Abstract
Antigen-specific interactions between B cells and T cells are essential for the generation of an efficient immune response. Since this requires peptide–MHC class II complexes (pMHC-II) on the B cell to interact with TCR on antigen-specific T cells, we have examined the mechanisms regulating the persistence, loss, and secretion of specific pMHC-II complexes on activated B cells. Using a mAb that recognizes specific pMHC-II, we found that activated B cells degrade approximately 50% of pMHC-II every day and release 12% of these pMHC-II from the cell on small membrane vesicles termed exosomes. These exosomes directly stimulate primed, but not naïve, CD4 T cells. Interestingly, engagement of antigen-loaded B cells with specific CD4 T cells stimulates exosome release in a manner that can be mimicked by pMHC-II crosslinking. Biochemical studies revealed that the pMHC-II released on exosomes was previously expressed on the plasma membrane of the B cells, suggesting that regulated exosome release from activated B cells is a mechanism to allow pMHC-II to escape intracellular degradation and decorate secondary lymphoid organs with membrane-associated pMHC-II complexes.
Keywords: antigen presentation, B cell, exosomes, MHC class II
Introduction
Antigen presentation by MHC class II molecules (MHC-II) to CD4 T cells regulates the onset and progression of an immune response. Recognition of antigenic peptide–MHC-II (pMHC-II) complexes by the clonotypic T cell receptor (TCR) initiates an activation cascade in the CD4 T cell, ultimately leading to its proliferation and differentiation into cytokine-secreting effector cells (Davis et al, 1998). These CD4 T cells, in turn, recognize and activate antigen-primed B cells to proliferate and undergo class-switch recombination and somatic hypermutation (Cahalan and Parker, 2005; Vinuesa et al, 2005). Since the specificity of this interaction is defined by the recognition of specific pMHC-II on the B cell by the TCR on the T cell, the availability of pMHC-II in secondary lymphoid tissues is an important issue to address.
For the formation of antigenic pMHC-II, MHC-II αβ dimers need to traffic to pre-lysosomal compartments in the endocytic pathway where antigenic peptides are generated (Watts, 1997). Newly synthesized MHC-II α- and β-chains assemble in the endoplasmic reticulum to form a nine-chain complex composed of three Invariant chain (Ii) molecules and three MHC-II αβ dimers (Hiltbold and Roche, 2002). Signals in the cytosolic tail of Ii guide MHC-II molecules to the endocytic pathway (Bakke and Dobberstein, 1990), where they sort into late endosomes with multivesicular morphology, called multivesicular bodies (MVBs). Curiously, FRET studies have shown that MHC-II interacts with the peptide editor HLA-DM only in the numerous intralumenal vesicles that fill the MVBs, suggesting that these vesicles serve as the sites of antigenic peptide loading onto MHC-II (Zwart et al, 2005). After peptide loading, the intralumenal vesicles can backfuse with the MVB limiting membrane in a process required for their delivery to the cell surface (Kleijmeer et al, 2001).
In apparent conflict with their role in the biogenesis of pMHC-II, MVBs also serve as sorting stations for internalized plasma membrane proteins destined for degradation in lysosomes (Katzmann et al, 2002). The finding that MHC-II can accumulate on intralumenal vesicles of MVB even in cells not expressing Ii suggests that pMHC-II can reach the MVB from the plasma membrane by an Ii-independent mechanism (Simonsen et al, 1997). While it is well known that surface MHC-II complexes recycle through early endocytic compartments (Salamero et al, 1990; Pinet et al, 1995; Pathak et al, 2001), little is known regarding the mechanisms regulating MHC-II trafficking in the late endocytic pathway. Recent studies have shown that MHC-II is a target of ubiquitination (Ohmura-Hoshino et al, 2006; Shin et al, 2006; van Niel et al, 2006b; Matsuki et al, 2007), a post-translational modification that identifies surface receptors destined for sorting into the intralumenal vesicles of the MVBs (Longva et al, 2002). Besides destruction in lysosomes, an alternative fate for the intralumenal vesicles of MVBs is their secretion from the cell after fusion of the limiting membrane of MVBs with the cell surface. The vesicles that are released from the cells by the process of MVB exocytosis are termed exosomes (Denzer et al, 2000a; van Niel et al, 2006a). Therefore, pMHC-II persistence on B cells depends on the balance between their generation in MVBs, expression on the plasma membrane, degradation in lysosomes, and secretion from the cell on MVB-derived exosomes.
