Abstract
Colicin Ia, a 626-residue bactericidal protein, consists of three domains, with the carboxy-terminal domain (C domain) responsible for channel formation. Whole colicin Ia or C domain added to a planar lipid bilayer membrane forms voltage-gated channels. We have shown previously that the channel formed by whole colicin Ia has four membrane-spanning segments and an ∼68-residue segment translocated across the membrane. Various experimental interventions could cause a longer or shorter segment within the C domain to be translocated, making us wonder why translocation normally stops where it does, near the amino-terminal end of the C domain (approximately residue 450). We hypothesized that regions upstream from the C domain prevent its amino-terminal end from moving into and across the membrane. To test this idea, we prepared C domain with a ligand attached near its amino terminus, added it to one side of a planar bilayer to form channels, and then probed from the opposite side with a water-soluble protein that can specifically bind the ligand. The binding of the probe had a dramatic effect on channel gating, demonstrating that the ligand (and hence the amino-terminal end of the C domain) had moved across the membrane. Experiments with larger colicin Ia fragments showed that a region of more than 165 residues, upstream from the C domain, can also move across the membrane. All of the colicin Ia carboxy-terminal fragments that we examined form channels that pass from a state of relatively normal conductance to a low-conductance state; we interpret this passage as a transition from a channel with four membrane-spanning segments to one with only three.
Keywords: voltage-gated channels, streptavidin, His-tag antibody, trypsin, single-channel conductance
INTRODUCTION
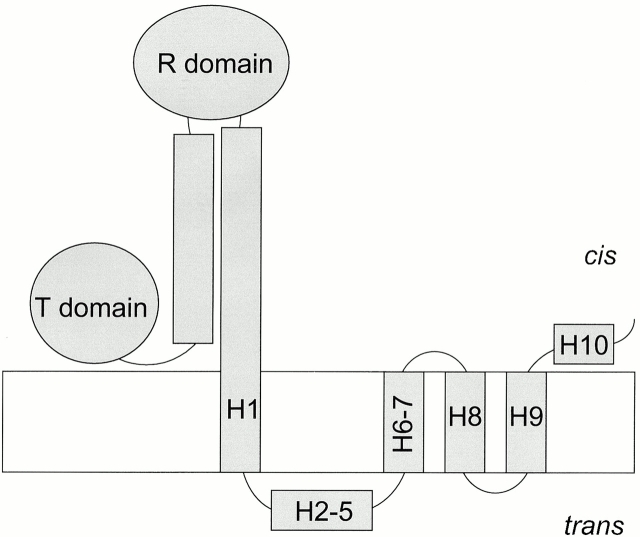
Colicin Ia belongs to a family of water-soluble bactericidal proteins that consist of three domains: the central R domain and the amino-terminal T domain are responsible for receptor-binding and translocation of the colicin across the outer membrane of the target cell, respectively, and the carboxy-terminal C domain forms a channel in the inner membrane to kill the cell (for general review, see Cramer et al. 1995). The crystal structure of the water-soluble form of colicin Ia reveals that the three domains are separated by two long α-helices in a coiled-coil, making a “Y”-shaped molecule (Wiener et al. 1997). Aside from the hydrophobic hairpin formed by helices 8 and 9 of the C domain, the rest of the molecule is highly charged (>30% of the residues) (Mankovich et al. 1986). Whole colicin or isolated C domain can also form channels in planar lipid bilayer membranes (Nogueira and Varanda 1988; Ghosh et al. 1993). When whole colicin Ia associates with a planar bilayer, it undergoes a series of conformational changes: after the hydrophobic hairpin inserts into the membrane (Kienker et al. 1997), the conducting channel is formed by the voltage-dependent insertion of two additional segments, with portions of helix 1 and helices 6–7 spanning the membrane, and helices 2–5 translocated completely across the membrane (Qiu et al. 1996) (Fig. 1). The T and R domains presumably remain on the cis side (the side to which the colicin was added).
Figure 1.
Schematic diagram of the whole colicin Ia molecule in the open channel state in a planar bilayer. Helices 1–10 of the carboxy-terminal C domain are labeled to show their topology in the membrane, with four membrane-spanning segments (helices 8 and 9 and portions of helices 1 and 6–7) and a translocated segment (helices 2–5). The C domain begins at about residue 451, in helix 1, which is located near the cis interface. The amino-terminal T domain and the central R domain, as well as the two long inter-domain helices, are presumed to reside on the cis side of the membrane.
We have shown that colicin Ia can still form channels (albeit somewhat aberrantly) when residues, located in helices 1–5, that normally move into or across the membrane are forced to stay on the cis side (Qiu et al. 1996). In addition, foreign sequences inserted between helices 3 and 4 move across the membrane, along with the normally translocated segment (Jakes et al. 1998). Apparently, the precise identities of the translocated segment and of the “upstream” membrane-spanning segment are not critically important. This led us to wonder why translocation normally stops where it does; that is, why doesn't all of helix 1 (residues 359–467) move across the membrane? We suspected that the T and R domains somehow serve to anchor the amino-terminal end of the C domain on the cis side. Thus, we have examined the isolated C domain (as well as longer carboxy-terminal fragments of colicin Ia) to determine whether or not its amino terminus can move across the membrane.
MATERIALS AND METHODS
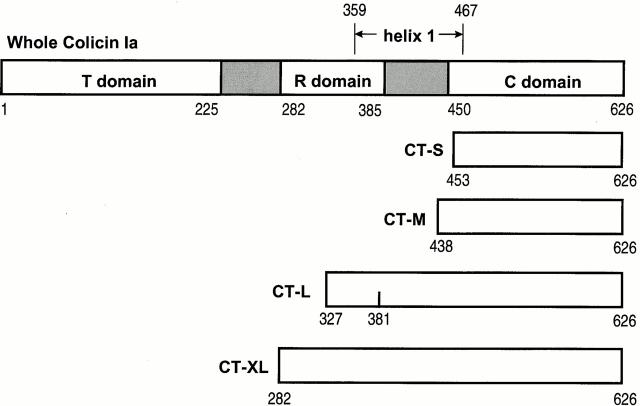
Preparation of Colicin Ia Carboxy-terminal Fragments
Whole colicin Ia was prepared as previously described (Qiu et al. 1994). We prepared carboxy-terminal fragments of colicin Ia in four different lengths, designated CT short (CT-S; residues 453–626), CT medium (CT-M; 438–626), CT long (CT-L; 327–626), and CT extra long (CT-XL; 282–626) (Fig. 2), as follows. The shortest fragment, CT-S, was cloned by using site-directed mutagenesis to create a BamHI site overlapping the codons for amino acids 450–452 in the colicin Ia gene cloned in pUC19 (Jakes et al., 1998). The resulting plasmid was digested with BamHI and the fragment containing the 3′ end of the colicin Ia gene, beginning with the codon for Ile 453 and ending with residue Ile 626, as well as the Ia immunity gene, was ligated at the BamHI site of the expression vector pET-15b (Novagen, Inc.) to create pKSJ120. The sequence at the amino terminus of the resulting protein is thus: MGSSHHHHHHSSGLVPRGSHMLEDP I453N454… Residues shown in italics are from the pET vector His-tag and cloning sequences; the beginning of the colicin sequence is shown in bold.
