Abstract
The present study tested the effects of EUK-134, a synthetic superoxide dismutase/catalase mimetic, on several indices of oxidative stress and neuropathology produced in the rat limbic system as a result of seizure activity elicited by systemic kainic acid (KA) administration. Pretreatment of rats with EUK-134 did not modify the latency for or duration of KA-induced seizure activity. It did produce a highly significant reduction in increased protein nitration, activator protein-1- and NF-κB-binding activity, and spectrin proteolysis as well as in neuronal damage resulting from seizure activity in limbic structures. These results support the hypothesis that kainate-induced excitotoxicity is caused, at least in part, by the action of reactive oxygen species. Furthermore, they suggest that synthetic superoxide dismutase/catalase mimetics such as EUK-134 might be used to prevent excitotoxic neuronal injury.
Various mechanisms have been proposed to account for the pathological manifestations observed after systemic administration of the excitotoxin kainic acid (KA). Because drugs blocking seizure activity prevent most of the neuronal damage resulting from KA injection (1, 2), it is clear that the pathology is not a direct consequence of the activation of KA or α-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) receptors, but rather is the result of events triggered by seizure activity. Excessive production of oxygen-free radicals and other radical species often has been proposed to play an important role in neuronal pathology resulting from excitotoxic insults (3–8). It generally is admitted that KA administration results in the activation of N-methyl-d-aspartate (NMDA) receptors in vulnerable neuronal populations (9, 10), an event that has been shown to cause the formation of superoxide radicals (11, 12). Moreover, we reported previously that levels of lipid peroxidation and protein oxidation, two parameters of oxidative stress, were increased significantly in hippocampus and piriform cortex at 8 and 16 h after KA-induced seizure activity in adult rats (13). We also showed that the DNA-binding activity of two transcription factors, namely activator protein-1 (AP-1) and NF-κB, generally considered to be markers of cellular insults (14, 15), was increased in these structures under these conditions (16). However, a causal relationship between oxidative stress and neuronal damage after systemic kainate injection has not been established unambiguously, because many agents used as antioxidants also interfere with seizure activity. In addition, because excitotoxicity also has been shown to be reduced by caspase inhibitors (17), oxidative stress could be a late consequence of the events triggered by seizure activity and leading to neuronal death.
Salen–manganese complexes are low-molecular-weight synthetic compounds that exhibit both superoxide dismutase (SOD) and catalase activities, catalytically eliminating both superoxide and hydrogen peroxide, respectively (18–20). Compared with proteinaceous antioxidant enzymes, these low-molecular-weight synthetic complexes should have better stability and bioavailability. In addition, their catalytic mode of action makes them more potent than traditional low-molecular-weight antioxidant compounds. Such properties make them useful not only as potential therapeutic agents, but also for the elucidation of the role of reactive oxygen species (ROS) in pathologies associated with diseases such as Alzheimer’s disease, Parkinson’s disease, and stroke. Indeed, salen–manganese complexes have shown efficacy in several models for ROS-associated diseases, for example, protecting pulmonary function in a stringent porcine model of adult respiratory distress syndrome (19). Of particular relevance to neurological diseases, they preserve synaptic function in hippocampal slices subjected to anoxia/reoxygenation (21), protect striatal dopaminergic neurons in two mouse models for Parkinson’s disease (20), and prevent paralysis in mouse experimental autoimmune encephalomyelitis (22). Furthermore, they protect organotypic hippocampal cultures from toxicity elicited by the β-amyloid peptide (23) and prevent rat brain damage in a rat focal cerebral ischemia model (24). We therefore used one of these compounds, EUK-134 (see ref. 24 for details of structure and activity), to establish further the contribution of ROS to KA excitotoxicity. We investigated the effects of EUK-134 on indices of oxidative stress as well as on the pathological manifestations produced by KA in the rat limbic system. In neuronal and other tissues, oxidative stress leads to macromolecular damage such as lipid peroxidation and protein oxidation, as well as the induction of several stress-related gene responses. One form of protein oxidation that has been implicated in neuronal damage is tyrosine nitration, caused by the action of peroxynitrite on tyrosine residues. Peroxynitrite is produced during oxidative stress through the reaction of superoxide ions with nitric oxide (NO) (25). Stress-related gene responses are mediated by such transcriptional factors as NF-κB and AP-1, both of which are activated by processes regarded as oxidative stress-dependent (14, 15). Thus, protein tyrosine nitration and AP-1 and NF-κB served as indices of oxidative stress in this study. Also investigated were known indicators of neuronal cell death, including spectrin proteolysis, which has been proposed to accompany apoptosis as a result of caspase activation (17). Finally, the extent of neuronal damage was localized and quantified based on changes in hematoxylin/eosin (H&E) staining (26). Our results indicate that EUK-134 prevents oxidative stress and attenuates rat brain damage induced by systemic administration of KA. This provides strong support for the hypothesis that ROS play a critical role in triggering neuronal damage resulting from seizure activity.