APC-derived exosomes are enriched in pMHC-II and co-stimulatory molecules and can function as a source of antigen for activation of naïve T cells by DCs in vitro and in vivo (Zitvogel et al, 1998; Thery et al, 2002; Segura et al, 2005a, 2005b). Interestingly, MHC-II-bearing exosomes have also been identified on the surface of follicular DCs in human tonsils (Denzer et al, 2000b). In addition, the immune-stimulatory capacity of exosomes is being exploited for use in cell-free antitumor vaccines (Kim et al, 2004; Taieb et al, 2005; Mignot et al, 2006). While such studies indirectly suggest that exosomes function in vivo, the precise physiological role of APC exosomes in immunity and the mechanisms that regulate their release from APCs remain elusive.
In this study we have asked whether secretion of pMHC-II complexes on exosomes can be modulated and whether exosome release allows antigenic pMHC-II to escape lysosomal degradation in B cells and remain available to interact with T cells. We found that activated spleen B cells secrete 12% of all specific pMHC-II on exosomes every day. Surprisingly, we found that engagement with antigen-specific CD4 T cells stimulated exosome release from activated B cells, and that simple pMHC-II crosslinking initiated signaling in the B cells that led to exosome release. Finally, we show that the recycled pMHC-II present on exosomes can directly stimulate primed (but not naïve) CD4 T cells, revealing a previously unrecognized feedback loop in the process of T cell/B cell collaboration.
Results
B cells secrete significant amounts of I-Ak-HEL46−61 complexes on exosomes
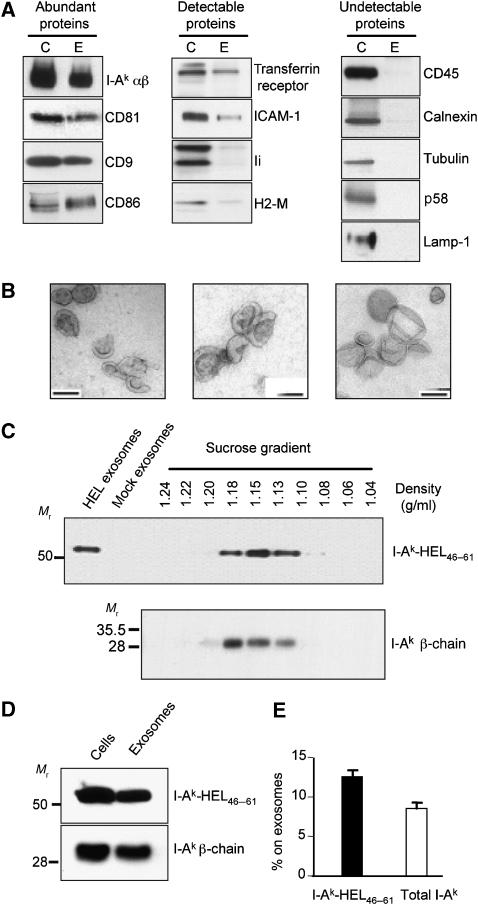
APCs such as activated B cells and mature DCs secrete exosomes that are enriched in MHC-II and CD86 (Raposo et al, 1996; Thery et al, 2002). Since MHC-II-bearing exosomes are present in secondary lymphoid organs (Denzer et al, 2000b), we investigated the source of the MHC-II molecules secreted on B cell-derived exosomes and the mechanisms underlying their release. Exosomes purified from a 24-h culture supernatant of LK35.2 B cells were analyzed for protein content, exosome density, and exosome morphology. Immunoblot analysis revealed that SDS-stable pMHC-II complexes, CD81, CD9, CD86 were relatively abundant in exosomes (Figure 1A). Transferrin receptor, ICAM-1, Ii, and H2-M were detectable, but not abundant, in our exosome preparations. By contrast, B220/CD45, calnexin, tubulin, Golgi p58, and Lamp-1 were not detected in the purified exosome preparations, indicating that our exosome preparations were free of contamination with non-exosome membrane proteins. Electron microscopy demonstrated that the vesicles present in our B cell exosome preparations were cup-shaped and measured approximately 60–100 nm in diameter (Figure 1B). These data confirm that the cellular material isolated from B cell supernatants possess the morphological and biochemical properties of B cell-derived exosomes (Raposo et al, 1996).