Figure 2.
Linear diagram showing the approximate lengths of whole colicin Ia and the four carboxy-terminal fragments, CT-S, CT-M, CT-L, and CT-XL. The boundaries of the T, R, and C domains are from Wiener et al. 1997. The inter-domain regions are shaded and the full length of helix 1 is indicated. Mutant fragments have a cysteine residue near the amino terminus (453C/CT-S, 439C/CT-M, 326C/CT-L, and “−3” C/CT-XL), or not so near it (381C/CT-L). The amino-terminal His-tags are not shown.
CT-S protein was expressed from pKSJ120-containing Escherichia coli BL21(DE3). Cells were grown to OD660 ∼ 1 at 37°C in Luria broth containing ampicillin at 100 μg/ml and stored overnight at 4°C. The cells were pelleted and resuspended in fresh medium and used to inoculate 100 ml of fresh Luria broth containing ampicillin. The culture was grown at 37°C to OD660 = 0.6, and then induced with 1 mM isopropyl-β-d-thiogalactoside (Labscientific, Inc.). Cells were harvested after 2 h of induction at 37°C. The soluble CT-S protein (with the amino-terminal His-tag) was purified on His-Bind metal chelation resin (Novagen, Inc.) as specified in the Novagen pET System Manual, except that the column was washed with 40 mM imidazole buffer. Purified protein eluted from the His-Bind resin in 1 M imidazole buffer was dialyzed extensively against 50 mM sodium borate, 300 mM NaCl, 2 mM EDTA, pH 9.0. Yields of pure soluble protein were generally 3–10 mg from 100 ml of culture. A comparable amount of protein was unrecoverable from inclusion bodies. Purified CT-S was aliquoted and stored at −80°C. When stored at 4°C over a period of weeks, the protein gradually lost its His-tag. This is probably due to the protease-sensitive arginine residue in the His-tag sequence.
Mutagenesis of CT-S to create I453C/CT-S was performed as described previously (Jakes et al. 1998). The mutant protein was purified as described above and biotinylated with N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide (Pierce Chemical Co.) as described in Qiu et al. 1996, except that dithiothreitol (DTT) (Boehringer) from the original reduction step was removed by dialysis, instead of on a G-25 column. Any unbiotinylated CT-S was separated from the biotinylated protein on an Ultralink monomeric avidin column (Pierce Chemical Co.), as described previously (Qiu et al. 1996).
A slightly longer carboxy-terminal fragment, CT-M, was made, containing 12 more residues than CT-S, with eight of them charged. For this construct, an XhoI site overlapping residues 437 and 438 of colicin Ia was introduced by site-directed mutagenesis. The XhoI–BamHI fragment containing the colicin Ia C domain and immunity protein genes was ligated into pET-15b, which had been digested with XhoI and BamHI, and then treated with calf intestine alkaline phosphatase. Transformants were screened and sequenced. The resulting protein has the same amino-terminal His-tag described above, and the sequence near the beginning of the colicin moiety is …GSHML E438E439KRKQDELKATKDA…, with residues from pET-15b in italics and those from colicin Ia in bold. The protein was expressed, purified, and stored as described above for CT-S. The yield was ∼4 mg from 100 ml of culture. Glutamic acid 439 was mutated to cysteine and the protein E439C/CT-M was purified and biotinylated as described above.
A much longer carboxy-terminal fragment of colicin Ia, CT-L, includes all of C domain helix 1, as well as R domain helices 2 and 3 (Wiener et al. 1997); it was cloned and expressed as follows. Plasmid pKSJ101, containing the wild-type colicin Ia and immunity protein genes cloned between the EcoRI and BamHI sites in pUC19 (Jakes et al., 1998), was digested with ClaI, which cuts colicin Ia at residue 327, in the R domain. Overhanging ends were filled using Klenow fragment of E. coli DNA polymerase I (GIBCO BRL), and BamHI linkers (New England Biolabs, Inc.) were ligated to the ClaI-digested DNA. After subsequent digestion with BamHI, the carboxy-terminal colicin fragment was ligated to phosphatased BamHI-digested pET-15b. Resulting transformants were screened with BamHI for the presence of the insert and then with SphI to ascertain the orientation of the insert. Clones with an insert in the correct orientation were sequenced to confirm the nature of the construct. The protein encoded by this plasmid (pKSJ149) was expressed, purified, and stored as described above for CT-S. The yield of this protein was ∼4 mg/100 ml of culture. The resulting protein has the same amino-terminal His-tag as described above, and the sequence near the beginning of the colicin moiety is …GSHMLEDRD327N328…, with residues in italics from pET-15b, bolded residues from colicin, and the arginine from the added linker sequence. That arginine was mutated to cysteine to give the protein R326C/CT-L (from plasmid pKSJ150), which was biotinylated as described above. Another mutant, L381C/CT-L, was prepared in the same way.
The biotinylated cysteine mutants of CT-L (either R326C or L381C) could not be separated from remaining unbiotinylated protein on monomeric avidin columns. For unknown reasons, both biotinylated and unbiotinylated CT-L stuck to the column and could only be removed with 8 M guanidine HCl. Biotinylated CT-L bound to streptavidin was therefore separated from unbiotinylated CT-L in a different way. Biotinylated, thrombin-cleaved (see below) L381C/CT-L (184 μg) was incubated with 1.8 mg streptavidin, which represents about a fivefold molar excess of streptavidin, in a total volume of 0.33 ml. The mixture was applied to a Superdex G-75 preparative column (Amersham Pharmacia Biotech) and eluted in 150 mM NaCl–20 mM Tris-Cl, pH 8.0. Sodium dodecyl sulfate gel electrophoresis confirmed that the void volume fractions from the column contained only high molecular weight complexes of biotinylated CT-L bound to streptavidin (≥90 kD), while unbound excess streptavidin (60 kD) and unbiotinylated CT-L (∼35 kD) came off the column in later included fractions. Material from the void volume was used for experiments that required streptavidin-bound CT-L.
CT-XL, which consists of the entire R and C domains of colicin Ia with the α-helix connecting them, was constructed and purified as follows. Site-directed mutagenesis was employed to introduce a unique XhoI site overlapping codons 280 and 281 of the colicin Ia sequence in pKSJ101. The 1,579-bp XhoI–BamHI fragment from the resulting plasmid, pKSJ160, containing the colicin R and C domains and the Ia immunity protein, was ligated into pET-15b, which had also been digested with XhoI and BamHI and treated with calf intestine alkaline phosphatase (Promega). Resulting transformants were screened for the presence of the colicin insert and sequenced. Protein from this new plasmid, pKSJ161, was expressed and purified as described above for all of the other His-tagged proteins. The protein has the same His-tag sequence as all of the other carboxy-terminal constructs; the sequence near the beginning of the colicin moiety is …GSHMLE K282N283T284…, with residues from pET-15b in italics and those from colicin Ia in bold. The yield from 100 ml of culture was ∼600 μg. The methionine in the pET-15b sequence linking the His-tag to the colicin sequence, at the −3 position before colicin residue K282, was mutated to cysteine to give plasmid pKSJ162. The protein “−3” C/CT-XL was expressed, purified, and stored as above; the yield was ∼3 mg from 200 ml culture. Biotin was attached to the cysteine with 3-(N-maleimidylpropionyl) biocytin (Molecular Probes, Inc.) as described by Qiu et al. 1996, except that DTT was removed from the initial reduction step by dialysis.
