibitor cantharidin in Zea mays L. J Exp Bot 57 2277–2289 [DOI] [PubMed] [Google Scholar]
- Corelli F, Federico R, Cona A, Venturini G, Schenone S, Botta M (2002) Solution and solid-phase synthesis of aminoalkylguanidines inhibiting polyamine oxidase and nitric oxide synthase. Med Chem Res 11 309–321 [Google Scholar]
- Daniel R, Guest D (2006) Defence responses induced by potassium phosphonate in Phytophthora palmivora-challenged Arabidopsis thaliana. Physiol Mol Plant Pathol 67 194–201 [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C (2001) Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA 98 13454–13459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federico R, Alisi C, Cona A, Angelini R (1988) Purification of polyamine oxidase from maize seedlings by immunoadsorbent column. In V Zappia, AE Pegg, eds, Advances in Experimental Medicine and Biology, Progress in Polyamine Research. Plenum Press, New York, pp 617–623 [DOI] [PubMed]
- Federico R, Leone L, Botta M, Binda C, Angelini R, Venturini G, Ascenzi P (2001) Inhibition of pig liver and Zea mays L. polyamine oxidase: a comparative study. J Enzyme Inhib 13 465–471 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JD, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422 442–446 [DOI] [PubMed] [Google Scholar]
- Ha HC, Woster PM, Yage JD, Casero RA (1997) The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci USA 94 11557–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PJ, Hartley RD (1976) Detection of bound ferulic acid in cell walls of the Gramineae by ultraviolet fluorescence microscopy. Nature 259 508–510 [Google Scholar]
- Jiang M, Zhang J (2003) Cross-talk between calcium and reactive oxygen species originated from NADPH oxidase in abscisic acid-induced antioxidant defense in leaves of maize seedlings. Plant Cell Environ 26 929–939 [DOI] [PubMed] [Google Scholar]
- Jih PJ, Chen YC, Jeng ST (2003) Involvement of hydrogen peroxide and nitric oxide in expression of the ipomoelin gene from sweet potato. Plant Physiol 132 381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126 1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseleau JP, Ruel K (1997) Study of lignification by noninvasive techniques in growing maize internodes: an investigation by Fourier transform infrared cross-polarization-magic angle spinning 13C-nuclear magnetic resonance spectroscopy and immunocytochemical transmission electron microscopy. Plant Physiol 114 1123–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Choi JL, Costa MA, An G (1992) Identification of G-box sequence as an essential element for methyl jasmonate response of potato proteinase inhibitor II promoter. Plant Physiol 99 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Kawakita K, Maeshima M, Doke N, Yoshioka H (2006) Subcellular localization of Strboh proteins and NADPH-dependent O2−-generating activity in potato tuber tissues. J Exp Bot 57 1373–1379 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy KV (1999) Methods in Cell Wall Cytochemistry. CRC Press, Boca Raton, FL
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane BG (2002) Oxalate, germins, and higher-plant pathogens. IUBMB Life 53 67–75 [DOI] [PubMed] [Google Scholar]
- León J, Rojo E, Sánchez-Serrano JJ (2001) Wound signalling in plants. J Exp Bot 52 1–9 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79 583–593 [DOI] [PubMed] [Google Scholar]
- Love AJ, Yun BW, Laval V, Loake GJ, Milner JJ (2005) Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol 139 935–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLusky SR, Bennett MH, Beale MH, Lewis MJ, Gaskin P, Mansfield JW (1999) Cell wall alterations and localized accumulation of feruloyl-3′-methoxytyramine in onion epidermis at sites of attempted penetration by Botrytis allii are associated with actin polarisation, peroxidase activity and suppression of flavonoid biosynthesis. Plant J 17 523–534 [Google Scholar]
- Møller SG, McPherson MJ (1998) Developmental expression and biochemical analysis of the Arabidopsis atao1 gene encoding an H2O2-generating diamine oxidase. Plant J 13 781–791 [DOI] [PubMed] [Google Scholar]
- Mur LA, Kenton P, Atzorn R, Miersch O, Wastemack C (2006) The outcomes of concentration-specific interactions between salycilate and jasmonate signalling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müsel G, Schindler T, Bergfeld R, Ruel K, Jacquet G, Lapierre C, Speth V, Schopfer P (1997) Structure and distribution of lignin in primary and secondary cell walls of maize coleoptiles analyzed by chemical and immunological probes. Planta 201 146–159 [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13 179–191 [PMC free article] [PubMed] [Google Scholar]