Figure 1.
B cells secrete 12% of their I-Ak-HEL46–61 complexes on exosomes in 24 h. (A) Exosomes were isolated from LK35.2 B cells. The exosome-donor B cells (C) were lysed, and the exosomes (E) were concentrated 10-fold relative to the volume of the B cell lysate. Equal proportions of B cell lysate and exosome lysate were analyzed by SDS–PAGE and immunoblotting to detect the indicated proteins. pMHC-II complexes were detected by incubating lysates with SDS–PAGE sample buffer at room temperature. The proteins analyzed were sorted into the following three categories: (i) those relatively abundant in exosomes, (ii) those detectable at low levels in exosomes, and (iii) those undetectable in exosomes. (B) Electron microscopic analysis of exosome preparations obtained by differential centrifugation from supernatants of LK35.2 cells. Bars, 100 nm. (C) LK35.2 cells were cultured in medium alone or medium containing 1 mg/ml HEL protein for 24 h. Equivalent portions of exosomes isolated from cells incubated with HEL (HEL-exosomes) or in medium alone (mock exosomes) as well as aliquots of each sucrose density gradient centrifugation fraction were analyzed by SDS–PAGE under non-boiling conditions and immunoblot analysis using mAb C4H3 to detect SDS-stable I-Ak-HEL46–61 complexes. The density of each fraction was determined using a refractometer and is shown above each fraction. (D, E) The exosome pellet obtained after differential centrifugation was concentrated 10-fold relative to that of the B cell lysate. The proportion of I-Ak-HEL46−61 complexes (determined by immunoblotting non-boiled samples with mAb C4H3) or total I-Ak β-chain (determined by immunoblotting boiled samples with an anti-I-Ak β-chain antibody) secreted on exosomes was expressed as a percentage of the total amount of immunoreactivity recovered on exosomes and cells. The graph shows the average±s.d. of data from five independent experiments.
To address the extent to which specific pMHC-II generated in APCs can be released on exosomes after antigen exposure has ceased, we followed the fate of I-Ak-HEL46−61 complexes generated in living cells using the pMHC-II-specific mAb C4H3 (Zhong et al, 1997; Reis e Sousa and Germain, 1999). B cells were pulsed with intact HEL protein, washed extensively, and exosomes released during a subsequent 24 h chase in HEL-free medium were isolated. No C4H3 immunoreactivity was observed in exosomes isolated from B cells that were not pulsed with HEL (Figure 1C, mock exosomes), confirming the specificity of C4H3 for I-Ak-HEL46−61 complexes. Sucrose density gradient centrifugation demonstrated that I-Ak-HEL46−61 complexes, like the bulk of exosome-associated MHC-II, migrated uniformly with a density of 1.15 g/ml, a value that is in excellent agreement with the density of human B cell exosomes (Raposo et al, 1996). Interestingly, quantitative immunoblot analysis revealed that 12% of all I-Ak-HEL46−61 complexes generated by the B cells were secreted on exosomes in only 24 h (Figure 1D and E), demonstrating that HEL-pulsed B cells secrete a significant amount of I-Ak-HEL46−61 complexes on exosomal membranes every day.