The amino-terminal His-tag could be completely removed from any of the above carboxy-terminal fragments by digesting the purified fragment with thrombin for 5 h at room temperature in 50 mM Na borate, 300 mM NaCl, 2 mM EDTA, pH 9.0, at a concentration of 1 ng thrombin per microgram fragment. Thrombin cuts after the unique arginine residue in the thrombin recognition sequence LVPRGS.
Planar Bilayer Experiments
Membrane formation was performed as previously described (Qiu et al. 1996). In brief, 20 μl asolectin (1% in pentane) was layered on top of the aqueous solutions in two compartments separated by a Teflon partition. The partition contained a 100–150-μm hole, which was pretreated on each side with ≈4 μl squalene (3% in petroleum ether). For experiments at pH 4.5 or 5.0, a comparable volume of 10 or 15% squalene was used instead, for greater membrane stability. After the solvents evaporated, the lipid layers were raised and lowered as required to form a membrane (Montal 1974).
For most macroscopic experiments, the aqueous solutions contained 100 mM KCl, 5 mM CaCl2, 1 mM EDTA, and an appropriate buffer: 20 mM HEPES, pH 8.0 or 7.2; 20 mM MES, pH 6.2; 100 mM MES, pH 5.0; or 20 mM fumaric acid, pH 4.5. The solutions for single-channel experiments were the same except that 1 M KCl was generally used. In addition, for experiments with unbiotinylated colicin mutants containing a cysteine residue, a reducing agent [5 mM DTT or 10 mM tris(2-carboxyethyl)-phosphine; Pierce Chemical Co.] was generally added. The volume of solution in each compartment was ≈1 ml.
Voltage-clamp recording was done as previously described (Jakes et al. 1990; Qiu et al. 1996), with an OPA102 operational amplifier (Burr Brown Corp.) configured as a current–voltage converter, the membrane voltage supplied by a homemade pulser, and the voltage and current filtered and recorded on a Physiograph chart recorder (Narco Biosystems, Inc.). The voltage is that of the cis compartment, defined as the side to which colicin was added, with respect to that of the opposite trans compartment. In later experiments, the pulser was replaced with a PCI-1200 I/O board (National Instruments) controlled by the Acquire program (X4.0.2; Bruxton Corp.) in a Pentium III computer (Micron Electronics, Inc.); the voltage from the D/A converter was passed through a voltage divider before going to the membrane. The output of the amplifier was boosted 10-fold with a noninverting amplifier and further boosted and low-pass filtered with a variable frequency filter (AP-255-5; A. P. Circuit Corp.) before going to the PCI-1200 for digitization (typically with a 6-ms sampling interval) and recording onto computer disk. The programs Review (X3.0.1; Bruxton Corp.) and Igor Pro (π; WaveMetrics, Inc.) were used for subsequent data analysis. The digitized results were confirmed by the Physiograph.
Colicin was sometimes mixed with a 1% solution of octyl glucoside to increase its channel-forming activity (Bullock and Cohen 1986); however, this was generally not necessary. Octyl glucoside and streptavidin were from Calbiochem-Novabiochem, the antibody to the His-tag was Penta-His mouse mAb (QIAGEN), anti–β-galactosidase mouse mAb was from Promega, and TPCK (l-1-tosylamide-2-phenylethyl chloromethyl ketone)-treated trypsin and soybean trypsin inhibitor were from Worthington Biochemical Corp.
RESULTS
The Amino-terminal End of the C Domain Moves Across the Membrane to the Trans Side
We prepared the colicin Ia channel-forming domain with biotin attached near its amino terminus. If the biotin moves across the membrane from the cis side to the opposite trans side when the channel opens, then it should serve as a target for binding by streptavidin in the trans solution. Streptavidin binding might be detected through some effect on the channels; for instance, it might be expected to interfere with channel closing by hindering the movement of the amino terminus back to the cis side.
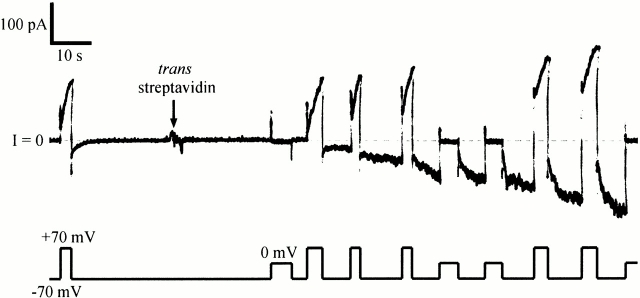
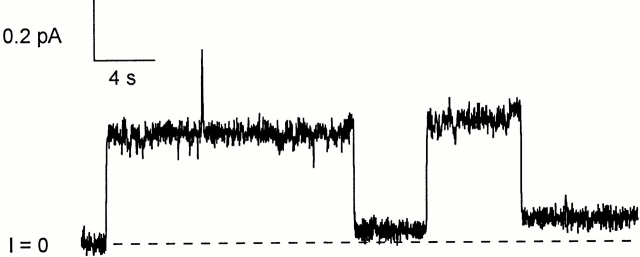
Fig. 3 shows a typical experiment. Biotinylated C domain was added to the cis solution and the normal pattern of gating was established. C domain channel gating is similar to that of whole colicin Ia channels, with turn-on at positive voltage and turn-off at negative voltage; however, turn-off is slower for C domain channels than for whole colicin Ia channels. After streptavidin was added to the trans solution, a new sort of conductance quickly appeared that turned on at negative voltage and off at zero or positive voltage–the reverse of the normal voltage dependence. This was not the anticipated effect of trans streptavidin, but it nevertheless shows that the amino-terminal biotin crossed the membrane to the trans side. The effect was the same for either C-domain fragment, CT-S or CT-M; it was also the same with or without the amino-terminal His-tag. The effect of trans streptavidin on biotinylated CT-S and CT-M was prevented by the earlier addition of excess biotin to the trans solution. Streptavidin had no effect on unbiotinylated C-domain fragments.
Figure 3.
