Abstract
The array of phagocytic receptors expressed by macrophages make them very efficient at pathogen clearance, and the phagocytic process links innate with adaptive immunity. Primary macrophages modulate antigen cross-presentation and T-cell activation. We assessed ex vivo the putative role of different phagocytic receptors in immune synapse formation with CD8 naïve T-cells from OT-I transgenic mice and compared this with the administration of antigen as a soluble peptide. Macrophages that have phagocytosed antigen induce T-cell microtubule-organizing center and F-actin cytoskeleton relocalization to the contact site, as well as the recruitment of proximal T-cell receptor signals such as activated Vav1 and PKCθ. At the same doses of loaded antigen (1 μM), “phagocytic” macrophages were more efficient than peptide-antigen–loaded macrophages at forming productive immune synapses with T-cells, as indicated by active T-cell TCR/CD3 conformation, LAT phosphorylation, IL-2 production, and T-cell proliferation. Similar T-cell proliferation efficiency was obtained when low doses of soluble peptide (3–30 nM) were loaded on macrophages. These results suggest that the pathway used for antigen uptake may modulate the antigen density presented on MHC-I, resulting in different signals induced in naïve CD8 T-cells, leading either to CD8 T-cell activation or anergy.
INTRODUCTION
Phagocytosis plays a crucial role in the clearance of pathogens at sites of infection. This phenomenon also has an important role in triggering the adaptive immune response, which requires the processing of microbial antigens and their presentation to CD4+ and CD8+ T-cells by antigen-presenting cells (APCs). It has been thought that the only cells able to prime naïve T lymphocytes are bone marrow-derived dendritic cells (DCs). However, recent studies show that macrophages stimulate not only effector T-cells but also naïve T-cells and can therefore participate in the activation of an adaptive primary immune response in vivo (Pozzi et al., 2005). Moreover, macrophages could be the dominant APCs at sites of inflammation or infection, because there is a large local increase in the number of these cells, in contrast to the low levels of DCs (Hamilton-Easton and Eichelberger, 1995; Usherwood et al., 1999). Both DCs and macrophages have been recently studied for their ability to cross-present antigens from phagocytosed pathogens through the major histocompatibility complex (MHC)-class I pathway (Pfeifer et al., 1993; Oliveira and Splitter, 1995; Reis e Sousa and Germain, 1995; Turner and Dockrell, 1996; Oh et al., 1997; Belkaid et al., 2002; Pozzi et al., 2005). Interestingly, one of the mechanisms for this cross-presentation is the involvement of the endoplasmic reticulum (ER) in the process of phagocytosis, a phenomenon that does not occur in neutrophils (Gagnon et al., 2002; Desjardins, 2003; Guermonprez et al., 2003; Houde et al., 2003). In addition to the phagocytosis of many pathogens, the ER-dependent mechanism contributes to the phagocytosis of latex beads, IgG-coated latex beads, and C3b-coated latex beads (Desjardins, 2003).
Macrophages express a wide variety of receptors that participate in the “phagocytic” uptake of antigens. In the early phase of infection, antigen uptake is mainly mediated by receptors for pathogen-associated molecular patterns (PAMPs), including mannose-(MR), C-type lectin-(LR), and Toll-like receptors. In contrast, receptors that bind opsonized antigens, including those for immunoglobulin Fc fragments (mainly FcγR) and activated complement factors, are involved at a later stage (Bajtay et al., 2006; Helmy et al., 2006). In the case of early phagocytic receptors, MR as well as LR, such as SIGNR1 and SIGNR3 (mouse homologues of DC-SIGN), laminarin, and DEC205 have been involved in phagocytosis of apoptotic cells as well as of bacteria, partially through the detection of mannan residues (Kindberg et al., 1990; Takahara et al., 2004; Burgdorf et al., 2006). Although it has been found that endocytosis through MR or DEC205 results in CD8+ T-cell activation and protection against tumor cells (Apostolopoulos et al., 2000; Bonifaz et al., 2002; Burgdorf et al., 2006), the involvement of macrophage phagocytosis through LR in the modulation of the immune response needs to be further explored.
Several studies indicate that the phagocytosis of particles opsonized by antibodies enhances the efficiency of MHC-I antigen presentation in DCs (Falo et al., 1995; Hsu and Komarovskaya, 2002; Tobar et al., 2004), although little work in this area has been done in macrophages (Abdul-Majid et al., 2002; Liu et al., 2006). Opsonization by the complement system reflects either the presence of antibodies bound to the particulate antigen (classic activation pathway) or the presence of PAMPs (collectin and alternative activation pathways; reviewed in Carroll, 2004). Complement activation products, such as C3b, target the antigenic particle to DCs or macrophages for uptake, mainly through CR3 and CR4 and the recently discovered CRIg receptors (Helmy et al., 2006). Nevertheless, it is not well known whether phagocytosis mediated by complement receptors (CRs) also enhances antigen presentation.
Antigen loaded-APCs form stable conjugates with specific T-cells and promote the relocalization of antigen recognition receptors as well as cell adhesion and signaling molecules to the contact site, to form what has been termed as the immune synapse (IS; Dustin et al., 1998; Monks et al., 1998). During the formation of the IS, the T-cell receptor (TCR) is engaged and activated for the recruitment and induction of proximal signaling molecules, including Lck, Zap70, LAT, Vav1, and PKCθ (Irving and Weiss, 1991; Straus and Weiss, 1992; van Oers et al., 1996; Zhang et al., 1998; Altman et al., 2000; Lin et al., 2000; Sun et al., 2000; Krawczyk et al., 2002). As a consequence, the microtubule-organizing center (MTOC) is reoriented for the efficient secretion of cytokines and killing granules (Kupfer et al., 1994), and the F-actin cytoskeleton is recruited to the contact site for the stabilization and reorganization of the IS in supramolecular activation clusters (SMACs; Monks et al., 1998; Grakoui et al., 1999; Dustin and Cooper, 2000; Bunnell et al., 2001).
To analyze the role of the pathogen entry pathway on the antigen presentation process, we have studied the effect of different macrophage phagocytosis pathways on the ability of macrophages to form ISs and to induce T-cell activation and proliferation. In this work we report that primary macrophages loaded through phagocytosis are able to activate naïve CD8+ T-cells for proliferation and T-cell cytokine production. Furthermore, we report that phagocytic macrophages were more efficient than macrophages loaded with soluble antigen peptide at forming productive ISs with T-cells, inducing T-cell active TCR/CD3 conformation, and phosphorylating proximal TCR-associated signaling molecules.
MATERIALS AND METHODS
Mice
All experiments were performed in 6- to 12-week-old mice. Mice were housed and bred in the Animal Unit of the School of Medicine, Universidad Autónoma of Madrid, Spain, in a pathogen-free facility. OT-I mice, an H-2Kb restricted anti-OVA TCR transgenic line under a C57BL/6 background, were used for T lymphocyte isolation (Hogquist et al., 1994). C57BL/6 mice (H-2b) were used for macrophage isolation.
Preparation of Macrophages and T-cells
Thioglycolate-elicited macrophages were obtained by plastic adherence of peritoneal exudate cells obtained through lavage of the peritoneal cavity of mice, injected intraperitoneally 4–5 d previously with 2 ml of thioglycolate broth (Sigma, St. Louis, MO). Contaminating granulocytes were depleted using magnetic beads for positive selection of Gr1+ cells, following the manufacturer's instructions (Miltenyi Biotech, Gladbach, Germany). CD8+ T-cells were isolated from spleen and lymph nodes of OT-I transgenic mice using magnetic beads for negative selection of CD8+ T-cells (Miltenyi Biotech). For naïve CD8+ T-cell isolation, anti-CD44 biotinylated antibody was added to the CD8+ isolation kit antibody cocktail.