Appearance of I-Ak-HEL46−61 complexes on exosomes correlates with their disappearance from B cells
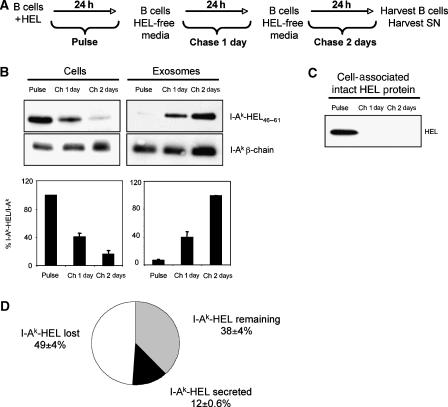
Since specific I-Ak-HEL46−61 complexes were being released from HEL-pulsed B cells, we examined the relationship between the persistence of I-Ak-HEL46−61 complexes in these cells and the kinetics of secretion of I-Ak-HEL46−61 complexes on exosomes. B cells were pulse-labeled with HEL, washed, and chased for either 1 or 2 days in exosome-free medium. At each time point, cell pellets were frozen and exosomes were isolated (Figure 2A). The maximum expression of I-Ak-HEL46−61 complexes on B cells occurred after the initial pulse exposure to HEL and quickly decreased over the next 2 days of chase in HEL/exosome-free medium. By contrast, little I-Ak-HEL46−61 complexes were found on exosomes isolated during the HEL pulse, but increased dramatically during the 2 days of chase (Figure 2B). It is noteworthy that intact HEL protein was only detected in the B cell lysate after the pulse and there was no intact HEL protein present on cells after 1 day of chase in HEL/exosome-free medium (Figure 2C), demonstrating that the I-Ak-HEL46−61 complexes present on exosomes obtained during the second day of chase were derived from B cells that did not possess detectable intact HEL protein. Although we observed a reciprocal trend on the loss of I-Ak-HEL46−61 complexes on donor B cells and their increase on exosomes, the amount of I-Ak-HEL46−61 complexes released on exosomes could not account for the total loss of these complexes from the B cell. The total loss of I-Ak-HEL46−61 complexes from B cells between the pulse and the chase 1 day time point was 61%, and during this time only 12% of the initial amount of I-Ak-HEL46−61 complexes present on the cell was released on exosomes (Figure 2D). We could not account for 49% of the initial amount of I-Ak-HEL46−61 complexes present in the cell, most likely due to their degradation in lysosomes or dissociation of HEL46−61 from I-Ak-HEL46−61 complexes. This observation demonstrates that secretion on exosomes allows a significant amount of specific pMHC-II to escape lysosomal degradation or disappearance from B cells.
Figure 2.
Appearance of I-Ak-HEL46−61 complexes on exosomes correlates with their disappearance from B cells. (A) To analyze the kinetics of appearance of I-Ak-HEL46−61 complexes on B cells and B cell-derived exosomes, LK35.2 B cells were incubated with 1 mg/ml HEL protein for 24 h before the cell supernatant (and an aliquot of the cells) was harvested (time=Pulse). The remaining cells were washed, replated in HEL/exosome-free medium, and after 1 day the supernatant (and an aliquot of the cells) was harvested (time=Chase 1 day). This process was repeated at 2 days post-pulse (time=Chase 2 day) and exosomes were purified by differential centrifugation. In each condition, the exosomes were concentrated 10-fold relative to the volume of the cell lysate. (B, C) Equal amounts of B cell lysate (B, C) or B cell-derived exosomes (B) were analyzed by immunoblot analysis for intact HEL protein, I-Ak-HEL46−61 complexes (using mAb C4H3), or total I-Ak (using a rabbit anti-I-Ak β-chain serum). The intensity of each band was determined by densitometry and the ratio of I-Ak-HEL46−61 complexes to total I-Ak β-chain was calculated for each time point (in arbitrary units). This value was expressed relative to the value of the Pulse sample (for cells), and to the value of the Chase 2 day sample (for exosomes). (D) The total recovery of I-Ak-HEL46−61 complexes from B cells between the Pulse and the Chase 1 day time point was quantified to be only 38%, and during this time 12% of the initial amount of I-Ak-HEL46−61 complexes present on the cell was released on exosomes. The data represent quantitative analysis of data (average±s.d.) obtained from three independent experiments.