The effect of trans streptavidin on C domain with biotin attached near its amino terminus. Before the start of the record, 20 ng of biotinylated mutant 453C/CT-S without a His-tag (plus 1 μg of octyl glucoside) were added to the cis compartment. Normal gating was seen, with the conductance turning on at +70 and off at −70 mV. At the arrow, 20 μg of streptavidin was added to the trans compartment. A new conductance rapidly developed that turned on at −70 and off at 0 or +70 mV—the reverse of the normal voltage dependence. (The turn off of the “reversed” conductance during a pulse to 0 or +70 mV was evident from the decreased conductance at −70 mV just after the pulse, relative to that just before the pulse.) After streptavidin addition, there were two populations of channel in the membrane: the original population of normal-gating channels that had not yet bound trans streptavidin, and a new population of reverse-gating channels that had. This shows that the amino-terminal biotin was accessible to trans streptavidin. The solution on both sides of the membrane was 100 mM KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM MES, pH 6.2. The record was filtered at 30 Hz.
Similar results were obtained for C domain with an amino-terminal His-tag when an antibody to the His-tag was added to the trans solution (data not shown). The effect of antibody developed more slowly than that of streptavidin, probably due to the faster and tighter binding of streptavidin to biotin, compared with antibody binding to His-tag. A control antibody (anti–β-galactosidase) had no effect.
A Short Sequence of Charged Residues Does Not Stop Translocation
For whole colicin Ia, a portion of C domain helix 1 (residues 450–470, approximately) forms a membrane-spanning segment in the open channel (Qiu et al. 1996). For C domain channels, as shown above, this segment moves across the membrane to the trans side. This indicates that in whole colicin Ia, the T and R domains somehow act as an anchor to hold the amino-terminal end of the C domain on the cis side. We wondered whether a smaller structure (for instance, a short sequence of charged residues) could do the same job. Our results with the C-domain fragments CT-S and CT-M suggest that this is not the case. Despite the high density of charged residues added near the amino terminus of CT-M (8 of the 12 additional residues) relative to CT-S, its amino terminus still moves across the membrane to the trans side.
We also took advantage of the amino-terminal His-tag on the C domain to see if it could act as a small anchoring structure. At a sufficiently low pH, the six consecutive histidine residues in the His-tag should each carry a positive charge. In experiments at pH 4.5 or 5.0, C domain with an amino-terminal His-tag and a biotin near the amino terminus was added to the cis solution to form channels, and the effect of trans streptavidin was monitored. A reverse-gating conductance still appeared under these conditions (data not shown). Assuming an effective pK of 6.0 (Patchornik et al. 1957), the six histidine residues should carry a charge of +5 or +6 almost 99% of the time at pH 4.5. Thus, these results indicate that a sequence of five positively charged residues is not sufficient to anchor the amino terminus of the C domain on the cis side.
It is possible (although not likely; see materials and methods) that some of the C domain molecules could have lost their His-tag due to proteolysis. An alternative experiment without this concern was to use antibody to probe directly for the presence of the His-tag on the trans side. Since antibody does not appear to bind well to His-tag at pH 5.0, experiments were performed with pH 5.0 on the cis side and pH 6.2 on the trans side. Under these conditions, the addition of His-tag antibody to the trans side induced reversed turn on, although more slowly than with pH 6.2 on both sides (data not shown). At pH 5.0, the six-histidine sequence should carry a charge of +5 or +6 ∼90% of the time.
The Amino-terminal Region of a Longer Carboxy-terminal Fragment Is also Translocated
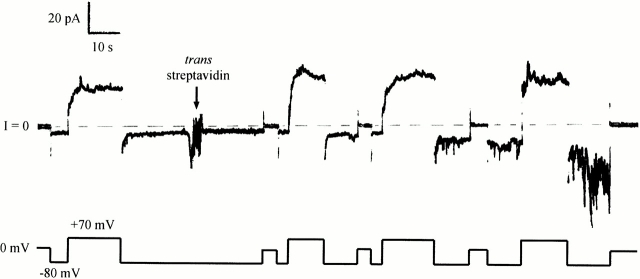
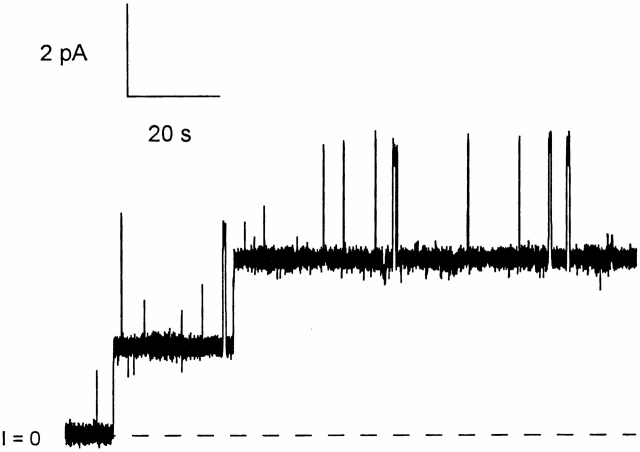
To determine whether a sequence upstream from the C domain acts as an anchor to stop translocation, we prepared a much longer carboxy-terminal fragment of colicin Ia (CT-L), including all of the long helix 1 extending from the C domain, plus part of the R domain, and attached biotin near its amino terminus at residue 326. This mutant also responded to trans streptavidin; the effect was an inhibition of turn off at negative voltage (more like the effect that we had originally anticipated for the shorter fragments), with the development of a noisy conductance (Fig. 4). Hence, the amino terminus of this long fragment moves across the membrane to the trans side. We obtained a similar result when the biotin was attached at residue 381, ∼80 residues from the amino terminus of this fragment (including 25 residues from the His-tag sequence) (data not shown), suggesting that not only the amino terminus, but probably all of the amino-terminal region, is translocated. The effect of trans streptavidin on both mutants was prevented by the earlier addition of excess biotin to the trans solution.
Figure 4.
The effect of trans streptavidin on a longer carboxy-terminal fragment with a biotin attached near the amino terminus. Before the start of the record, 240 ng of biotinylated mutant 326C/CT-L (with an amino-terminal His-tag) were added to the cis compartment. The conductance turned on at +70 mV and off at −80 mV. At the arrow, 20 μg of streptavidin were added to the trans compartment. This produced an inhibition of turn off at negative voltage, with the development of a noisy conductance. This shows that the amino-terminal biotin of this long fragment was accessible to trans streptavidin. The solution on both sides of the membrane was 100 mM KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM HEPES, pH 7.2. The record was filtered at 30 Hz.