MATERIALS AND METHODS
Animal Treatments.
All studies were conducted in accordance with the National Research Council “Guide for the Care and Use of Laboratory Animals” and under the auspices of the University Animal Use Committee. Sprague–Dawley rats were housed under standard laboratory conditions with free access to food and water. Male adult rats (weighing 200–250 g) were injected s.c. with (i) 0.9% NaCl (control group), (ii) 10 mg/kg KA (KA group), (iii) 10 mg/kg KA and EUK-134 (24 and 0.5 h before systemic administration of KA) (KA & EUK group), and (iv) 10 mg/kg EUK-134 (24 and 0.5 h before systemic administration of saline) (EUK group). These doses of KA produced a reliable pattern of recurrent seizure activity lasting >4 h, which has been well documented in numerous studies. At 8 h, 16 h, or 5 days after KA treatment, animals (n = 6 in each group) were killed by decapitation after methoxyflurane anesthesia. Their brains were removed for further processing.
Immunohistochemistry.
Immunohistochemistry was performed by using the avidin–biotinylated horseradish peroxidase complex (ABC) method. Perfused rat brains were embedded in paraffin and sectioned (8 μm). After deparaffinizing and rehydrating, sections first were incubated in 10% normal horse serum diluted in PBS for 1 h at room temperature, followed by incubation with a rabbit polyclonal anti-nitrotyrosine antibody (10 μg/ml; Calbiochem) overnight at 4°C. After several washes in PBS, sections were incubated in biotinylated goat anti-rabbit IgG 1:200 (Vector) for 2 h and then in ABC diluted in PBS for 30 min. Peroxidase reaction was carried out with 3,3′-diaminobenzidine tetrahydrochloride (0.05% in 50 mM Tris⋅HCl buffer, pH 7.4) as chromogen and 0.03% H2O2 as oxidant. Sections then were dehydrated in graded ethanol and, finally, covered with Permount. Control experiments for immunohistochemistry were performed with control rabbit IgG replacing the primary antibody, which resulted in no detectable staining. Results of immunohistochemistry were analyzed and documented with a Zeiss light microscope.
Electrophoretic Mobility-Shift Assay (EMSA).
Nuclear extracts were prepared as described by Staal et al. (27) with some modifications. Dissected brain regions were homogenized in ice-cold homogenization buffer (0.1 M Tris⋅HCl, pH 7.4/0.32 M sucrose/25 mM KCl/5 mM MgCl2/0.5 mM DTT/0.5 mM PMSF/2 μg/ml antipain/2 μg/ml leupeptin) with a motor-driven Dounce homogenizer and 10 strokes at 1,700 rpm. Homogenates were centrifuged at 4°C for 30 sec at 12,000 × g, and the supernatants were discarded. Pellets were resuspended in 0.4 ml of lysis buffer (10 mM Hepes/1.5 mM MgCl2/10 mM KCl/0.5 mM DTT/0.5 mM PMSF/2 μg/ml antipain/2 μg/ml leupeptin) and incubated on ice for 10 min. Ten microliters of 10% NP-40 solution was added. The mixture was mixed vigorously for 30 sec and recentrifuged for 30 sec at 12,000 × g. Pelleted nuclei were resuspended in 50 μl of extraction buffer (20 mM Hepes/25% glycerol/1.5 mM MgCl2/300 mM NaCl/0.25 mM EDTA/0.5 mM DTT/0.5 mM PMSF/2 μg/ml leupeptin/2 μg/ml antipain), incubated on ice for 20 min, and then centrifuged again for 20 min at 12,000 × g at 4°C. The supernatants containing the nuclear proteins were collected and stored at −70°C. Protein concentration was determined by using the Bio-Rad protein assay reagent. Double-stranded NF-κB and AP-1 consensus oligonucleotides were end-labeled with [γ-32P]ATP. EMSAs were performed essentially as described earlier (27). Binding-reaction mixtures [5 μg of nuclear protein/1 μg of poly(dI-dC)/32P-labeled probe (10,000 cpm)/50 mM NaCl/0.2 mM EDTA/0.5 mM DTT/2% glycerol/210 mM Tris⋅HCl] were incubated for 20 min at room temperature. DNA–protein complexes were separated from unbound DNA probes by electrophoresis through a 6% nondenaturing polyacrylamide gel in 0.5× TBE (44.5 mM Tris⋅HCl, pH 8.0/44.5 mM boric acid/1 mM EDTA) for 1.5 h at 225 V. Gels were vacuum-dried and exposed to a phosphorimaging screen and analyzed by using imagequant software (Molecular Dynamics).
H&E Staining.
Frozen coronal brain sections (8 μm) were postfixed in 4% paraformaldehyde in PBS, stained with H&E. After staining, the slides were coverslipped and examined for cell damage. For each animal, several sections were rated and an average score was calculated. These ratings were applied to areas CA1 and CA3 of the hippocampus and the piriform cortex.