Opsonization and Phagocytosis Assays
Latex beads (3 μm, Sigma) were opsonized as previously described (May et al., 2000; Olazabal et al., 2002) with slight modifications. Briefly, low endotoxin OVA protein (with <0.03 EU/ml endotoxin at the highest concentration used; Calbiochem, Darmstadt, Germany) or BSA protein (Sigma) were incubated overnight with latex beads at a concentration of 5 or 10 mg/ml, respectively. The amount of OVA and BSA bound to the beads was determined based on the difference between protein concentration in the solution before and after adsorption (Kovacsovics-Bankowski et al., 1993). Opsonized beads were added to macrophages at an approximate BSA or OVA protein concentration of 50 μg/ml (1 μM). OVA and BSA possess mannan side chains and when bound to beads were used as ligands of LR-mediated phagocytosis (Kindberg et al., 1990; Takahara et al., 2004; Burgdorf et al., 2006). For FcγR- and LR-phagocytosis, OVA- or BSA-coated beads were incubated with rabbit anti-OVA (Rockland, Gilbertsville, PA) or rabbit anti-BSA (Sigma) IgG antibodies during 30 min at RT (BSA- or OVA-IgG beads). For CR- and LR-phagocytosis, OVA- or BSA-beads were opsonized as described using phenol glycolipid-1 (PGL-1) of the Mycobacterium leprae membrane (kindly provided by the NIH Leprosy Research Support program, Colorado). Macrophages were mixed with beads for 1 h to allow optimal phagocytosis. In the case of phagocytosis assays mediated by CR, macrophages were pretreated with 20 nM fMLP for 20 min.
Antigen Presentation Assays
Differently opsonized latex beads were added to macrophages (3 × 105) previously seeded on 24-well plates at the indicated concentrations. After incubation for 1 h, cells were extensively washed and incubated again for 2–3 h. Afterward, CD8+ T-cells (1 × 106) were added to macrophages for different periods. Two hours before T-cell addition, macrophages were loaded as indicated with peptides derived from OVA (257-264, SIINFEKL) or BSA (294-301, RNITYEKL) in the following concentration ranges: 3 pM–30 pM (low), 100 pM–100 nM (medium), or 1 μM (high).
T-Cell Activation Markers and Cytokine Production
Cells were recovered and stained for CD69 and CD25 using specific labeled monoclonal antibodies (BD Biosciences, Erembodegem, Belgium) after 24 or 48 h of conjugate formation, respectively. For cytokine analysis after 48 h of antigen presentation, as described before (Casey and Mescher, 2007), cells were stimulated with phorbol 12-myristate acetate (PMA; 20 ng/ml) plus ionomycin (500 ng/ml) for 6 h and incubated with 50 μg/ml brefeldin A (Sigma) during the last 4 h. Collected cells were fixed and permeabilized with 0.3% saponin and stained with APC-labeled anti-IL-2 or anti-IFN-γ antibodies (BD Biosciences), and analyzed in a FACSCalibur flow cytometer (BD Biosciences) on gated CD8+ cells.
Proliferation Assays
Antigen presentation assays were performed with CFSE-labeled T-cells (5 μm, Molecular Probes, Nasdaq, IVGN) during 50 h. Cells were recovered and analyzed for carboxyfluorescein succinimidyl ester (CFSE) levels by flow cytometry on gated CD8+ cells.
Immunofluorescence Assays
Cell conjugates formed after 20 min were fixed in 4% paraformaldehyde and 4% sucrose, blocked in TBS containing 0.1 mg/ml human γ-globulin and 1% BSA, and stained with the following reagents: phalloidin (Molecular Probes), anti-α-tubulin-FITC mAb (Sigma), anti-phosphorylated Y174 Vav (polyclonal antibody, kindly provided by Dr. X. Bustelo, Centro de Investigación del Cancer, Salamanca, Spain; Lopez-Lago et al., 2000), anti-PKCθ (polyclonal antibody, Sc-13, Santa Cruz Biotechnology, Santa Cruz, CA), and APA 1/1 mAb, which detects the activated conformation of CD3ε (Risueno et al., 2005, 2006). Highly cross-adsorbed secondary antibodies labeled with Rhodamine-X were from Molecular Probes. During microscope examination, T-cells and macrophages were identified based on cell morphology, and conjugate formation was quantified. Cells were visualized with a DMR epifluorescence photomicroscope (Leica, Heidelberg, Germany) with a 63×/1.40–0.7 NA oil CS objective, coupled to a CCD Camera (Cohu, San Diego, CA). For confocal images, a Leica TCS-SP confocal laser scanning unit with Ar and He beams was used, attached to a Leica DMIRBE inverted epifluorescence microscope (Leica Microsystems). The acquisition software was Leica QFISH V2.1, and images were processed with Adobe Photoshop 7.0 (San Jose, CA).
Immunoprecipitation and Western Blot Assays
Cells were lysed with 50 mM Tris-HCl buffer, pH 7.5, containing 1% NP-40, 0.2% Triton X-100, 150 mM NaCl, and phosphatase and protease inhibitors at 4°C for 40 min. Cell lysates were spun to remove cell debris and nuclei, and in the case of CD3 analyses, supernatants were precleared with protein A-Sepharose beads at 4°C overnight, immunoprecipitated with rabbit anti-CD3ζ (Sahuquillo et al., 1998) for 2 h, and recovered with protein A-Sepharose beads at 4°C for 20 min. Immunoprecipitates were washed six times with lysis buffer and analyzed by Western blot for phosphotyrosine content with 4G10 antibody and anti-CD3ζ for loading control. For LAT and ZAP70 analyses, cell lysates were resolved by SDS-PAGE, transferred to membranes, and blotted with the following antibodies: rabbit polyclonal anti-Vav Y174, anti Vav (rabbit polyclonal, Upstate Biotechnology, Lake Placid, NY), rabbit polyclonal anti-phospho LAT Y132 (rabbit polyclonal, Abcam, Cambridge, MA), anti-LAT (rabbit polyclonal, Upstate Biotechnology), anti-phospho Y315 ZAP70 (rabbit polyclonal, Abcam) and anti-ZAP70 (rabbit polyclonal, Abcam), all in TBS-Tween (0.1%). Bound antibodies were detected with horseradish peroxidase–conjugated secondary antibodies, followed by visualization with SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL).
Statistical Analysis
The Mann-Whitney test (p < 0.01 or p < 0.05) was performed.