Carboxy-terminal Fragments Form Smaller Channels than Those of Whole Colicin Ia
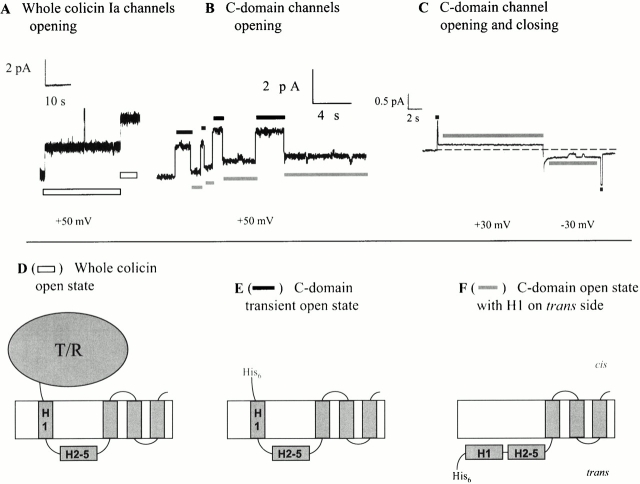
We next turn to a distinctive feature, observed in carboxy-terminal fragment single-channel currents, which may provide an independent assay for translocation of the amino terminus. This does not involve probing from the trans solution with streptavidin or antibody, but rather depends on the spontaneous behavior of the fragment channels. Based on our previous experiments (Qiu et al. 1996), whole colicin Ia forms channels with four membrane-spanning segments from the C domain: helices 8 and 9 and portions of helices 1 and 6–7 (Fig. 1). If the helix 1 portion of isolated C domain moves completely across the membrane to the trans side, then C domain channels should be left with only three membrane-spanning segments. One would expect these channels to have a conductance different from that of the channels formed by whole colicin Ia. We examined C domain single channels to see if this was the case.
The conductance of whole colicin Ia channels is 40–44 pS in 1 M KCl, pH 6.2 (Fig. 5 A). At a voltage of +50 mV or more, channels remain in this conductance state “indefinitely” (that is, the patience of the channel is greater than that of the experimenter), with occasional brief flickers to a fully closed state. C domain channels show a different behavior. These channels open initially with about the same conductance (33–44 pS) as that of whole colicin Ia channels, but then quickly pass to a state of lower conductance (5–10 pS) (Fig. 5 B). This low-conductance open state is stable: channels do not return to the normal-conductance state while the positive voltage is maintained. Our interpretation of these results is that we have resolved the transition of the C domain portion of helix 1 from a membrane-spanning conformation to a completely translocated state. According to this view, the transient higher-conductance state corresponds to a channel with four membrane-spanning segments, resembling the whole colicin Ia channel (Fig. 5 E), and the low-conductance state comes from a channel with only three membrane-spanning segments (F). Qualitatively similar results were obtained with all the carboxy-terminal fragments discussed so far (CT-S, CT-M, and CT-L) and did not depend on the presence of an amino-terminal His-tag or biotin. Sometimes, the channels passed back through the higher-conductance state before closing at negative voltage (Fig. 5 C). This suggests that en route to closing, the amino terminus of the C domain can reinsert into the membrane from the trans side, while the downstream membrane-spanning segments are still in place.
Figure 5.
Comparison of single channels of whole colicin Ia with its carboxy-terminal fragments. (A) Two whole colicin Ia channels opened with a relatively high conductance (42–44 pS) and stayed at this level. (B) Four C domain channels in succession opened with a conductance comparable with whole colicin channels (33–44 pS), but then dropped to a state of lower conductance (5–7 pS). (C) Example of one C domain channel that passed back through the higher-conductance state (here 33 pS) before closing at negative voltage. (D) Schematic model of whole colicin Ia in its open state, with four membrane-spanning segments. (E) Model of C domain in the transient, higher-conductance state, also with four membrane-spanning segments. (F) Model of C domain in the low-conductance open state, with only three membrane-spanning segments, and helix H1 translocated across the membrane. The segments labeled H1 in D–F represent the part of helix 1 that is within the C domain. The white bars in A indicate the open channel state of whole colicin Ia that is diagrammed in D. In B and C, the black bars indicate when the channel is in the transient open state of E, and the gray bars indicate when it is in the low-conductance open state of F. The amount of colicin added to the cis compartment was: (A) 3 ng whole colicin Ia, (B) 0.37 ng CT-M without a His-tag, and (C) 10 ng biotinylated mutant 453C/CT-S with an amino-terminal His-tag. The records were filtered at (A) 30 Hz, (B) 20 Hz, (C) 10 Hz, and (D) none of the above. The solution on both sides of the membrane for A–C was 1 M KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM MES, pH 6.2. B begins with four channels already open in the low-conductance state; there are no channels open at the beginning of A or C.
We now consider the channels formed by our longest carboxy-terminal fragment of colicin Ia, CT-XL, which includes all of the R and C domains, plus the long helix that connects them. These channels showed the drop in single-channel conductance that we believe is diagnostic of translocation of the amino terminus (Fig. 6 A). This did not depend on the presence of an amino-terminal His-tag or biotin. There was, however, a subtle difference between the CT-XL channels and the channels formed by the shorter fragments (CT-S, CT-M, and CT-L). For the shorter fragments, after the channels entered the low-conductance state, they could be turned off at negative voltage; when they subsequently reopened at positive voltage, they opened initially, as before, to the transient, higher-conductance state, before dropping to the low-conductance state. For CT-XL, channels in the low-conductance state could also be turned off at negative voltage; upon the return to positive voltage, however, the channels reopened directly to the low-conductance state (Fig. 6 B). This held true even when large negative voltages (−200 mV) were used to turn the channels off. Our interpretation of this result is that, although the amino terminus is translocated across the membrane to the trans side at positive voltage, it does not generally move back to the cis side at negative voltage. Thus, the closings that we observe reflect the entry into some new closed state, in which the amino terminus is still on the trans side.
Figure 6.
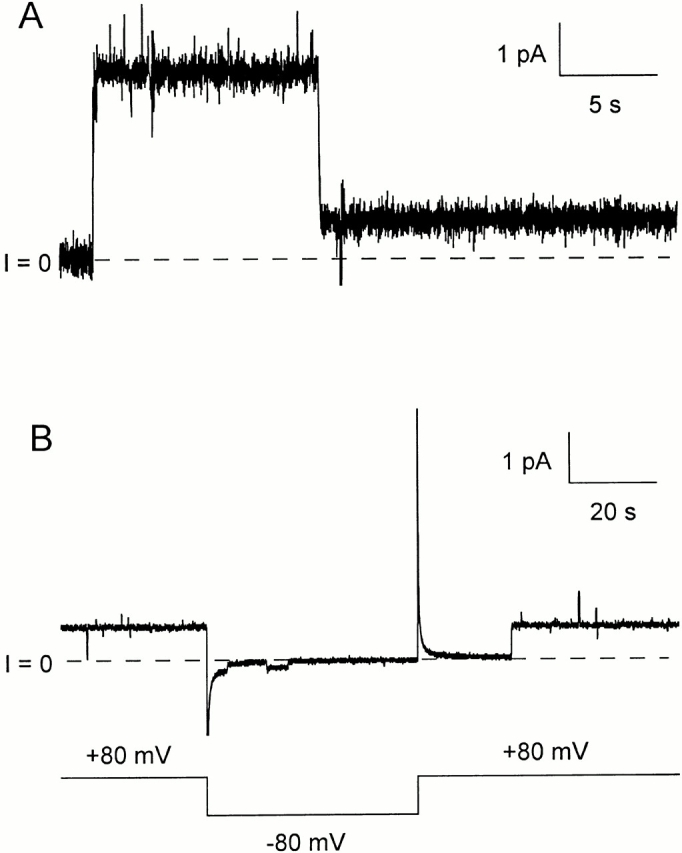
Single channels formed by the colicin Ia carboxy-terminal fragment, 282–626. Before the start of each record, 46 ng of mutant “−3” C/CT-XL (without a His-tag) were added to the cis compartment. (The colicin mutant was biotinylated via a maleimide linker, but streptavidin was not used for these experiments.) (A) A single channel opens to the normal conductance (44 pS), and then drops to a low-conductance state (9 pS). The voltage was held at +80 mV for the duration of this record. (B) A channel that has already passed through the normal conductance state is shown in the low-conductance state (8 pS) at the beginning of the record. When the voltage was switched to −80 mV, the channel flickered once (the conductance at this voltage is only 1.6 pS) and closed. After the return to +80 mV, the channel reopened directly to the low-conductance state, apparently without passing through the normal conductance state. The solution on both sides of the membrane was the same as in Fig. 5, with the addition of 2.6 mM tris(2-carboxyethyl)-phosphine. The records were filtered at (A) 30 Hz and (B) 10 Hz.