Western Blots.
Brain tissues obtained from treated rats were homogenized in ice-cold homogenization buffer (0.01 M Tris⋅HCl, pH 7.4/0.32 M sucrose/2 mM EDTA/1 mM EGTA/0.5 mM DTT/0.5 mM PMSF/2 μg/ml antipain/2 μg/ml leupeptin). One volume of 2× SDS/PAGE sample buffer (0.1 M Tris⋅HCl, pH 6.8/4% SDS/20% glycerol/30 mM 2-mercaptoethanol/0.2% bromphenol blue) was added to the homogenate, and the mixture was heated by using a boiling-water bath for 5 min. Aliquots (40 μg of protein) were subjected to SDS/PAGE (8% polyacrylamide) and transferred onto nitrocellulose membranes. After incubation in Tris-buffered saline (TBS) containing 3% gelatin at room temperature, blots were incubated overnight with an anti-α-spectrin antibody (1:1,000 dilution) (Chemicon) in TBS containing 1% gelatin and 0.05% Tween-20. Spectrin breakdown products (SBDP) were detected by using alkaline phosphatase-conjugated secondary antibody (Bio-Rad). Blots were scanned into a computer and analyzed by using imagequant software.
Statistical Analyses.
Data are presented as mean ± SD. Statistical analysis was performed by using one-way ANOVA followed by Tukey’s multiple-range test for honestly significant differences. All statistical procedures were performed with statgraphics 5.1 software.
RESULTS
About 45 min after receiving KA, rats exhibited masticatory movements and nodding. At about the same time, numerous, intermittent “wet-dog” shakes were observed, accompanied by stereotypic movements of the forepaws, described as “piano playing.” This behavior was replaced later by clonic convulsions with rearing and falling-down episodes and by generalized clonic convulsions with tonic extension episodes (28). In KA and KA & EUK groups, all the rats displayed a similar pattern of seizure activity, with no difference in latency or in seizure intensity and duration.
Nitrotyrosine Immunohistochemistry.
For nitrotyrosine immunohistochemistry, animals were sacrificed 8 h after KA or saline injection, i.e., at a time when minimal neuronal damage is evident in most vulnerable neuronal populations. In the control group, most neurons in hippocampus and piriform cortex were weakly and homogeneously labeled (Fig. 1). No labeling was observed when control rabbit IgG replaced the primary antibody. A similar pattern of staining was observed in the EUK group (not shown). In the KA group, extensive immunoreactivity was observed in CA1 and CA3 regions, but not in the granule cells of the dentate gyrus. In particular, pyramidal neurons were stained heavily, and increased staining also was evident in stratum oriens and stratum radiatum of CA1 and CA3. In piriform cortex, although numerous neurons had already died at this time point, the neuropil exhibited increased staining (not shown). Staining in the nucleus was lighter than in the cytoplasm, suggesting that nitrotyrosine-containing proteins were present mainly in the cytoplasm. In the KA & EUK group, only one of six rat brains exhibited some staining qualitatively similar to that observed in all rats in the KA group. Staining in the other five animals was very similar to that found in control animals (Fig. 1).
Figure 1.
Effects of EUK-134 treatment on KA-induced changes in nitrotyrosine immunoreactivity. Rats were treated with KA or KA & EUK as described in Materials and Methods. They were killed 8 h after treatment and perfused with paraformaldehyde. Coronal brain sections (8 μm thick) were processed for immunohistochemistry with an antibody specific for nitrotyrosinated proteins. Note the large increase in immunoreactivity in pyramidal cell bodies of CA1 and CA3 in the KA group (arrows) and the complete blockade of the effect in the KA & EUK group.
EMSA.