RESULTS
“Phagocytic” Macrophages Induce T-Cell Activation
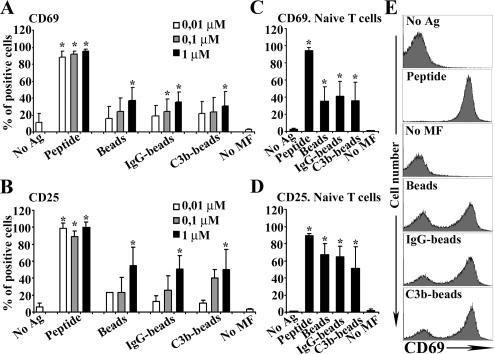
The effect of different types of phagocytosis on the antigen presentation potential of primary macrophages was analyzed by their ability to activate CD8+ T-cells. Peritoneal mouse macrophages were loaded with increasing doses of soluble peptides (10 nM-1 μM) or were incubated with beads that had been previously coated with proteins (BSA or OVA), such that the amount of protein coating the beads was equivalent to 10 nM-1 μM, as indicated in the legend of Figure 1. Additionally, the latex beads were opsonized in different ways to facilitate entry through LR, LR plus FcγR, or LR plus CR. As expected, phagocytosis through LR plus FcγR and LR plus CR was more efficient in the uptake of IgG- and C3b-beads than LR in the uptake of beads (not shown); therefore, to assure that a similar amount of antigen was being uptaken through the different receptors, phagocytosis assays were performed during 1 h for saturation. Activation of OT-I CD8+ T-cells, bearing a transgenic TCR specific for OVAp, was analyzed by measuring the expression of the T-cell activation markers CD69 and CD25. Macrophages that take up antigen via phagocytosis were called “phagocytic” macrophages. When loaded with the specific antigen (OVA-beads), phagocytic macrophages induced CD69 and CD25 expression in OT-I T-cells in a dose-dependent manner (Figure 1, A and B). This induction from 10% to ∼40% of expressing cells was significant at the highest concentration tested (1 μM; p < 0.01). In comparison, OVAp-loaded macrophages were much more efficient at inducing CD69 and CD25 expression, ∼90%, even at the lowest concentrations tested (Figure 1, A and B). We found that macrophages loaded with the nonspecific antigen BSA, in the form of peptide or of differently opsonized beads, did not induce CD69 or CD25 expression (data not shown). The difference between phagocytic macrophages and OVAp-loaded macrophages persisted when macrophages were depleted of granulocytes and when purified naïve T-cells were used (p < 0.05, Figure 1, C and D, and representative histograms in panel E).
Figure 1.
Phagocytic and OVAp-loaded macrophages induce CD69 and CD25 expression in T lymphocytes. CD8+ cells were analyzed for the surface expression of CD69 or CD25 molecules by flow cytometry. Dose-response assays of CD69 (A) and CD25 expression (B) determined as the percentage of positive cells. (C and D) Same as in A and B but granulocyte-depleted macrophages and naïve CD8+ T-cells were used. Macrophages without antigen (No Ag). T-cells without macrophages (No MF). (A–D) Data are expressed as arithmetic means ± SEM of four independent experiments; statistical comparisons were by the Mann-Whitney test (*p < 0.05). (E) Histograms of CD69 expression in CD8+ cells are shown from a representative experiment.
CD8+ T-Cell Cytokine Production and Proliferation Induced by Phagocytic or Peptide-loaded Macrophages
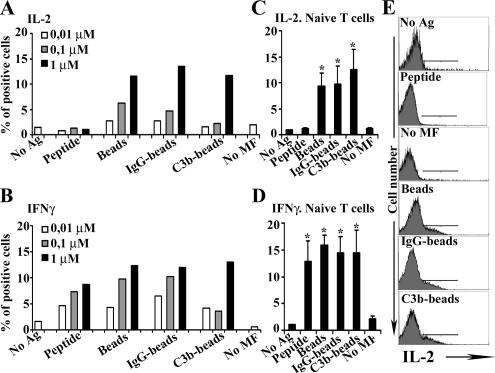
Next, the influence of macrophage antigen uptake by either phagocytosis or extracellular peptide loading on T-cell cytokine production was assessed by intracellular staining of IL-2 and IFN-γ. In a dose-response assay, phagocytic macrophages activated up to ∼10–15% of CD8+ T-cells to produce IL-2 and IFN-γ, compared with unloaded macrophages (p < 0.05, representative experiments in Figure 2, A and B). Interestingly, macrophages loaded with soluble OVAp stimulated IL-2 synthesis minimally at the concentrations tested. However, the production of IFN-γ was induced at similar levels by both OVAp-loaded and phagocytic macrophages. Similar results were obtained when purified naïve CD8+ T-cells and macrophages depleted of granulocytes were used (Figure 2, C and D, and representative histograms in panel E). Therefore, phagocytic macrophages induce T-cell IL-2 production more efficiently than macrophages-loaded with soluble OVAp, not only in resting T-cells, but also in naïve T-cells.
Figure 2.
Induction of IL-2 and IFN-γ production in T lymphocytes by phagocytic or OVAp-loaded macrophages. CD8+ cells were analyzed by flow cytometry for the intracellular production of IL-2 and IFN-γ. Representative dose-response assays of the production of IL-2 (A) and IFNγ (B), determined as the percentage of positive cells. (C and D) Same as in A and B, granulocyte-depleted macrophages and naïve CD8+ T-cells were used. Data are expressed as arithmetic means ± SEM of four independent experiments; statistical comparisons were by the Mann-Whitney test (*p < 0.05). (E) Histograms of IL-2 production in CD8+ cells are shown from a representative experiment.
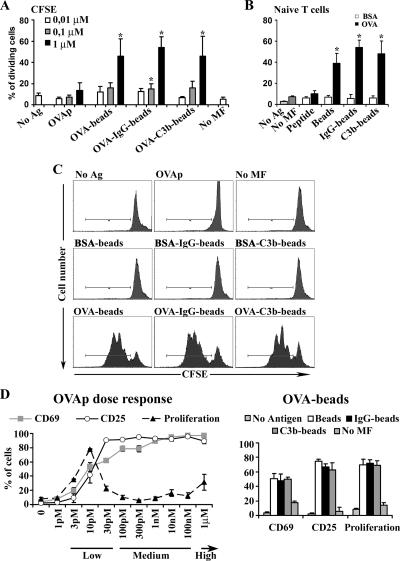
T-cell proliferation was assessed by CFSE staining and estimation of the number of cell divisions by flow cytometry. Data were quantified and represented as the cumulative number of cell divisions. Dose-response experiments were performed. We found that phagocytic macrophages loaded with an unspecific antigen (BSA-beads) did not induce T-cell proliferation (Figure 3, B and C), whereas macrophages loaded with the specific antigen (OVA-beads), induced up to 50% of T-cells to proliferate (p < 0.05, Figure 3A). In contrast, soluble OVAp-loaded macrophages did not induce T-cell proliferation at the concentrations tested. Similar results were obtained when macrophages depleted of granulocytes and naïve T-cells were used (Figure 3B and representative histograms in C). Therefore, phagocytic macrophages, but not macrophages loaded with soluble antigen peptide, induce proliferation of naïve CD8+ T-cells, in accordance with their effect on IL-2 synthesis.
Figure 3.
Phagocytic macrophages induce CD8+ T-cell proliferation. CFSE-labeled T-cells were analyzed by flow cytometry for CFSE expression after 50 h of antigen presentation to estimate the number of T-cell divisions. (A) Dose-response assays of CFSE staining determined as the percentage of dividing cells. (B) Macrophages loaded with 1 μM BSA (□) or OVA (■) peptide or with differently opsonized beads. Macrophages depleted of granulocytes and naïve CD8+ T-cells were used. Data are expressed as arithmetic means ± SEM of four independent experiments; statistical comparisons were made with the Mann-Whitney test (*p < 0.05). Fold inductions of unloaded macrophages are presented. (C) Histograms of CFSE staining in CD8+ cells are shown from a representative experiment. (D) Dose-response assays comparing the numbers of CD69 and CD25 positive and proliferating T-cells after exposure to macrophages loaded with different concentrations of OVAp (left) or with 1 μM of differently opsonized OVA-beads (right).