MHC-II molecules access the exosome pathway from the cell surface
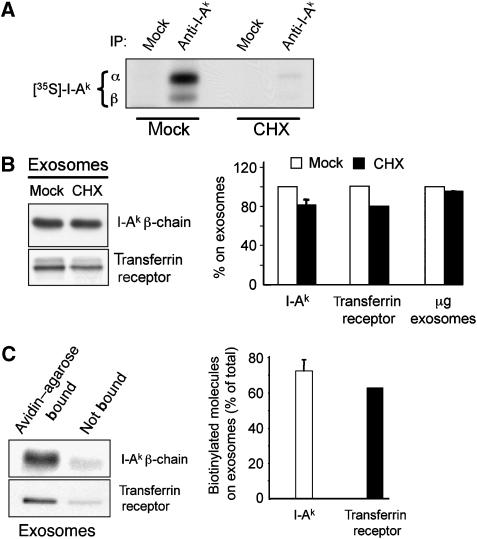
Since we observed a kinetic delay in the appearance of I-Ak-HEL46−61 complexes on exosomes compared with their generation in the host cells, we asked whether pMHC-II secreted on exosomes represented newly generated pMHC-II complexes or pMHC-II recycled from the plasma membrane. While treatment of B cells with the protein synthesis inhibitor cycloheximide blocked MHC-II protein synthesis by >95% (Figure 3A), this treatment did not significant affect the amount of either MHC-II or transferrin receptor released on exosomes (Figure 3B), demonstrating that newly synthesized MHC-II are not likely to be immediately targeted for release on exosomes.
Figure 3.
Class II molecules access the exosome pathway from the cell surface. (A) LK35.2 B cells were mock treated or treated with cycloheximide (CHX) during a 1 h pulse label with [35S]methionine at 37°C. I-Ak molecules were isolated from cell lysates by immunoprecipitation and analyzed by reducing SDS–PAGE and autoradiography. (B) Exosomes were isolated from LK35.2 B cells that were mock treated or incubated in the presence of CHX for 4 h. Equivalent proportions of exosomes from each condition were analyzed by immunoblot analysis to detect total I-Ak β-chain and transferrin receptor. The amount of I-Ak and transferrin receptor present in each exosome preparation was determined by quantitative densitometry of immunoblots. The data represents results (average±s.d.) from three independent experiments. The total amount of protein present in each exosome preparation was also determined. (C) LK35.2 B cells were biotinylated on ice as described in Materials and methods. Viable cells were isolated and equivalent aliquots of cells were incubated at 37°C in exosome-free medium. Exosomes were isolated from the cell supernatants after 4 h of culture and biotinylated proteins were isolated on streptavidin–agarose beads (non-biotinylated proteins were present in the non-bound streptavidin–agarose supernatant). Aliquots of material bound to streptavidin—agarose, as well as the non-bound supernatant were analyzed by SDS–PAGE and immunoblotting with anti-I-Ak β-chain or anti-transferrin receptor antibodies. The amount of biotinylated MHC-II or transferrin receptor present on exosomes was expressed as a percentage of the total amount of MHC-II or transferrin receptor present in the sample (total amount equals the amount bound to streptavidin–agarose beads plus the amount present in non-bound supernatant). The graph shows the average±s.d. from data obtained from two independent experiments.
In addition to serving as compartments for peptide loading onto MHC-II, MVBs act as endocytic sorting stations for molecules internalized from the plasma membrane (Raiborg et al, 2003). Since newly synthesized MHC-II did not appear to contribute significantly to the MHC-II pool released on exosomes and our initial results demonstrated that half of the surface pool of MHC-II was lost each day, we next examined whether surface MHC-II could enter the MVB pathway for secretion on exosomes. B cells were biotinylated on ice, washed, and exosomes collected during a 4-h ‘chase' in medium at 37°C. Greater than 70% of all I-Ak molecules present on exosomes isolated during this chase bound to avidin–agarose beads, revealing that they were derived from the cell surface pool of MHC-II (Figure 3C). A very similar result was observed when we used this assay to examine the trafficking of the transferrin receptor, a molecule that has been shown to enter to the exosome pathway from the cell surface (Vidal et al, 1997). Biotinylation of the pool of Ii-associated MHC-II that transiently exists on the plasma membrane prior to endocytosis was not responsible for this result, since B cells derived from Ii-deficient mice secreted wild-type amounts of exosomes (data not shown). These data demonstrate that the majority of MHC-II on exosomes were expressed on the plasma membrane and were not merely sorted into intralumenal vesicles of MVB immediately after peptide loading onto newly synthesized MHC-II.