We did not observe an effect of trans streptavidin on CT-XL biotinylated at the amino terminus (data not shown). This is not surprising in light of the above interpretation. For a residue that would normally move from the trans side back to the cis side, trans streptavidin would hinder this movement. In contrast, for a residue that resides stably on the trans side, trans streptavidin might bind, but it would not be expected to have any major effect on channel gating.
Trans Trypsin Turns Whole Colicin Ia Channels into Carboxy-terminal Fragment Channels
As an independent approach to creating a colicin Ia fragment with only three membrane-spanning segments, we took advantage of the sensitivity of colicin Ia to protease in the trans solution (Kagan 1981). After adding whole colicin Ia to the cis compartment, we attempted to cut off the fourth, amino-terminal–most segment of the open channel (along with the T and R domains) using trypsin in the trans compartment. Based on our model of the whole colicin Ia open channel (Fig. 1), the only trypsin sites exposed on the trans side of the membrane should be the 15 or 16 basic residues in the translocated segment, from K470 or R476 to R537. As shown in Fig. 7, trans trypsin caused the conductance of single channels to drop from the normal conductance (in 100 mM KCl, pH 8.0) of 9 pS to a low-conductance state of 1 pS, in a manner reminiscent of the spontaneous behavior of C domain channels. This effect was not observed in the absence of trypsin and could be prevented by the addition of soybean trypsin inhibitor to the trans solution, demonstrating that it is a specific enzymatic effect. In 1 M KCl, pH 8.0, trans trypsin made the conductance drop from the normal conductance (at this pH and salt concentration) of 60 pS down to 8 pS. Unpublished experiments with a whole colicin Ia mutant indicate that the major trypsin site is the pair of lysine residues (485–486) in the loop between C domain helices 2 and 3.
Figure 7.
The effect of trans trypsin on whole colicin Ia channels. Before the start of the record, 100 ng of whole colicin Ia were added to the cis compartment, and 5 μg of trypsin were added to the trans compartment. The record shows two channels opening at +40 mV. Each channel opened with the normal conductance (9 pS) for this salt condition, but then dropped to a low-conductance open state (0.9–1.0 pS). This decrease in conductance presumably reflects the cutting by trypsin of a site in the translocated segment and the subsequent separation of the helix 1 membrane-spanning segment from the rest of the channel. The solution on both sides of the membrane was 100 mM KCl, 5 mM CaCl2, 1 mM EDTA, 20 mM HEPES, pH 8.0. The record was filtered at 20 Hz.
Finally, a Big Enough Anchor
If the T and R domains of whole colicin Ia normally serve to anchor the amino-terminal end of the C domain on the cis side, then it should be possible to reconstitute this function in the isolated C domain by using streptavidin as a substitute anchor. Thus, a biotinylated carboxy-terminal fragment of colicin Ia (either 439C/CT-M or 381C/CT-L) was preincubated with streptavidin before the mixture was added to the cis compartment. The resulting single channels showed conductance and gating properties characteristic of whole colicin Ia channels (Fig. 8). (With biotinylated 439C/CT-M, channels that dropped to a low-conductance state were still sometimes observed; probably these arose from a small amount of unbiotinylated C domain remaining after purification on the monomeric avidin column, or else from biotinylated C domain that for some reason was not bound by streptavidin. Low-conductance channels appeared more rarely with biotinylated 381C/CT-L, which was first incubated with streptavidin before purification on a Superdex G-75 sizing column.) As expected, trans trypsin made the streptavidin-preincubated, biotinylated 439C/CT-M channels drop to a low-conductance state (data not shown).
Figure 8.
C domain with its amino terminus anchored on the cis side by streptavidin made normal channels. A preincubated mixture containing 4 ng of biotinylated mutant 439C/CT-M without a His-tag and 150 ng of streptavidin was added to the cis compartment. The record shows two channels that opened and stayed in the higher-conductance state (37–38 pS) at +50 mV. The solution on both sides of the membrane and filtering were the same as in Fig. 5 B.
DISCUSSION
In this paper, we address the question of why translocation of colicin Ia across a planar bilayer normally stops where it does, near the amino-terminal end of the channel-forming C domain. We speculated that the two domains (R and T) upstream from the C domain are somehow responsible for terminating translocation, and that without these, the amino terminus of the C domain will end up on the trans side of the membrane. To test this hypothesis, we examined the channel-forming properties of four carboxy-terminal constructs of colicin Ia, with particular reference to whether their amino termini ended up on the cis or trans side of the membrane in the open channel state. Two of these constructs, CT-S (residues 453–626) and CT-M (438–626), consisted of the C domain plus a small piece of the very long helix 1 that connects it to the R domain; the third, CT-L (327–626), contained in addition all of helix 1 plus helices 2 and 3 of the R domain; and the fourth, CT-XL (282-626), included all of that plus the rest of the R domain, where helix assignments are from the x-ray crystal structure (Wiener et al. 1997). We found that all four of these fragments formed channels in planar bilayer membranes, and that in each case the amino terminus was translocated across the membrane to the trans side.
Evidence for the Location of the Amino Terminus in the Channel's Open State
The presence on the trans side of the membrane of the amino terminus of the carboxy-terminal fragments CT-S, CT-M, and CT-L was assayed by addition to the trans solution of either His-tag antibody, which could bind to the amino-terminal His-tag on our constructs, or streptavidin, which could bind to biotin attached to a cysteine introduced near the amino terminus. We expected that if the amino terminus was translocated to the trans side when the channel opened at positive voltages, and was translocated back to the cis side when the channel closed at negative voltages, then its binding to trans His-tag antibody or trans streptavidin would prevent channel closure, just as the binding of trans streptavidin to biotinylated residues within the translocated region of whole colicin Ia prevented channel closure (Qiu et al. 1996). This is essentially what we observed for CT-L (Fig. 4). For the shorter fragments, however, much to our surprise, the binding of the amino-terminal region by trans antibody or trans streptavidin caused the channels to turn on at negative and off at positive voltages, the reverse of the normal voltage dependence (Fig. 3). This phenomenon is of interest in its own right, but the important point here is that, unlike the case for whole colicin Ia, the amino terminus of C domain channels is on the trans side instead of the cis side in the final open channel state.