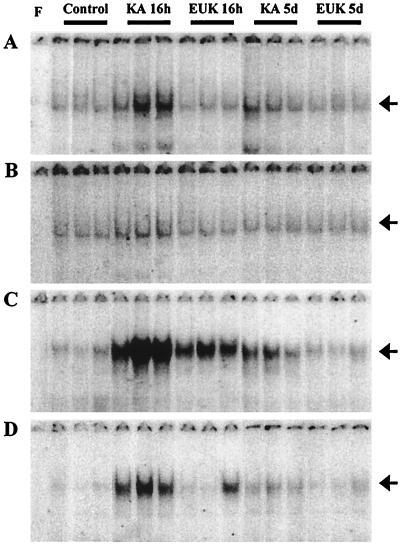
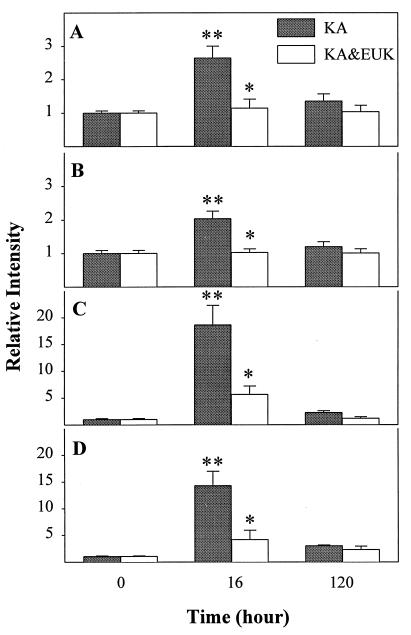
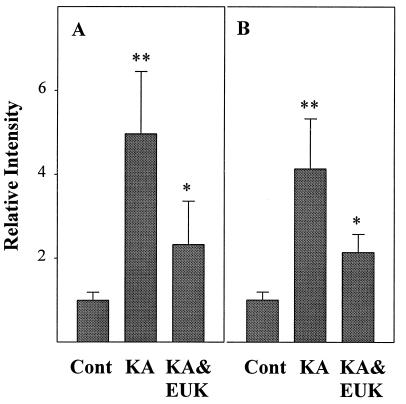
The levels of activated NF-κB and AP-1 were assessed based on each transcription factor’s ability to bind to its consensus DNA sequences. Rats were killed at 16 h or 5 days after saline, KA, or KA & EUK injection. Nuclear protein extracts were prepared from piriform cortex and hippocampus. The relative amounts of transcription factor–DNA complex, indicative of levels of active transcription factor, were measured with EMSAs by using labeled oligonucleotides containing the NF-κB and AP-1 consensus sequences, respectively (Figs. 2 and 3). For NF-κB, KA treatment resulted in the induction of a major band migrating with an apparent molecular weight similar to that of the complex p65/p50 found in HeLa cell nuclear extracts (this band was used for all quantitative analyses). Quantitative analysis of the gels indicated that, in piriform cortex and hippocampus, the binding activity of NF-κB exhibited similar patterns after KA treatment (Fig. 3). Induction of NF-κB was significant at 16 h after KA treatment, as active NF-κB was increased about 3-fold in piriform cortex and 2-fold in hippocampus. EUK-134 treatment prevented the induction of NF-κB elicited by systemic administration of KA. Five days posttreatment, active NF-κB had returned to control levels in the KA and KA & EUK groups in both piriform cortex and hippocampus.
Figure 2.
Effects of EUK-134 treatment on KA-induced changes in NF-κB- and AP-1-binding activity. Control and KA-treated rats were killed at 16 h or 5 days after KA injection. Nuclear extracts from control, KA, and KA & EUK groups were prepared from piriform cortex (A and C) and hippocampus (B and D). Aliquots (5-μg proteins) were incubated with labeled NF-κB (A and B) or AP-1 (C and D) probes, and bound nucleotides were separated from free probes by EMSA. Gels were dried and analyzed with a PhosphorImager (Molecular Dynamics). For each group, samples from three representative animals are shown. Lane F contained no nuclear extracts as a negative control. Arrows on the right correspond to NF-κB- or AP-1-specific binding complexes.
Figure 3.
Quantitative analysis of changes in NF-κB and AP-1 DNA-binding activity after KA treatment. Control and KA-treated rats were killed at 16 h or 5 days after KA injection. The DNA-binding activities of NF-κB (A and B) and AP-1 (C and D) in piriform cortex (A and C) and hippocampus (B and D) were quantified by using imagequant software. Data were expressed as ratios between values found in control and treated animals and represent the means ± SD of six animals in each group. Statistically significant differences between control and KA treatment are indicated by ∗∗, and differences between KA and KA & EUK groups are indicated by ∗.
Similar changes in AP-1 expression were found in piriform cortex and hippocampus (Fig. 3). Sixteen hours after KA treatment, active AP-1 was increased 20-fold in piriform cortex and 14.5-fold in hippocampus. EUK-134 treatment significantly reduced the induction of AP-1 in piriform cortex and hippocampus. Five days after KA treatment, active AP-1 levels still remained above control values in piriform cortex and hippocampus. In the KA & EUK group, active AP-1 had returned to control levels in piriform cortex and was only slightly above the control level in hippocampus. EUK-134 treatment did not produce any change in either NF-κB or AP-1 DNA-binding activity.
H&E Staining.