Exosome secretion increases upon B cell activation
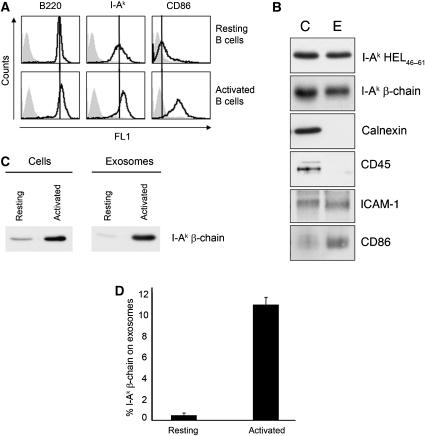
All of the experiments presented thus far examined exosome release from an activated B cell lymphoma cell line. To assess the physiological relevance of the secretion of pMHC-II on exosomes and to determine if the relative amount of MHC-II released on exosomes varies during the process of B cell activation, we examined the secretion of pMHC-II on exosomes derived from resting and anti-IgM/CD40-activated B cells. The activation status of spleen B cells was confirmed by flow cytometry (Figure 4A), and the exosomes derived from activated spleen B cells were found to be biochemically indistinguishable from exosomes isolated from the activated LK35.2 B cell line (compare Figure 1A and Figure 4B). Resting spleen B cells release very small amounts of their total pool of MHC-II on exosomes. By contrast, activation with anti-IgM/CD40 resulted in the release of 11% of all I-Ak from the cells in 48 h (Figure 4C and D). Release of specific pMHC-II on exosomes was examined using HEL pulsed activated B cells. Like the secretion of all MHC-II on exosomes, approximately 12% of all I-Ak-HEL46−61 complexes were released from fully activated primary B cells in 48 h (Figure 4B). The increase in MHC-II release upon B cell activation was a consequence of augmented exosome release and not merely enhanced efficiency of MHC-II transport to intralumenal vesicles of MVB, since the increase in MHC-II secretion upon B cell activation mirrored the increase in total protein present in the exosome preparations from resting and activated B cells (data not shown). These results reveal activation-dependent exosome release from primary B cells and demonstrate that a significant proportion of pMHC-II is secreted from activated primary B cells.
Figure 4.
Activated spleen B cells preferentially secrete MHC-II-bearing exosomes. Resting spleen B cells were incubated with 1 mg/ml HEL protein overnight and aliquots of the culture were maintained in medium alone (resting) or stimulated with anti-IgM/anti-CD40 (activated). After 48 h the cells and culture supernatants were harvested. (A) B220, I-Ak, and CD86 expression on cells was monitored by flow cytometry to confirm the activation status of the cultures. (B) An aliquot of the activated cell culture and a 10-fold concentrated aliquot of the exosomes isolated from this culture were analyzed by immunoblot analysis for I-Ak-HEL46−61 complexes, total I-Ak β-chain, calnexin, CD45, ICAM-1, and CD86. (C, D) Exosomes were isolated from 48-h culture supernatants of resting or activated B cells. Aliquots containing five times more exosome lysate than cell lysate were analyzed by immunoblotting using an anti-I-Ak antibody. The percentage of total I-Ak β-chain released on exosomes isolated from these cells was calculated by densitometric analysis of the blots. The graph shows the average±s.d. from data obtained from two independent experiments.