- Paschalidis KA, Roubelakis-Angelakis KA (2005) Sites and regulation of polyamine catabolism in the tobacco plant: correlations with cell division/expansion, cell cycle progression, and vascular development. Plant Physiol 138 2174–2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406 731–734 [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191 123–128 [Google Scholar]
- Perez-Amador MA, Leon J, Green PJ, Carbonell J (2002) Induction of the arginine decarboxylase ADC2 gene provides evidence for the involvement of polyamines in the wound response in Arabidopsis. Plant Physiol 130 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, Van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7 456–464 [DOI] [PubMed] [Google Scholar]
- Razem FA, Bernards MA (2002) Hydrogen peroxide is required for polyphenolic domain formation during wound-induced suberization. J Agric Food Chem 50 1009–1015 [DOI] [PubMed] [Google Scholar]
- Rea G, de Pinto MA, Tavazza R, Biondi S, Gobbi V, Ferrante P, De Gara L, Federico R, Angelini R, Tavladoraki P (2004) Ectopic expression of maize polyamine oxidase and pea copper amine oxidase in the cell wall of tobacco plants. Plant Physiol 134 1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea G, Metoui O, Infantino A, Federico R, Angelini R (2002) Copper amine oxidase expression in defense responses to wounding and Ascochyta rabiei invasion. Plant Physiol 128 865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers HI (2005) Cell death and organ development in plants. Curr Top Dev Biol 71 225–261 [DOI] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R (2004) Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell 16 616–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Sherf BA, Bajar AM, Kolattukudy PE (1993) Abolition of an inducible highly anionic peroxidase activity in transgenic tomato. Plant Physiol 101 201–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Plas F, Elmayan T, Blein JP (2002) The plasma membrane oxidase NtrbohD is responsible for AOS production in elicited tobacco cells. Plant J 31 137–147 [DOI] [PubMed] [Google Scholar]
- Slocum RD, Flores HE, Galston AW, Einstein LH (1989) Improved method for HPLC analysis of polyamines, agmatine and aromatic monoamines in plant tissue. Plant Physiol 89 512–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TA, Davies PJ (1985) Separation and quantitation of polyamines in plant tissue by high performance liquid chromatography of their dansyl derivatives. Plant Physiol 78 89–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M, Belenghi B, Delledonne M, Menachem E, Levine A (1999) The involvement of cysteine proteases and protease inhibitor genes in the regulation of programmed cell death in plants. Plant Cell 11 431–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Berberich T, Miyazaki A, Seo S, Ohashi Y, Kusano T (2003) Spermine signalling in tobacco: activation of mitogen-activated protein kinases by spermine is mediated through mitochondrial dysfunction. Plant J 36 820–829 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Uehara Y, Berberich T, Ito A, Saitoh H, Miyazaki A, Terauchi R, Kusano T (2004) A subset of hypersensitive response marker genes, including HSR203J, is the downstream target of a spermine signal transduction pathway in tobacco. Plant J 40 586–595 [DOI] [PubMed] [Google Scholar]
- Tavladoraki P, Schininà ME, Cecconi F, Di Agostino S, Manera F, Rea G, Federico R, Mariottini P, Angelini R (1998) Maize polyamine oxidase: primary structure from protein and cDNA sequencing. FEBS Lett 426 62–66 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JDG (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37 1130–1134 [DOI] [PubMed] [Google Scholar]
- Walters D (2003) Polyamines and plant disease. Phytochemistry 64 97–107 [DOI] [PubMed] [Google Scholar]
- Walters D, Cowley T, Mitchell A (2002) Methyl jasmonate alters polyamine metabolism and induces systemic protection against powdery mildew infection in barley seedlings. J Exp Bot 53 747–756 [DOI] [PubMed] [Google Scholar]
- Woodward MP, Young WW, Bloodgood RA (1985) Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods 78 143–153 [DOI] [PubMed] [Google Scholar]
- Xu F, Zhong XC, Sun RC, Lu Q (2006) Anatomy, ultrastructure and lignin distribution in cell wall of Caragana korshinskii. Ind Crops Prod 24 186–193 [Google Scholar]
- Yoda H, Hiroi Y, Sano H (2006) Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol 142 193–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Yamaguchi Y, Sano H (2003) Induction of hypersensitive cell death by hydrogen peroxide produced through polyamine degradation in tobacco plants. Plant Physiol 132 1973–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JDG, Doke N (2003) Nicotiana benthamiana gp91(phox) homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.