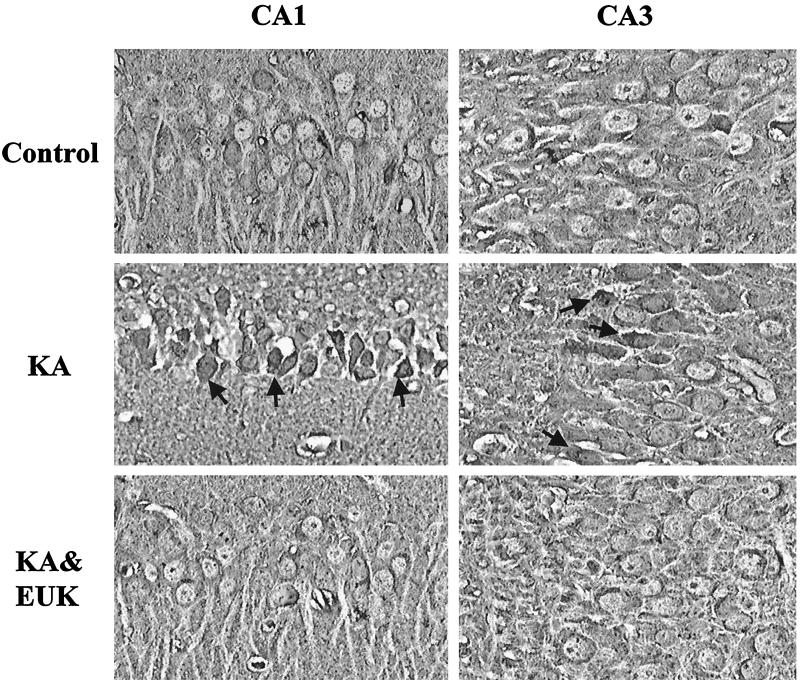
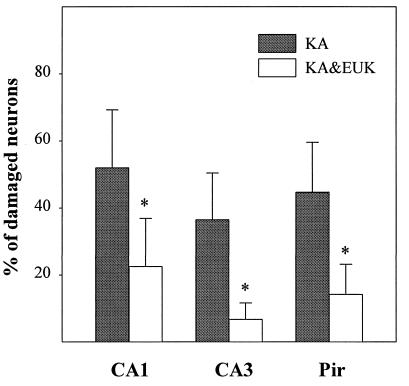
Rats were killed 5 days after treatment. Fresh-frozen sections were stained with H&E and examined with light microscopy. Damaged neurons were identified as those exhibiting eosinophilic cytoplasm and condensed nuclei. The fraction of morphologically damaged neurons was determined in hippocampal fields CA1 and CA3 and in piriform cortex. KA treatment induced severe damage in CA1 (52% of total neurons), CA3 (37%), and piriform cortex (45%) (Fig. 4). EUK-134 treatment provided a significant reduction (P < 0.05) of KA-induced neuronal damage in CA1 (22% of total neurons) and an almost complete protection in CA3 (7%) and piriform cortex (14%). EUK-134 treatment did not result in any neuronal damage (not shown).
Figure 4.
Effects of EUK-134 treatment on KA-induced neuronal damage. Control and KA-treated rats were killed 5 days after KA injection. Frozen brain sections were stained with H&E and examined by light microscopy. Damaged neurons were identified as those exhibiting eosinophilic cytoplasm and condensed nuclei. For each rat, the percentage of damaged neurons was determined in two separate sections (all pyramidal cells in CA1 and CA3 or 500 cells in piriform cortex were counted, respectively) and the values were averaged. Results represent the means ± SD of data from six rats in each group. Statistically significant differences between KA and KA & EUK groups are indicated by ∗.
Spectrin Proteolysis.
Spectrin proteolysis often has been used as a quantitative marker of neuronal damage, because spectrin is a substrate for caspase and calpain-like proteases activated during neuronal cell death (17, 29). The amounts of SBDP were determined by immunoblots in homogenates of piriform cortex and hippocampus of animals sacrificed 16 h after KA treatment. The blots were scanned, and relative amounts of SBDP with molecular masses of 150 and 145 kDa were quantified by using imagequant software. In the control group, α-spectrin was found to be mostly in the intact 240-kDa form, with some minor signals for various breakdown products (not shown). In the KA group, SBDP levels increased significantly in both piriform cortex and hippocampus as compared with the control group. EUK-134 treatment markedly inhibited SBDP formation in piriform cortex and hippocampus. Quantification of the levels of SBDP (150 and 145 kDa) in KA and KA & EUK groups indicated that the decreases in SBDP were highly significant (P < 0.001) (Fig. 5).
Figure 5.
Quantitative analysis of spectrin proteolysis after KA-induced seizure activity. Control and KA-treated rats were killed 16 h after KA injection. The amount of the 150- and 145-kDa bands in immunoblots stained with antispectrin antibodies was quantified. Data were expressed as ratios between values found in control and treated animals and represent the means ± SD of six animals. Statistically significant differences between control and KA treatment are indicated by ∗∗, and differences between KA and KA & EUK groups are indicated by ∗.