Antigen-specific T cells induce exosome secretion from primed B cells
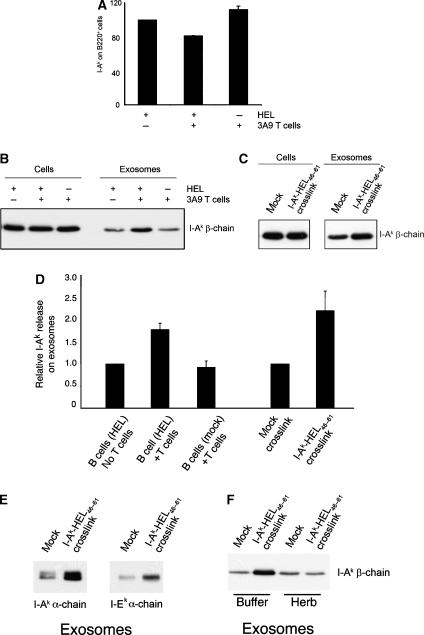
Mature B cell activation is accomplished following the encounter of antigen-primed B cells with antigen-specific T cells (Cahalan and Parker, 2005; Vinuesa et al, 2005). To determine if the interaction with antigen-specific T cells affects exosome release from activated B cells, we incubated HEL-pulsed or mock-treated activated spleen B cells with CD4 T cells isolated from 3A9 mice for 24 h and then harvested the cells and exosomes from each culture. T cells from the 3A9 mouse express a clonotypic TCR that recognizes I-Ak-HEL46−61 complexes, the very same pMHC-II recognized by the C4H3 pMHC-II-specific mAb used in our studies. Phenotypic changes of I-Ak levels on B220+ B cells were monitored by flow cytometry and quantitated (Figure 5A). Co-culture with 3A9 T cells did not alter surface expression of MHC-II on mock-treated B cells (Figure 5A) and had no effect on the release of MHC-II on exosomes from these B cells (Figure 5B). When HEL-pulsed B cells were used in the assay we observed a modest (20%) reduction in surface MHC-II on the B cells (Figure 5A). Remarkably, incubation of antigen-loaded B cells with 3A9 T cells resulted in a two-fold increase in the amount of MHC-II secreted from the B cell on exosomes (Figure 5B and D).
Figure 5.
T cells induce exosome release from activated B cells. (A, B, D) Anti-CD40/IgM-activated spleen B cells were incubated with or without HEL protein, washed, and cultured either alone or in the presence of equal numbers of 3A9 CD4 T cells. After 24 h the cultured cells and culture supernatants were harvested and exosomes were isolated by differential centrifugation. (A) I-Ak expression on the B220+ cells present in each culture was monitored by FACS analysis. (B) Aliquots containing five-fold concentrated exosome lysate than cell lysate were analyzed by immunoblotting for total MHC-II using an anti-I-Ak β-chain antibody. The results shown are representative of three independent experiments. (C) Activated spleen B cells were incubated with HEL protein, washed, and replated in medium containing (or not) the anti-I-Ak-HEL46−61 mAb C4H3. Culture supernatants were harvested after 48 h and exosomes were isolated by differential centrifugation. Aliquots containing exosome lysate five-fold concentrated than the cell lysate were analyzed by immunoblotting using an anti-I-Ak β-chain antibody. (D) The relative amount of I-Ak recovered on exosomes under the conditions described in panel B (normalized to HEL-pulsed B cells cultured alone; data from two independent experiments) or in panel C (normalized to mock-crosslinked cells; data from three independent experiments) was determined by densitometry. (E) Activated spleen B cells were incubated with HEL protein and crosslinked with mAb C4H3 as described in panel C, and analyzed by immunoblotting for different isoforms of MHC-II using either anti I-Ak α-chain or anti I-Ek α-chain antibody. (F) Activated spleen B cells were incubated with HEL protein and crosslinked with mAb C4H3 in the absence (buffer) or presence of 10 μg/ml herbimycin (Herb) in the culture and analyzed by immunoblotting for total MHC-II as described in panel C.