Different doses of antigen have been shown to induce either CTL stimulation or anergy (Oved et al., 2007). We therefore assessed whether macrophages loaded with different antigen doses would show this dual behavior. Dose-response experiments were performed starting with picomolar peptide concentrations, and T-cell CD69 and CD25 expression and proliferation were assessed. At low peptide concentrations (3 pM–30 pM) CD69 and CD25 were induced to levels comparable to those in T-cells primed with phagocytically loaded macrophages (Figure 3D). Interestingly, only this range of peptide concentrations was able to induce T-cell proliferation; the ability to proliferate was lost at medium (100 pM–100 nM) or high (1 μM) doses of peptide. Thus, macrophages exposed to low amounts of peptide can induce CD8 proliferation, but macrophages exposed to medium or high amounts fail to do so.
Conjugate and IS Formation of Phagocytic and Peptide-loaded Macrophages with Naive CD8+ T Lymphocytes
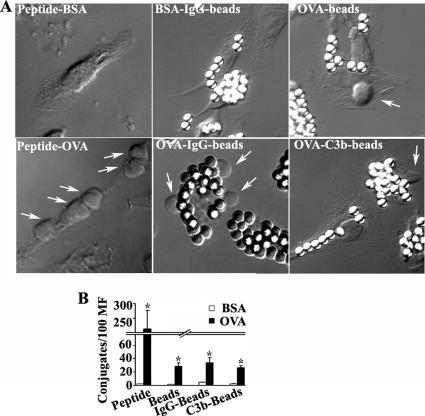
Since phagocytic macrophages were able to fully activate T-cells, in contrast to the incomplete stimulatory effect produced by macrophages loaded with medium or high doses of peptide, we next investigated whether these differences might reflect the nature and characteristics of the cellular interactions between these two types of antigen-loaded macrophages with naïve CD8+ T-cells. Macrophages depleted of granulocytes were conjugated with naïve CD8+ T-cells and analyzed using a differential interphase contrast microscope. We found that macrophages loaded with either OVAp (from 3 pM to 1 μM) or differently opsonized OVA-beads were able to form a significant number of conjugates with naive CD8+ T-cells compared with macrophages loaded with BSA (controls; p < 0.01, Figure 4, A and B, and data not shown). Moreover, macrophages loaded with low OVAp doses (3–30 pM) formed conjugates with T-cells with similar efficiency to macrophages loaded with OVA-beads, OVA-IgG-beads, or OVA-C3b-beads (data not shown). However, when macrophages were loaded with medium-high OVAp doses (100 pM–1 μM), the efficiency of conjugate formation was significantly higher than with phagocytic macrophages loaded with the differently opsonized OVA-beads (p < 0.01, Figure 4, A and B, and data not shown). Therefore, the poor ability of macrophages loaded with medium-high peptide doses (100 pM–1 μM) to induce T-cell proliferation and IL-2 production was not caused by a defective formation of T-cell/macrophage conjugates.
Figure 4.
Macrophages form peptide-specific conjugates with naive CD8+ T-cells. (A) Differential interphase contrast images of conjugates formed between differently loaded macrophages and CD8+ T-cells. Arrows indicate conjugated T-cells. (B) Number of conjugates formed between CD8+ T-cells and macrophages loaded with 1 μM BSA (□) or OVA (■) peptide or with differently opsonized beads. Results are presented as total T-cells per 100 macrophages and shown as arithmetic means ± SEM of five independent experiments; statistical comparison of BSA- and OVA-loaded macrophages was performed with the Mann-Whitney test (*p < 0.05).
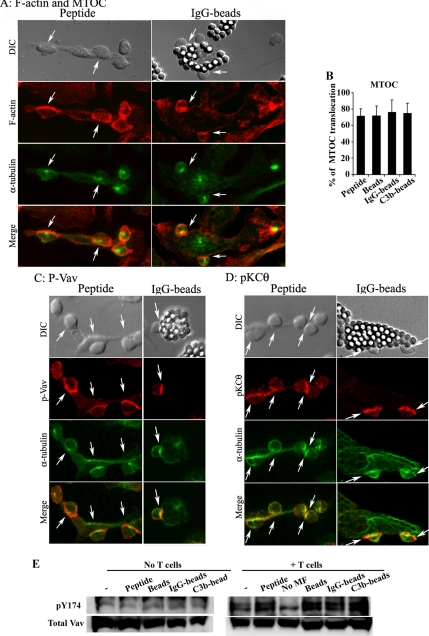
Macrophage-induced IS formation was then characterized by analyzing events induced in T-cells. T-cell cytoskeleton rearrangement was detected by F-actin clustering at the contact site and by MTOC relocation (Figure 5A). Macrophages loaded with 1 μM of either soluble OVAp or of differently opsonized OVA-beads were able to induce these events (Figure 5A). In particular, OVAp-loaded macrophages induced MTOC translocation with a similar efficiency to phagocytic macrophages (Figure 5B). Moreover, the recruitment of the TCR early signaling proteins phosphorylated Vav1 and PKCθ was also detected in T-cells conjugated with either OVAp-loaded or phagocytic macrophages (Figure 5, C and D, and Western blot in E).
Figure 5.
Phagocytic macrophages induce T-cell F-actin clustering, MTOC translocation, and recruitment of TCR proximal signaling proteins. Conjugates formed with macrophages loaded with OVAp (left panels) or OVA IgG-beads (right panels) are shown. DIC images are shown in the top panels. Cells were stained for F-actin with phalloidin (A); for phosphorylated Vav (C), or PKCθ (D; red), and for the MTOC, using the α-tubulin antibody (green). Merged images are shown in the bottom panels. Arrows indicate T-cell conjugates positive for MTOC and other molecules. (B) The number of conjugates in which the T-cell MTOC was translocated was quantified and expressed as the percentage of the total number of conjugates formed. Results are presented as the arithmetic means ± SD of five experiments; statistical comparisons were made with the Mann-Whitney test (*p < 0.05). (E) Immunoblots showing phosphorylated Y174 Vav and total Vav expression in macrophages loaded with 1 μM of OVA peptide or OVA differently opsonized to beads (left) and in conjugates formed between T-cells and these macrophage populations (right).
Phagocytic Macrophages Form Productive ISs with Naïve CD8+ T-Cells
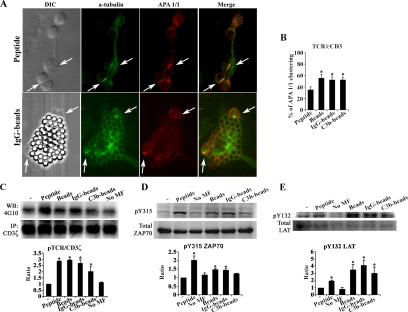
To compare early differential signals induced in T-cells conjugated with phagocytic macrophages or macrophages loaded with high (nonproliferative) peptide doses, we analyzed markers of productive ISs on naïve CD8+ T-cells. The induction of the conformational change in the TCR/CD3 in T-cells near the contact area was analyzed and quantified in conjugates formed with macrophages loaded with 1 μM of either soluble OVAp or differently opsonized OVA-beads. TCR engagement induces a conformational change that is transmitted to the CD3ε cytoplasmic tail. This can be detected with the conformation-specific antibody APA 1/1 and is a useful indicator of productive IS formation (Risueno et al., 2005, 2006). Despite their high capacity to form conjugates with T-cells, macrophages loaded with OVAp were less efficient at inducing the active TCR/CD3ε conformation than phagocytic macrophages (p < 0.01, Figure 6, A and B).
Figure 6.