DISCUSSION
These results indicate that pretreatment of rats with the synthetic catalytic scavenger of ROS, EUK-134, prevents oxidative stress and produces a significant degree of protection against the pathological manifestations of KA-induced seizure activity in hippocampus and piriform cortex. Although numerous studies have suggested that ROS are critically involved in excitotoxicity (see refs. 5, 30, and 31 for reviews), very few studies have demonstrated convincingly, in an in vivo excitotoxic model, a causal relationship between increased production of ROS and neuronal damage (7, 32). A clear demonstration of the crucial role of ROS in excitotoxicity should include: (i) evidence for increased production of ROS specifically in neuronal populations at risk with excitotoxicity before overt signs of neuronal damage and (ii) evidence that manipulations aimed at preventing ROS accumulation reduce neuronal damage without interfering with excitatory amino acid receptor functions. The former has been difficult in in vivo experiments because techniques to directly measure or visualize ROS production are not readily available. Most studies have used surrogate markers such as the determination of protein oxidation or lipid peroxidation to infer that increased ROS production was associated with excitotoxicity (13). Alternatively, the localization of certain oxidative stress-related genes has been used to quantify and to map out the distribution of cells exhibiting oxidative stress under certain conditions. A number of studies have shown that a variety of ROS scavengers afford some degree of protection against excitotoxic damage (7, 32–34), but the lack of evidence showing the specificity and selectivity of these agents has made the conclusion that ROS critically participate in excitotoxicity uncertain. In particular, numerous manipulations or agents used to reduce oxidative stress interfere with seizure activity, thus preventing unambiguous interpretations of the results. In the present study, we provide evidence for increased ROS production in KA-treated rats by examining indices of oxidative stress, namely, protein nitration and NF-κB and AP-1 activation, demonstrating the occurrence of oxidative stress in neuronal populations selectively vulnerable to KA. Furthermore, we show that eliminating ROS accumulation with a synthetic SOD/catalase mimetic markedly reduces this selective KA-induced neuronal death.
It generally is accepted that oxidative stress increases the production of not only ROS, but also NO, thus resulting in the formation of the toxic peroxynitrite ions (24). A recent study also indicated increased accumulation of nitroxide radicals in hippocampus after KA injection (35). These radicals are responsible for the nitrotyrosylation of numerous proteins, an effect that has been proposed to account for several features of excitotoxicity (36). The immunohistochemical localization of nitrotyrosylated proteins using antibodies specific for nitrotyrosine therefore provides a convenient method to map out the distribution of cells exhibiting increased production of peroxynitrite. This method has been used, for example, to localize increased oxidative stress in neurons from brain of patients with Alzheimer’s disease (37, 38). After KA-induced seizure activity, increased staining was evident in CA1 and CA3 areas but not in the dentate gyrus of the hippocampus. It also was present in piriform cortex, although the more rapid degeneration of neurons in this structure precluded a clear mapping of the cells exhibiting increased staining. Thus, the increased staining for nitrotyrosine precedes neuronal damage, and its distribution matches well that of the vulnerable populations.
AP-1 and NF-κB are two transcription factors often considered part of oxidative stress responses of numerous cell types (14, 15). We previously showed that expression of AP-1 and NF-κB was increased markedly in hippocampus and piriform cortex of adult animals after KA-induced seizure activity (16). Because these transcription factors were not induced after KA-induced seizure activity in developing animals, that is, at a developmental stage when neurons in the limbic system are resistant to KA-induced damage, we concluded that increased expression of these two transcription factors was a correlate of neuronal damage (16). This conclusion was in good agreement with numerous reports indicating that delayed induction of several members of the immediate early gene family is a marker of selective neuronal vulnerability (39, 40). Finally, recent reports have indicated that increased formation of several breakdown products of the cytoskeletal protein, spectrin, reflects the activation of the calcium-dependent protease calpain and/or that of some members of the caspase family and, therefore, is an additional marker of neuronal damage (17, 29, 41, 42).
Our results clearly indicate that oxidative stress occurs before significant neuronal death in hippocampus. They also confirm our earlier results indicating delayed induction of AP-1 and NF-κB in the selectively vulnerable regions of the limbic system (16). Furthermore, they confirm that calpain and/or caspase is activated and produces spectrin breakdown. All the markers of oxidative stress were prevented completely by pretreatment of the rats with the synthetic catalytic SOD/catalase mimetic, EUK-134. Because this compound did not modify the onset or the intensity and duration of KA-induced seizure activity, it is likely that the main effect of this ROS scavenger is to prevent oxidative stress by eliminating oxygen-free radicals and/or hydrogen peroxide generated as a result of the activation of NMDA receptors. In addition, an SOD mimetic such as EUK-134 would be expected to prevent the accumulation of peroxynitrite through removal of its precursor superoxide and, thus, prevent nitrotyrosylation of proteins. We do not have evidence indicating that EUK-134 directly interacts with NO or with NOS generation, but we cannot exclude the possibility that such an effect also could contribute to its neuroprotective effect because NOS inhibitors protect kainate-induced neuronal damage (43, 44). The prevention by EUK-134 treatment of spectrin proteolysis might reflect the fact that this event is a late event in the cascade initiated by seizure activity. Alternatively, it could be that ROS production results in disturbance in intracellular calcium, leading to calpain and/or caspase activation.