Topology of the Chanel Formed by Carboxy-terminal Fragments
In the course of these experiments, we developed a second assay for the translocation of the amino terminus of the fragments across the membrane to the trans side: the transition of the single-channel conductance from the normal level to a low (nonzero) level. Whole colicin Ia channels open to the normal level at voltages of at least +50 mV and stay there, except for brief flickers to other levels (Fig. 5 A). In contrast, all of the carboxy-terminal fragments examined showed a novel behavior: after a channel opened and spent some time at the normal conductance level, it made an abrupt transition to a low-conductance state, which it then occupied stably while the positive voltage was maintained (Fig. 5B and Fig. C, and Fig. 6 A).
The channel formed by whole colicin Ia has four membrane-spanning segments (Fig. 1). The hydrophobic hairpin (residues 580–612) is inserted into the membrane in a non–voltage-dependent manner, and contributes the two carboxy-terminal membrane-spanning segments (Kienker et al. 1997). The other two membrane-spanning segments (extending approximately between residues 544–572 and 450–470) assume their membrane-spanning conformation at positive voltages and move back to the cis side of the membrane at negative voltages; along with their movement, the region between them (residues 474–541) is translocated back and forth across the membrane (Slatin et al. 1994; Qiu et al. 1996). The low-conductance channel formed by the carboxy-terminal fragments does not have the amino-terminal segment (residues 450–470) spanning the membrane, since it has been translocated to the trans side, leaving only three membrane-spanning segments (Fig. 5 F). We presume that these three segments are identical (or very similar) to the three corresponding segments in whole colicin Ia. Thus, everything upstream from residue 541 is translocated across the membrane to the trans side. In the case of the CT-XL fragment, this would mean that all 260 residues from 282 to 541 (plus 25 residues of the amino-terminal His-tag) are translocated.
The channel ultimately formed by the carboxy-terminal fragments, having three membrane-spanning segments, is different from that formed by whole colicin Ia, which has four. This channel has a smaller conductance (∼7 pS in 1 M KCl, pH 6.2) than the whole colicin Ia channel has (∼40 pS). Of particular interest, the fragment channel first opens with a conductance approximately the same as that of the whole colicin channel, before falling to the lower-conductance state (Fig. 5 B). We believe that the transient higher-conductance state corresponds to a channel with four membrane-spanning segments, similar to the whole colicin channel (Fig. 5 E). Thus, we are seeing the passage of helix 1 from its membrane-spanning conformation to its completely translocated state (Fig. 5 F). A lower-conductance channel can also be produced from whole colicin Ia by cutting in the translocated segment (probably between helices 2 and 3) with trans trypsin (Fig. 7); in this case, the drop in single-channel conductance corresponds to the separation of helices 1 and 2 (plus the T and R domains) from the channel-forming fragment. We have also sometimes observed that before carboxy-terminal fragment channels close at negative voltages, they first rapidly pass through the higher conductance state (Fig. 5 C). This suggests that en route to channel closing, their amino-terminal region can first reinsert into the membrane as a membrane-spanning segment before it, along with the translocated region and the third membrane-spanning segment (residues 544–572), moves back through the membrane to the cis side. Since we only occasionally see this rapid transient of higher conductance before channel closure, it may represent just one of several kinetically possible closing paths. On the other hand, it could be the major or only closing pathway, but we observe it only infrequently because of our limited time resolution.
What Normally Stops Translocation?
The results presented in this paper, along with earlier results, raise the question of why translocation stops where it does in whole colicin Ia, leaving the amino-terminal end of the channel-forming domain on the cis side and thereby creating a channel with four, instead of three, membrane-spanning segments. We have previously shown that an inserted FLAG epitope, DYKDDDDK, with seven of eight residues charged, is translocated across the membrane (Jakes et al. 1998). In the present work, we have seen that the highly charged amino-terminal end of the 438–626 fragment, EEKRKQDE, is translocated, as is a sequence of five or six positive charges that are present at pH 4.5 in the amino-terminal His-tag. In addition, all 260 residues comprising helices 2–5 of the C domain, the long helix 1, and all of the R domain are translocated. Apparently, therefore, “any” hydrophilic peptide can be translocated across the membrane by the three membrane-spanning segments of the carboxy-terminal domain of colicin Ia (residues 542–626). The only anchor that we have found sufficient to stop translocation of the amino terminus of the carboxy-terminal fragments is streptavidin, a 60-kD tetrameric protein (Fig. 8). Whether an inserted long hydrophobic sequence would get stuck in the membrane and thereby stop translocation remains to be seen; however, no such sequence exists in colicin Ia, apart from the hydrophobic hairpin. Nothing in the primary sequence of colicin Ia is an obvious candidate for a “stop translocation” sequence. Nor is anything in its secondary structure such a candidate, given that the ∼160 Å α-helix 1 somehow gets translocated (although not necessarily as an α-helix), as does the two-stranded β-sheet (residues 298–326) in the R domain. We therefore favor the hypothesis that an aspect of the whole colicin's tertiary structure halts translocation. In particular, the coiled-coil formed by the two long α-helices connecting the T and C domains to the R domain (Wiener et al. 1997) might not unwind, and therefore could act as a brake on translocation. It is not clear how much of this applies to other channel-forming colicins. Both whole colicin A and its C domain, for instance, form relatively low-conductance channels (13 pS in 1 M KCl, pH 6.2) (Martinez et al. 1983); perhaps both of these molecules form channels with only three membrane-spanning segments.
The above discussion applies to colicin in planar bilayers; it is likely that there are other factors that stop translocation in vivo. The R domain binds to a receptor in the outer membrane; the T domain interacts with the TonB-Exb machinery that is in the periplasmic space and is anchored in the inner membrane. There is evidence, at least for colicin A, that a part of the colicin molecule remains exposed to the external medium when the pore has formed in the inner membrane (Bénédetti et al. 1992). That anchoring, presumably, would prevent wholesale translocation of parts of the colicin upstream of the channel-forming domain.
The mechanism of translocation remains a mystery. How does a long, highly charged protein segment get across a lipid bilayer? A scenario that we favor is that the “fourth” segment slides through the channel (of which it is a part), followed by the upstream polar regions attached to it. That is, the channel itself provides a hydrophilic pathway for the translocation of part of itself. Once these regions have passed through the channel to the trans side, there remain only three membrane-spanning segments, which arrange themselves to form the low-conductance channel.