Phagocytic macrophages induce productive immune synapses: phosphorylation of CD3ζ, ZAP70, and LAT. (A) Conjugates formed with OVAp (top panels) or OVA-IgG-beads loaded macrophages (bottom panels) are shown. DIC images are shown in the left panels. Cells were stained for MTOC using the α-tubulin antibody (green) and for the active conformation of TCR/CD3ε chain using the APA 1/1 antibody (red). Merged images are shown on the right. Arrows indicate T-cell conjugates positive for MTOC and APA 1/1. (B) Histogram showing the number of APA 1/1 positive T-cells, expressed as the percentage of the conjugates formed. Data are the arithmetic means ± SEM of five experiments; statistical comparisons were made with the Mann-Whitney test (*p < 0.05). (C) Conjugates were processed and immunoprecipitated with anti-CD3ζ antibody, followed by immunoblot for anti-phosphotyrosine residues (4G10 antibody, top panel) or CD3ζ (bottom panel). (D and E) Representative immunoblots of phosphorylated ZAP70 Y315 and LAT Y132 (top panels) and of total ZAP70 and LAT (bottom panels). Histograms below C–E show the results of densitometric analysis, presented as the ratio of phosphorylated to total protein bands. Data are the arithmetic means ± SEM of three experiments; statistical comparisons were made with the Mann-Whitney test (*p < 0.05).
Other TCR proximal activation signals were analyzed on conjugated naïve CD8+ T-cells. Because CD3ζ, ZAP70 and LAT are specifically expressed in T-cells, activation of these molecules by tyrosine phosphorylation was assayed in cell conjugates by immunoprecipitation and Western blot analysis. In all cases, OVAp-loaded macrophages and phagocytic macrophages induced the phosphorylation of CD3ζ, ZAP70, and LAT. Quantitative analysis showed that OVAp-loaded and phagocytic macrophages induced CD3ζ and ZAP70 Y315 phosphorylation at comparable levels (Figure 6, C and D), although LAT Y132 phosphorylation was more efficiently induced by phagocytic macrophages (Figure 6E). Altogether, these data indicate that phagocytic macrophages induce some early signaling events at higher efficiency than high-dose (1 μM) OVAp-loaded macrophages. In particular, CD3ε active conformation and the specific phosphorylation of LAT Y132 are highly induced by phagocytic macrophages, which is indicative of a higher efficiency in the formation of productive ISs.
DISCUSSION
This study shows that the way macrophages are primed with antigen has a profound effect on their capacity to fully activate naïve CD8 T-cells. LR-, FcR-, and CR- phagocytic macrophages fully activated CD8 T-cells, being potent inducers of IL-2 secretion and T-cell proliferation. Similar effects were detected with macrophages loaded with low doses of soluble antigenic peptide (3–30 pM). In contrast, although macrophages loaded with medium (100 pM–100 nM) or high (1 μM) doses of peptide antigen were very efficient at inducing CD69 and CD25 expression, they were poor inducers of IL-2 secretion and cell proliferation.
It has been reported that the source and characteristics of antigen uptake may determine the efficiency of the immune response, since particulate antigens are processed and presented more efficiently than soluble antigens (Harding and Song, 1994; Kovacsovics-Bankowski and Rock, 1995; Reis e Sousa and Germain, 1995; Rescigno et al., 1998; Yada et al., 2003). In this regard, our work shows that macrophages loaded with high doses of antigen (1 μM) in the form of soluble peptide form a higher number of conjugates with CD8+ T-cells than macrophages loaded with antigen in the form of opsonized particles that are taken up by phagocytosis. However, macrophages loaded with high antigenic peptide doses are inefficient activators of T lymphocytes, as measured by IL-2 production and T-cell proliferation, as described before (Kovacsovics-Bankowski et al., 1993; Harding and Song, 1994). Although it has been widely accepted that antigen presentation occurs mainly in spleen and lymph nodes, macrophages may play an important role in antigen presentation in nonlymphoid tissues such as sites of infection (Porgador et al., 1997; Krogsgaard et al., 2000). Furthermore, macrophages and DCs may use different receptors to take up the same antigen (Syme et al., 2002; Woelbing et al., 2006). Our data show that phagocytosis through LR or LR and FcR or LR and CR increases efficient MHC-I presentation to naïve CD8+ T-cells. Receptors involved in apoptotic cell uptake, however, do not induce MHC-I presentation, and it is only when antigens are opsonized by immunoglobulins or iC3b or C1q that FcR and CR play a relevant role in facilitating antigen clearance (Abdul-Majid et al., 2002; Dhodapkar et al., 2002; Mold et al., 2002; Verbovetski et al., 2002; Akiyama et al., 2003; Ogden et al., 2005; Skoberne et al., 2006). Likewise, antigens from tumor cells induce a protective response when they are targeted by antibodies (Falo et al., 1995; Hsu and Komarovskaya, 2002; Tobar et al., 2004; Schmidt et al., 2006).
Our data indicate that LR, FcR, and CR phagocytic macrophages form productive ISs very efficiently and have an important effect on IL-2 production and proliferation of CD8+ T-cells. The ISs formed by the three types of phagocytic macrophages, in comparison with macrophages loaded with high doses of peptide, are specifically efficient in the induction of the active conformation of CD3ε, as well as in the phosphorylation of LAT Y132. Several mechanisms can be proposed to explain the different T-cell responses obtained after stimulation with low (3–30 pM) or medium-high (100 pM–1 μM) doses of soluble peptide. On one hand, phagocytic macrophages and macrophages exposed to low peptide doses may present similar amounts of antigen on MHC-I, because both types of macrophages induce T-cell CD69 and CD25 expression and proliferation to similar extents. Therefore, the antigen density on the macrophage surface may be a regulatory check point, as has been proposed before for CTL (Alexander-Miller et al., 1998; Oved et al., 2007). It is conceivable that phagocytosis controls the amount of peptide expressed on the surface of APC to ensure activation, and that in the absence of phagocytosis similar ranges of uptaken antigen may lead to a tolerogenic T-cell response. In this regard, we show that when medium-high doses of peptide are added to macrophages, the T-cell response varies: CD69 and CD25 expression remain high, but cells are unable to proliferate. In contrast, other APCs such as DCs elicit OT-I CD8+ T-cell proliferation when loaded with medium-high doses of OVAp (Machy et al., 2002; Galea-Lauri et al., 2004; Taraban et al., 2004). Another mechanism that may account for the effect of phagocytosis on the efficiency of T-cell antigen presentation by macrophages could be an up-regulation of costimulatory or adhesion molecules; however, we could not detect any up-regulation of CD40, CD80, or CD86 or of several adhesion molecules at early (1–4 h) or late times (24 and 48 h) after phagocytosis (data not shown and Table 1 of Supplementary Material). However, because basal levels of costimulatory molecules are already high in unprimed peritoneal macrophages, it is possible that a balance may be required between TCR signals and costimulatory signals. This balance may be obtained by phagocytic macrophages and macrophages loaded with low (3–30 pM) doses of peptide. In contrast, at higher peptide doses, the TCR signal may be functionally unbalanced and may therefore lead to an anergic response. This possibility is in agreement with the recently proposed asymmetric distribution of agonist peptide-MHC complexes on the plasma membrane, which could account for the different stimulatory potential of phagocytic and low (3–30 pM) peptide-loaded macrophages versus medium-high (100 pM–1 μM) peptide-loaded macrophages (Schamel et al., 2006). Alternatively, the secretion of inflammatory cytokines by phagocytic macrophages may be another mechanism underlying their ability to form productive ISs, because phagocytosis is known to induce TNF-α and IL-6 secretion, which enhances macrophage activation and provides a higher capacity for antigen presentation (Debets et al., 1988; Hirsch et al., 1994; Refici et al., 2001; Trinidad et al., 2006).