EUK-134 prevented most but not all neuronal damage resulting from KA-induced seizure activity. This would suggest that mechanisms other than ROS production also are activated as a result of seizure activity and contribute to neuronal death. Interestingly, the effect of EUK-134 was different in CA3 and CA1, suggesting that different mechanisms of excitotoxicity are present in these different neuronal populations. The nature of these mechanisms is not known but could involve the induction of specific genes that are different from oxidative-stress responses or second-messenger pathways. More experiments will be needed to resolve this question. Nonetheless, it is clear that EUK-134 protects most of the vulnerable neurons from excitotoxic cell death in this model. This is consistent with previous findings that salen–manganese complexes are highly protective in various other models for neurological diseases, in particular, β-amyloid toxicity (23). These observations, overall, warrant further evaluation of the therapeutic potential of these SOD/catalase mimetics in neurodegenerative diseases.
Acknowledgments
This work was supported in part by a grant from Eukarion, Inc.
ABBREVIATIONS
- KA
kainic acid
- AP-1
activator protein-1
- EMSA
electrophoretic mobility-shift assay
- ROS
reactive oxygen species
- SBDP
spectrin breakdown products
- H&E
hematoxylin/eosin
- SOD
superoxide dismutase
References
- 1.Tremblay E, Nitecka L, Berger M L, Ben-Ari Y. Neuroscience. 1984;13:1051–1072. doi: 10.1016/0306-4522(84)90288-4. [DOI] [PubMed] [Google Scholar]
- 2.Sloviter R S. Adv Exp Med Biol. 1986;203:659–671. doi: 10.1007/978-1-4684-7971-3_50. [DOI] [PubMed] [Google Scholar]
- 3.Flamm E S, Demopoulos H B, Seligman M L, Poser R G, Ransohoff J. Stroke. 1978;9:445–447. doi: 10.1161/01.str.9.5.445. [DOI] [PubMed] [Google Scholar]
- 4.Granger, D. N., Hollwarth, M. E. & Parks, D. A. (1986) Acta. Physiol. Scand.548 (Suppl.), 47–63. [PubMed]
- 5.Monyer H, Hartly D M, Choi D W. Neuron. 1990;5:121–126. doi: 10.1016/0896-6273(90)90302-v. [DOI] [PubMed] [Google Scholar]
- 6.Bondy S C, Lee D K. Brain Res. 1993;610:229–233. doi: 10.1016/0006-8993(93)91405-h. [DOI] [PubMed] [Google Scholar]
- 7.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 8.Schulz J B, Henshaw D R, Siwek D, Jenkins B G, Ferrante R J, Bencipolloni P, Kowall N W, Rosen B R, Beal M F. J Neurochem. 1995;64:2239–2247. doi: 10.1046/j.1471-4159.1995.64052239.x. [DOI] [PubMed] [Google Scholar]
- 9.Berg M, Bruhn T, Johansen F F, Diemer N H. Pharmacol Toxicol. 1993;73:262–268. doi: 10.1111/j.1600-0773.1993.tb00582.x. [DOI] [PubMed] [Google Scholar]
- 10.Baran H, Loscher W, Mevissen M. Brain Res. 1994;652:195–200. doi: 10.1016/0006-8993(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 11.Lafon-Cazal M, Culcasi M, Gaven F, Pietri S, Bockaert J. Neuropharmacology. 1993;32:1259–1966. doi: 10.1016/0028-3908(93)90020-4. [DOI] [PubMed] [Google Scholar]
- 12.Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. Nature (London) 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 13.Bruce A, Baudry M. Free Radical Biol Med. 1995;18:993–1002. doi: 10.1016/0891-5849(94)00218-9. [DOI] [PubMed] [Google Scholar]
- 14.Pinkus R, Weiner L W, Daniel V. J Biol Chem. 1996;271:13422–13429. doi: 10.1074/jbc.271.23.13422. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz M L, Baeuerle P A. Immunobiology. 1995;193:116–127. doi: 10.1016/s0171-2985(11)80534-6. [DOI] [PubMed] [Google Scholar]
- 16.Rong Y, Baudry M. J Neurochem. 1996;67:662–668. doi: 10.1046/j.1471-4159.1996.67020662.x. [DOI] [PubMed] [Google Scholar]
- 17.Nath R, Probert A, Jr, McGinnis K M, Wang K K. J Neurochem. 1998;71:186–195. doi: 10.1046/j.1471-4159.1998.71010186.x. [DOI] [PubMed] [Google Scholar]