A major difficulty in envisioning the structure of the original channel is the strong likelihood that it is formed from a single colicin molecule; i.e., it is not a multimeric structure (see references in Qiu et al. 1996). The possibility of forming a large channel from four membrane-spanning segments (as in our case) along with associated lipids is suggested by molecular dynamics simulations of a melittin pore (Lin and Baumgaertner 2000). Conceivably, a similar structure could be formed from three membrane-spanning segments to create the low-conductance channel. Our task of visualizing channel structure and translocation would of course be much easier if the channels formed by the colicin Ia fragments considered in this paper, unlike the channel formed by whole colicin Ia, were multimeric. We have no evidence, however, to favor this.
The end state that we have described for the channel formed by the colicin Ia fragments is a structure with three membrane-spanning segments created by the carboxy-terminal portion (residues 542–626), with all of the upstream residues on the trans side of the membrane. A related situation exists for diphtheria toxin, where the carboxy-terminal ∼110 residues of its translocation domain form a structure consisting of three membrane-spanning segments, with the ∼270 upstream residues on the trans side of the membrane (Oh et al. 1999; Senzel et al. 2000).
Acknowledgments
We thank Jean-Claude Schwartz for assistance with the Superdex column.
This work was supported by National Institutes of Health grant GM29210.
Footnotes
Portions of this work were previously published in abstract form (Kienker, P., S. Slatin, K. Jakes, and A. Finkelstein. 1999. Biophys. J. 76:A120).
Abbreviations used in this paper: CT-L, colicin Ia carboxy-terminal fragment 327–626; CT-M, CT fragment 438–626; CT-S, CT fragment 453–626; CT-XL, CT fragment 282–626; DTT, dithiothreitol.
The nature of the reverse-gating channel is of interest in its own right, but it is not essential to the present study; we plan to examine this topic in greater detail in a subsequent communication.
This “normal” channel behavior was observed for streptavidin-preincubated C domain from which the amino-terminal His-tag had been removed. If the His-tag was left on, the channels flickered to a nonconducting “blocked” state in a voltage-dependent manner. This interesting phenomenon will be considered in a subsequent paper.
How much of the long helix 1 “belongs” to the C domain is somewhat arbitrary. Trypsin digestion of colicin Ia results in a channel-forming carboxy-terminal fragment that begins at residue 451 (Ghosh et al. 1993), which is conventionally taken as the amino terminus of the C domain.
References
- Bénédetti H., Lloubès R., Lazdunski C., Letellier L. Colicin A unfolds during its translocation in Escherichia coli cells and spans the whole cell envelope when its pore has formed. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:441–447. doi: 10.1002/j.1460-2075.1992.tb05073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock J.O., Cohen F.S. Octyl glucoside promotes incorporation of channels into neutral planar phospholipid bilayers. Studies with colicin Ia. Biochim. Biophys. Acta. 1986;856:101–108. doi: 10.1016/0005-2736(86)90015-5. [DOI] [PubMed] [Google Scholar]
- Cramer W.A., Heymann J.B., Schendel S.L., Deriy B.N., Cohen F.S., Elkins P.A., Stauffacher C.V. Structure-function of the channel-forming colicins. Annu. Rev. Biophys. Biomol. Struct. 1995;24:611–641. doi: 10.1146/annurev.bb.24.060195.003143. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Mel S.F., Stroud R.M. A carboxy-terminal fragment of colicin Ia forms ion channels. J. Membr. Biol. 1993;134:85–92. doi: 10.1007/BF00232745. [DOI] [PubMed] [Google Scholar]
- Jakes K.S., Abrams C.K., Finkelstein A., Slatin S.L. Alteration of the pH-dependent ion selectivity of the colicin E1 channel by site-directed mutagenesis. J. Biol. Chem. 1990;265:6984–6991. [PubMed] [Google Scholar]
- Jakes K.S., Kienker P.K., Slatin S.L., Finkelstein A. Translocation of inserted foreign epitopes by a channel-forming protein. Proc. Nat. Acad. Sci. USA. 1998;95:4321–4326. doi: 10.1073/pnas.95.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B.L. Voltage-dependent Channels Formed by Colicins 1981. Ph.D. Thesis, Albert Einstein College of Medicine; Bronx, NY: pp. 86 pp [Google Scholar]
- Kienker P.K., Qiu X.-Q., Slatin S.L., Finkelstein A., Jakes K.S. Transmembrane insertion of the colicin Ia hydrophobic hairpin. J. Membr. Biol. 1997;157:27–37. doi: 10.1007/s002329900213. [DOI] [PubMed] [Google Scholar]
- Lin J.-H., Baumgaertner A. Stability of a melittin pore in a lipid bilayera molecular dynamics study. Biophys. J. 2000;78:1714–1724. doi: 10.1016/S0006-3495(00)76723-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankovich J.A., Hsu C.H., Konisky J. DNA and amino acid sequence analysis of structural and immunity genes of colicins Ia and Ib. J. Bacteriol. 1986;168:228–236. doi: 10.1128/jb.168.1.228-236.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M.C., Lazdunski C., Pattus F. Isolation, molecular and functional properties of the C-terminal domain of colicin A. EMBO (Eur. Mol. Biol. Organ.) J. 1983;2:1501–1507. doi: 10.1002/j.1460-2075.1983.tb01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Nogueira R.A., Varanda W.A. Gating properties of channels formed by colicin Ia in planar lipid bilayer membranes. J. Membr. Biol. 1988;105:143–153. doi: 10.1007/BF02009167. [DOI] [PubMed] [Google Scholar]
- Oh K.J., Senzel L., Collier R.J., Finkelstein A. Translocation of the catalytic domain of diphtheria toxin across planar phospholipid bilayers by its own T domain. Proc. Nat. Acad. Sci. USA. 1999;96:8467–8470. doi: 10.1073/pnas.96.15.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchornik A., Berger A., Katchalski E. Poly-l-histidine. J. Am. Chem. Soc. 1957;79:5227–5230. [Google Scholar]
- Qiu X.-Q., Jakes K.S., Finkelstein A., Slatin S.L. Site-specific biotinylation of colicin Iaa probe for protein conformation in the membrane. J. Biol. Chem. 1994;269:7483–7488. [PubMed] [Google Scholar]
- Qiu X.-Q., Jakes K.S., Kienker P.K., Finkelstein A., Slatin S.L. Major transmembrane movement associated with colicin Ia channel gating. J. Gen. Physiol. 1996;107:313–328. doi: 10.1085/jgp.107.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzel L., Gordon M., Blaustein R.O., Oh K.J., Collier R.J., Finkelstein A. Topography of diphtheria toxin's T domain in the open channel state. J. Gen. Physiol. 2000;115:421–434. doi: 10.1085/jgp.115.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatin S.L., Qiu X.-Q., Jakes K.S., Finkelstein A. Identification of a translocated protein segment in a voltage-dependent channel. Nature. 1994;371:158–161. doi: 10.1038/371158a0. [DOI] [PubMed] [Google Scholar]
- Wiener M., Freymann D., Ghosh P., Stroud R.M. Crystal structure of colicin Ia. Nature. 1997;385:461–464. doi: 10.1038/385461a0. [DOI] [PubMed] [Google Scholar]