The defective T-cell response induced by macrophages loaded with high doses (1 μM) of soluble peptide provides a control for investigating the mechanism that underlies the choice between the formation of productive ISs or tolerance. The induction in this condition of CD25 and CD69 expression without concomitant full lymphocyte activation (IL-2 production, cell proliferation, etc.) may suggest the generation of regulatory T-cells (Nordstrom et al., 2005; Jarnicki et al., 2006). However, we failed to detect foxp3 expression, a marker of regulatory T-cells, on these CD25+ T-cells (Olazabal, I. M., and Sanchez-Madrid, F., unpublished data). Therefore, it is more likely that high-dose peptide-loaded macrophages induce a partial activation of T-cells that results in the generation of anergic lymphocytes, which are unable to synthesize IL-2 and proliferate, despite their ability to efficiently induce IFNγ production and MTOC translocation (DeSilva et al., 1991). The absence, or less efficient induction, of some early signaling events such as TCR/CD3ε conformational change, LAT Y132 phosphorylation, and of late events such as IL-2 secretion, in comparison with phagocytic macrophages, may suggest the induction of a pathway leading to anergy. Defects in IL-2 production and in LAT localization or phosphorylation have been previously associated with anergy (Schwartz, 1992, 2003). Hence, in the case of ionomycin-induced T-cell anergy, TCR/CD3ζ ITAM phosphorylation is increased as well as recruitment and phosphorylation of ZAP70, matching our observations with high-dose peptide-loaded macrophages. However, defects in palmitoylation and phosphorylation of LAT make ZAP70 unable to continue the signal and result in defects in Ca2+ influx, IL-2 secretion, and cell proliferation (Hundt et al., 2006). This mechanism may also explain our data showing reduced LAT phosphorylation after stimulation with high-dose peptide-loaded macrophages. Similarly, partial activation signals are induced by antagonistic peptides, where the MTOC is efficiently translocated but IL-2 production is defective (Martin-Cofreces et al., 2006). Additionally, the mechanism of anergy induction involves a defective costimulatory signal in T-cells (June et al., 1987; Thompson et al., 1989; Buhtoiarov et al., 2005). As stated above, it is possible that dysregulated costimulatory and TCR signals may lead to T-cell anergy. The induction of T-cell anergy is an important regulatory response in the control of inflammation. Because soluble antigenic peptides may be encountered at sites of tissue damage or inflammation, one possibility is that presentation of these peptides by macrophages may be a mechanism to limit further damage (Pulle et al., 2006). In summary, our work demonstrates that primary macrophages are able to form productive ISs with naïve T-cells through receptor-mediated phagocytosis, inducing differential T-cell signaling events.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. R. Gonzalez-Amaro, I. Gonzalez-Alvaro, M. Gomez, and A. Urzainqui for critical reading of the manuscript. This work was supported by the BFU 2005-08435/BMC and “Ayuda a la Investigación Básica Juan March 2002” (F.S.-M). I.O. is an investigator from the “Ministerio de Ciencia y Tecnología Programa Ramón y Cajal.”
Abbreviations used:
- LR
C-type lectin receptor
- CR
complement receptor
- DC
dendritic cell
- IS
immune synapse
- MTOC
microtubule-organizing center.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E07-07-0650) on December 12, 2007.
REFERENCES
- Abdul-Majid K. B., Stefferl A., Bourquin C., Lassmann H., Linington C., Olsson T., Kleinau S., Harris R. A. Fc receptors are critical for autoimmune inflammatory damage to the central nervous system in experimental autoimmune encephalomyelitis. Scand. J. Immunol. 2002;55:70–81. doi: 10.1046/j.1365-3083.2002.01024.x. [DOI] [PubMed] [Google Scholar]
- Akiyama K., Ebihara S., Yada A., Matsumura K., Aiba S., Nukiwa T., Takai T. Targeting apoptotic tumor cells to Fc gamma R provides efficient and versatile vaccination against tumors by dendritic cells. J. Immunol. 2003;170:1641–1648. doi: 10.4049/jimmunol.170.4.1641. [DOI] [PubMed] [Google Scholar]
- Alexander-Miller M. A., Derby M. A., Sarin A., Henkart P. A., Berzofsky J. A. Supraoptimal peptide-major histocompatibility complex causes a decrease in bc1–2 levels and allows tumor necrosis factor alpha receptor II-mediated apoptosis of cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1391–1399. doi: 10.1084/jem.188.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman A., Isakov N., Baier G. Protein kinase Ctheta: a new essential superstar on the T-cell stage. Immunol. Today. 2000;21:567–573. doi: 10.1016/s0167-5699(00)01749-7. [DOI] [PubMed] [Google Scholar]
- Apostolopoulos V., Barnes N., Pietersz G. A., McKenzie I. F. Ex vivo targeting of the macrophage mannose receptor generates anti-tumor CTL responses. Vaccine. 2000;18:3174–3184. doi: 10.1016/s0264-410x(00)00090-6. [DOI] [PubMed] [Google Scholar]
- Bajtay Z., Csomor E., Sandor N., Erdei A. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol. Lett. 2006;104:46–52. doi: 10.1016/j.imlet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Von Stebut E., Mendez S., Lira R., Caler E., Bertholet S., Udey M. C., Sacks D. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 2002;168:3992–4000. doi: 10.4049/jimmunol.168.8.3992. [DOI] [PubMed] [Google Scholar]
- Bonifaz L., Bonnyay D., Mahnke K., Rivera M., Nussenzweig M. C., Steinman R. M. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhtoiarov I. N., Lum H., Berke G., Paulnock D. M., Sondel P. M., Rakhmilevich A. L. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J. Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- Bunnell S. C., Kapoor V., Trible R. P., Zhang W., Samelson L. E. Dynamic actin polymerization drives T cell receptor-induced spreading: a role for the signal transduction adaptor LAT. Immunity. 2001;14:315–329. doi: 10.1016/s1074-7613(01)00112-1. [DOI] [PubMed] [Google Scholar]
- Burgdorf S., Lukacs-Kornek V., Kurts C. The mannose receptor mediates uptake of soluble but not of cell-associated antigen for cross-presentation. J. Immunol. 2006;176:6770–6776. doi: 10.4049/jimmunol.176.11.6770. [DOI] [PubMed] [Google Scholar]
- Carroll M. C. The complement system in regulation of adaptive immunity. Nat. Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- Casey K. A., Mescher M. F. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J. Immunol. 2007;178:7640–7648. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- Debets J. M., Van der Linden C. J., Dieteren I. E., Leeuwenberg J. F., Buurman W. A. Fc-receptor cross-linking induces rapid secretion of tumor necrosis factor (cachectin) by human peripheral blood monocytes. J. Immunol. 1988;141:1197–1201. [PubMed] [Google Scholar]
- DeSilva D. R., Urdahl K. B., Jenkins M. K. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J. Immunol. 1991;147:3261–3267. [PubMed] [Google Scholar]
- Desjardins M. ER-mediated phagocytosis: a new membrane for new functions. Nat. Rev. Immunol. 2003;3:280–291. doi: 10.1038/nri1053. [DOI] [PubMed] [Google Scholar]
- Dhodapkar K. M., Krasovsky J., Williamson B., Dhodapkar M. V. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 2002;195:125–133. doi: 10.1084/jem.20011097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Cooper J. A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Falo L. D., Jr, Kovacsovics-Bankowski M., Thompson K., Rock K. L. Targeting antigen into the phagocytic pathway in vivo induces protective tumour immunity. Nat. Med. 1995;1:649–653. doi: 10.1038/nm0795-649. [DOI] [PubMed] [Google Scholar]