- 18.Baudry M, Etienne S, Bruce A, Palucki M, Jacobsen E, Malfroy B. Biochem Biophys Res Commun. 1993;192:964–968. doi: 10.1006/bbrc.1993.1509. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez P K, Zhuang J, Doctrow S R, Malfory B, Benson P F, Menconi M J, Fink M P. J Pharmacol Exp Ther. 1995;275:798–806. [PubMed] [Google Scholar]
- 20.Doctrow S R, Huffman K, Marcus C B, Musleh W, Bruce A, Baudry M, Malfroy B. In: Antioxidants in Disease Mechanisms and Therapeutic Strategies. Sies H, editor. New York: Academic; 1996. pp. 247–269. [Google Scholar]
- 21.Musleh W, Bruce A, Malfroy B, Baudry M. Neuropharmacology. 1994;33:929–934. doi: 10.1016/0028-3908(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 22.Malfroy B, Doctrow S R, Orr P L, Tocco G, Fedoseyeva E V, Benichou G. Cell Immunol. 1997;177:62–68. doi: 10.1006/cimm.1997.1091. [DOI] [PubMed] [Google Scholar]
- 23.Bruce A, Malfroy B, Baudry M. Proc Natl Acad Sci USA. 1996;93:2312–2316. doi: 10.1073/pnas.93.6.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker K, Marcus C B, Huffman K, Kruk H, Malfroy B, Doctrow S R. J Pharmacol Exp Ther. 1998;284:215–221. [PubMed] [Google Scholar]
- 25.Beckman J S, Koppenol W H. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 26.Bruce A, Sakhi S, Schreiber S S, Baudry M. Exp Neurol. 1995;132:209–219. doi: 10.1016/0014-4886(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 27.Staal F J, Roederer M, Herzenberg L A. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sperk G. Prog Neurobiol. 1994;42:1–32. doi: 10.1016/0301-0082(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 29.Saido T C, Yokota M, Nagao S, Yamaura I, Tani E, Tsuchiya T, Suzuki K, Kawashima S. J Biol Chem. 1993;268:25239–25243. [PubMed] [Google Scholar]
- 30.Trotti D, Danbolt N C, Volterra A. Trends Pharmacol Sci. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 31.Facchinetti F, Dawson V L, Dawson T M. Cell Mol Neurobiol. 1998;18:667–682. doi: 10.1023/A:1020685903186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bruce A, Najm I, Malfroy B, Baudry M. Neurodegeneration. 1992;1:265–271. [Google Scholar]
- 33.Ciani E, Groneng L, Voltattorni M, Rolseth V, Contestabile A, Paulsen R E. Brain Res. 1996;728:1–6. [PubMed] [Google Scholar]
- 34.Scanlon J M, Aizenman E, Reynolds I J. Eur J Pharmacol. 1997;326:67–74. doi: 10.1016/s0014-2999(97)00137-4. [DOI] [PubMed] [Google Scholar]
- 35.Ueda Y, Yokoyama H, Ohyanishiguchi H, Kamada H. Magn Reson Med. 1998;40:491–493. doi: 10.1002/mrm.1910400321. [DOI] [PubMed] [Google Scholar]
- 36.Atlante A, Gagliardi S, Marra E, Calissano P. Neurosci Lett. 1998;245:127–130. doi: 10.1016/s0304-3940(98)00195-5. [DOI] [PubMed] [Google Scholar]
- 37.Murphy M P, Packer M A, Scarlett J L, Martin S W. Gen Pharmacol. 1998;31:179–186. doi: 10.1016/s0306-3623(97)00418-7. [DOI] [PubMed] [Google Scholar]
- 38.Su J H, Deng G, Cotman C W. Brain Res. 1997;774:193–199. doi: 10.1016/s0006-8993(97)81703-9. [DOI] [PubMed] [Google Scholar]
- 39.Smith M A, Harris P L R, Sayre L M, Beckman J S, Perry G. J Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber S S, Tocco G, Najm I, Thompson R F, Baudry M. J Mol Neurosci. 1993;4:149–159. doi: 10.1007/BF02782498. [DOI] [PubMed] [Google Scholar]
- 41.Wang K K, Posmantur R, Nadimpalli R, Nath R, Mohan P, Nixon R A, Talanian R V, Keegan M, Herzog L, Allen H. Arch Biochem Biophys. 1998;356:187–196. doi: 10.1006/abbi.1998.0748. [DOI] [PubMed] [Google Scholar]
- 42.Wang K K, Posmantur R, Nath R, McGinnis K, Whitton M, Talanian R V, Glantz S B, Morrow J S. J Biol Chem. 1998;273:22490–22497. doi: 10.1074/jbc.273.35.22490. [DOI] [PubMed] [Google Scholar]
- 43.Schulz J B, Matthews R T, Jenkins B G, Ferrante R J, Siwek D, Henshaw D R, Cipolloni P B, Mecocci P, Kowall N W, Rosen B R. J Neurosci. 1995;15:8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones P A, Smith R A, Stone T W. Neurosci Lett. 1998;249:75–78. doi: 10.1016/s0304-3940(98)00372-3. [DOI] [PubMed] [Google Scholar]