- Gagnon E., Duclos S., Rondeau C., Chevet E., Cameron P. H., Steele-Mortimer O., Paiement J., Bergeron J. J., Desjardins M. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- Galea-Lauri J., Wells J. W., Darling D., Harrison P., Farzaneh F. Strategies for antigen choice and priming of dendritic cells influence the polarization and efficacy of antitumor T-cell responses in dendritic cell-based cancer vaccination. Cancer Immunol. Immunother. 2004;53:963–977. doi: 10.1007/s00262-004-0542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Bromley S. K., Sumen C., Davis M. M., Shaw A. S., Allen P. M., Dustin M. L. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Guermonprez P., Saveanu L., Kleijmeer M., Davoust J., Van Endert P., Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- Hamilton-Easton A., Eichelberger M. Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice. J. Virol. 1995;69:6359–6366. doi: 10.1128/jvi.69.10.6359-6366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J. Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- Helmy K. Y., Katschke K. J., Jr, Gorgani N. N., Kljavin N. M., Elliott J. M., Diehl L., Scales S. J., Ghilardi N., van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Hirsch C. S., Ellner J. J., Russell D. G., Rich E. A. Complement receptor-mediated uptake and tumor necrosis factor-alpha-mediated growth inhibition of Mycobacterium tuberculosis by human alveolar macrophages. J. Immunol. 1994;152:743–753. [PubMed] [Google Scholar]
- Hogquist K. A., Jameson S. C., Heath W. R., Howard J. L., Bevan M. J., Carbone F. R. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Houde M., Bertholet S., Gagnon E., Brunet S., Goyette G., Laplante A., Princiotta M. F., Thibault P., Sacks D., Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- Hsu F. J., Komarovskaya M. CTLA4 blockade maximizes antitumor T-cell activation by dendritic cells presenting idiotype protein or opsonized anti-CD20 antibody-coated lymphoma cells. J. Immunother. 2002;25:455–468. doi: 10.1097/00002371-200211000-00002. [DOI] [PubMed] [Google Scholar]
- Hundt M., Tabata H., Jeon M. S., Hayashi K., Tanaka Y., Krishna R., De Giorgio L., Liu Y. C., Fukata M., Altman A. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–522. doi: 10.1016/j.immuni.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Irving B. A., Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64:891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- Jarnicki A. G., Lysaght J., Todryk S., Mills K. H. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J. Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Gillespie M. M., Lindsten T., Thompson C. B. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol. Cell. Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindberg G. M., Magnusson S., Berg T., Smedsrod B. Receptor-mediated endocytosis of ovalbumin by two carbohydrate-specific receptors in rat liver cells. The intracellular transport of ovalbumin to lysosomes is faster in liver endothelial cells than in parenchymal cells. Biochem. J. 1990;270:197–203. doi: 10.1042/bj2700197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Clark K., Benacerraf B., Rock K. L. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc. Natl. Acad. Sci. USA. 1993;90:4942–4946. doi: 10.1073/pnas.90.11.4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics-Bankowski M., Rock K. L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- Krawczyk C., Oliveira-dos-Santos A., Sasaki T., Griffiths E., Ohashi P. S., Snapper S., Alt F., Penninger J. M. Vav1 controls integrin clustering and MHC/peptide-specific cell adhesion to antigen-presenting cells. Immunity. 2002;16:331–343. doi: 10.1016/s1074-7613(02)00291-1. [DOI] [PubMed] [Google Scholar]
- Krogsgaard M., Wucherpfennig K. W., Cannella B., Hansen B. E., Svejgaard A., Pyrdol J., Ditzel H., Raine C., Engberg J., Fugger L. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85–99 complex. J. Exp. Med. 2000;191:1395–1412. doi: 10.1084/jem.191.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer H., Monks C. R., Kupfer A. Small splenic B cells that bind to antigen-specific T helper (Th) cells and face the site of cytokine production in the Th cells selectively proliferate: immunofluorescence microscopic studies of Th-B antigen-presenting cell interactions. J. Exp. Med. 1994;179:1507–1515. doi: 10.1084/jem.179.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., O'Mahony A., Mu Y., Geleziunas R., Greene W. C. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Mol. Cell. Biol. 2000;20:2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Gao X., Masuda E., Redecha P. B., Blank M. C., Pricop L. Regulated expression of FcgammaR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J. Immunol. 2006;177:8440–8447. doi: 10.4049/jimmunol.177.12.8440. [DOI] [PubMed] [Google Scholar]
- Lopez-Lago M., Lee H., Cruz C., Movilla N., Bustelo X. R. Tyrosine phosphorylation mediates both activation and downmodulation of the biological activity of Vav. Mol. Cell. Biol. 2000;20:1678–1691. doi: 10.1128/mcb.20.5.1678-1691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machy P., Serre K., Baillet M., Leserman L. Induction of MHC class I presentation of exogenous antigen by dendritic cells is controlled by CD4+ T cells engaging class II molecules in cholesterol-rich domains. J. Immunol. 2002;168:1172–1180. doi: 10.4049/jimmunol.168.3.1172. [DOI] [PubMed] [Google Scholar]
- Martin-Cofreces N. B., Sancho D., Fernandez E., Vicente-Manzanares M., Gordon-Alonso M., Montoya M. C., Michel F., Acuto O., Alarcon B., Sanchez-Madrid F. Role of Fyn in the rearrangement of tubulin cytoskeleton induced through TCR. J. Immunol. 2006;176:4201–4207. doi: 10.4049/jimmunol.176.7.4201. [DOI] [PubMed] [Google Scholar]
- May R. C., Caron E., Hall A., Machesky L. M. Involvement of the Arp2/3 complex in phagocytosis mediated by FcgammaR or CR3. Nat. Cell Biol. 2000;2:246–248. doi: 10.1038/35008673. [DOI] [PubMed] [Google Scholar]
- Mold C., Baca R., Du Clos T. W. Serum amyloid P component and C-reactive protein opsonize apoptotic cells for phagocytosis through Fcgamma receptors. J. Autoimmun. 2002;19:147–154. doi: 10.1006/jaut.2002.0615. [DOI] [PubMed] [Google Scholar]
- Monks C. R., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Nordstrom I., Nurkkala M., Collins L. V., Eriksson K. CD8+ T-cells suppress antigen-specific and allogeneic CD4+ T-cell responses to herpes simplex virus type 2-infected human dendritic cells. Viral Immunol. 2005;18:616–626. doi: 10.1089/vim.2005.18.616. [DOI] [PubMed] [Google Scholar]
- Ogden C. A., Kowalewski R., Peng Y., Montenegro V., Elkon K. B. IGM is required for efficient complement mediated phagocytosis of apoptotic cells in vivo. Autoimmunity. 2005;38:259–264. doi: 10.1080/08916930500124452. [DOI] [PubMed] [Google Scholar]
- Oh Y. K., Harding C. V., Swanson J. A. The efficiency of antigen delivery from macrophage phagosomes into cytoplasm for MHC class I-restricted antigen presentation. Vaccine. 1997;15:511–518. doi: 10.1016/s0264-410x(97)00221-1. [DOI] [PubMed] [Google Scholar]
- Olazabal I. M., Caron E., May R. C., Schilling K., Knecht D. A., Machesky L. M. Rho-kinase and myosin-II control phagocytic cup formation during CR, but not FcgammaR, phagocytosis. Curr. Biol. 2002;12:1413–1418. doi: 10.1016/s0960-9822(02)01069-2. [DOI] [PubMed] [Google Scholar]
- Oliveira S. C., Splitter G. A. CD8+ type 1 CD44hi CD45 RBlo T lymphocytes control intracellular Brucella abortus infection as demonstrated in major histocompatibility complex class I- and class II-deficient mice. Eur. J. Immunol. 1995;25:2551–2557. doi: 10.1002/eji.1830250922. [DOI] [PubMed] [Google Scholar]
- Oved K., et al. A novel postpriming regulatory check point of effector/memory T cells dictated through antigen density threshold-dependent anergy. J. Immunol. 2007;178:2307–2317. doi: 10.4049/jimmunol.178.4.2307. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Porgador A., Yewdell J. W., Deng Y., Bennink J. R., Germain R. N. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715–726. doi: 10.1016/s1074-7613(00)80447-1. [DOI] [PubMed] [Google Scholar]
- Pozzi L. A., Maciaszek J. W., Rock K. L. Both dendritic cells and macrophages can stimulate naive CD8 T cells in vivo to proliferate, develop effector function, and differentiate into memory cells. J. Immunol. 2005;175:2071–2081. doi: 10.4049/jimmunol.175.4.2071. [DOI] [PubMed] [Google Scholar]
- Pulle G., Vidric M., Watts T. H. IL-15-dependent induction of 4-1BB promotes antigen-independent CD8 memory T cell survival. J. Immunol. 2006;176:2739–2748. doi: 10.4049/jimmunol.176.5.2739. [DOI] [PubMed] [Google Scholar]
- Refici M. L., Metzger D. W., Arulanandam B. P., Lennartz M. R., Loegering D. J. Fcgamma-receptor signaling augments the LPS-stimulated increase in serum tumor necrosis factor-alpha levels. Am J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1037–R1044. doi: 10.1152/ajpregu.2001.280.4.R1037. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C., Germain R. N. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J. Exp. Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M., Citterio S., Thery C., Rittig M., Medaglini D., Pozzi G., Amigorena S., Ricciardi-Castagnoli P. Bacteria-induced neo-biosynthesis, stabilization, and surface expression of functional class I molecules in mouse dendritic cells. Proc. Natl. Acad. Sci. USA. 1998;95:5229–5234. doi: 10.1073/pnas.95.9.5229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risueno R. M., Gil D., Fernandez E., Sanchez-Madrid F., Alarcon B. Ligand-induced conformational change in the T-cell receptor associated with productive immune synapses. Blood. 2005;106:601–608. doi: 10.1182/blood-2004-12-4763. [DOI] [PubMed] [Google Scholar]
- Risueno R. M., van Santen H. M., Alarcon B. A conformational change senses the strength of T cell receptor-ligand interaction during thymic selection. Proc. Natl. Acad. Sci. USA. 2006;103:9625–9630. doi: 10.1073/pnas.0601785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuquillo A. G., Roumier A., Teixeiro E., Bragado R., Alarcon B. T cell receptor (TCR) engagement in apoptosis-defective, but interleukin 2 (IL-2)-producing, T cells results in impaired ZAP70/CD3-zeta association. J. Exp. Med. 1998;187:1179–1192. doi: 10.1084/jem.187.8.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schamel W. W., Risueno R. M., Minguet S., Ortiz A. R., Alarcon B. A conformation- and avidity-based proofreading mechanism for the TCR-CD3 complex. Trends Immunol. 2006;27:176–182. doi: 10.1016/j.it.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Schmidt J., Klempp C., Buchler M. W., Marten A. Release of iC3b from apoptotic tumor cells induces tolerance by binding to immature dendritic cells in vitro and in vivo. Cancer Immunol. Immunother. 2006;55:31–38. doi: 10.1007/s00262-005-0690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T cell anergy. Annu. Rev. Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- Skoberne M., Somersan S., Almodovar W., Truong T., Petrova K., Henson P. M., Bhardwaj N. The apoptotic-cell receptor CR3, but not alphavbeta5, is a regulator of human dendritic-cell immunostimulatory function. Blood. 2006;108:947–955. doi: 10.1182/blood-2005-12-4812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- Sun Z., et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- Syme R. M., Spurrell J. C., Amankwah E. K., Green F. H., Mody C. H. Primary dendritic cells phagocytose Cryptococcus neoformans via mannose receptors and Fcgamma receptor II for presentation to T lymphocytes. Infect. Immun. 2002;70:5972–5981. doi: 10.1128/IAI.70.11.5972-5981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahara K., Yashima Y., Omatsu Y., Yoshida H., Kimura Y., Kang Y. S., Steinman R. M., Park C. G., Inaba K. Functional comparison of the mouse DC-SIGN, SIGNR1, SIGNR3 and Langerin, C-type lectins. Int. Immunol. 2004;16:819–829. doi: 10.1093/intimm/dxh084. [DOI] [PubMed] [Google Scholar]
- Taraban V. Y., Rowley T. F., Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J. Immunol. 2004;173:6542–6546. doi: 10.4049/jimmunol.173.11.6542. [DOI] [PubMed] [Google Scholar]
- Thompson C. B., Lindsten T., Ledbetter J. A., Kunkel S. L., Young H. A., Emerson S. G., Leiden J. M., June C. H. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl. Acad. Sci. USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobar J. A., Gonzalez P. A., Kalergis A. M. Salmonella escape from antigen presentation can be overcome by targeting bacteria to Fc gamma receptors on dendritic cells. J. Immunol. 2004;173:4058–4065. doi: 10.4049/jimmunol.173.6.4058. [DOI] [PubMed] [Google Scholar]
- Trinidad A. G., de la Puerta M. L., Fernandez N., Bayon Y., Crespo M. S., Alonso A. Coupling of C3bi to IgG inhibits the tyrosine phosphorylation signaling cascade downstream Syk and reduces cytokine induction in monocytes. J. Leukoc. Biol. 2006;79:1073–1082. doi: 10.1189/jlb.1205701. [DOI] [PubMed] [Google Scholar]
- Turner J., Dockrell H. M. Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro. Immunology. 1996;87:339–342. doi: 10.1046/j.1365-2567.1996.512590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood E. J., Hogg T. L., Woodland D. L. Enumeration of antigen-presenting cells in mice infected with Sendai virus. J. Immunol. 1999;162:3350–3355. [PubMed] [Google Scholar]
- van Oers N. S., Killeen N., Weiss A. Lck regulates the tyrosine phosphorylation of the T cell receptor subunits and ZAP-70 in murine thymocytes. J. Exp. Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbovetski I., Bychkov H., Trahtemberg U., Shapira I., Hareuveni M., Ben-Tal O., Kutikov I., Gill O., Mevorach D. Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down-regulates DR and CD86, and up-regulates CC chemokine receptor 7. J. Exp. Med. 2002;196:1553–1561. doi: 10.1084/jem.20020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelbing F., et al. Uptake of Leishmania major by dendritic cells is mediated by Fcgamma receptors and facilitates acquisition of protective immunity. J. Exp. Med. 2006;203:177–188. doi: 10.1084/jem.20052288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada A., Ebihara S., Matsumura K., Endo S., Maeda T., Nakamura A., Akiyama K., Aiba S., Takai T. Accelerated antigen presentation and elicitation of humoral response in vivo by FcgammaRIIB- and FcgammaRI/III-mediated immune complex uptake. Cell Immunol. 2003;225:21–32. doi: 10.1016/j.cellimm.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Zhang W., Sloan-Lancaster J., Kitchen J., Trible R. P., Samelson L. E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.