Since T cell-induced exosome release from B cells was dependent upon the presence of appropriate pMHC-II, we investigated whether engagement of I-Ak-HEL46−61 complexes on the B cell would stimulate exosome release. Crosslinking these complexes on activated B cells using mAb C4H3 resulted in a two-fold increase in the MHC-II released on exosomes (Figure 5C and D). Notably, this increase was not observed in the absence of MHC-II crosslinking (Figure 5C, mock), or when irrelevant mAb (anti-B220) were incubated with the cells (data not shown). Surprisingly, we found that even I-Ek molecules were released on exosomes after I-Ak-HEL46−61 crosslinking (Figure 5E), demonstrating that MHC-II crosslinking led to a global release of MHC-II from intralumenal vesicles and was not specific to the crosslinked MHC-II molecules itself. Furthermore, the tyrosine kinase inhibitor herbimycin was able to completely block the MHC-II crosslinking-induced stimulation of exosome release from the activated B cells, but had no effect on basal exosome release (Figure 5F). These data demonstrate that engagement of specific pMHC-II on the surface of activated B cells by the TCR of antigen-specific T cells stimulates the release of MHC-II-bearing exosomes and strongly suggests that MHC-II crosslinking stimulates signaling in the B cell that leads to the release of MHC-II-bearing exosomes.
B cell exosomes activate primed CD4 T cells
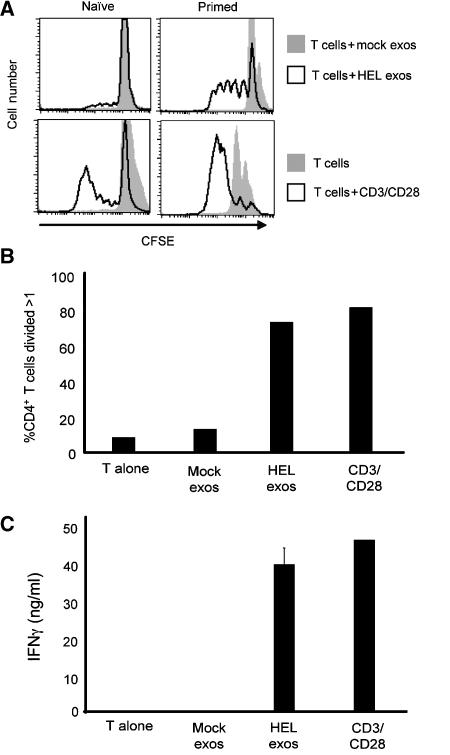
Primed antigen-specific CD4 T cells engaged in T cell/B cell collaboration possess a relatively low activation threshold (Vinuesa et al, 2005). Since pMHC-II-bearing exosomes alone do not efficiently activate naïve CD4 T cells (Thery et al, 2002; Segura et al, 2005a), we considered the possibility that exosomes alone might stimulate T cells with a low activation threshold. Naïve and primed CD4 T cells were screened as targets of exosome function, since they represent two types of T cells with different thresholds for activation. Naïve or primed 3A9 CD4 T cells were labeled with CFSE and incubated in the presence of exosomes derived from LK35.2 B cells or activated spleen B cells cultured in medium alone (mock exosomes) or previously pulsed in medium containing HEL protein (HEL-exosomes). HEL-exosomes were unable to activate naïve 3A9 CD4 T cells, but were able to stimulate primed 3A9 T cells to undergo several cycles of cell division (Figure 6A and B). Furthermore, exosomes isolated from HEL-pulsed activated spleen B cells stimulated primed CD4 T cells to differentiate into interferon-γ-secreting effector cells (Figure 6C), a process that requires continuous antigenic stimulation (Gudmundsdottir et al, 1999; Bajenoff et al, 2002). Exosomes isolated from HEL-pulsed resting B cells were very poor stimulators of primed 3A9 T cells and the limited T cell proliferation observed was likely due to the presence of exosomes secreted from small amounts of activated B cells present in the culture (Supplementary Figure 1, and data not shown). Similar results were obtained when we examined the activation of AND T cells by exosomes derived from activated B cells pulsed with pigeon cytochrome C, demonstrating that exosomes possess the MHC-II and antigenic peptide required for primed CD4 T cell activation (Supplementary data; Supplementary Figure 2). These data confirm that B cell-derived exosomes can directly provide the specific pMHC-II complexes required for proliferation and cytokine secretion from CD4 T cells that possess a low activation threshold.
Figure 6.
B cell-derived exosomes activate primed CD4 T cells. (A) A 1-μg weight of exosomes isolated from LK35.2 B cells incubated in medium alone (mock-exosomes) or medium containing 1 mg/ml of HEL protein (HEL-exosomes) was incubated with either naïve or primed CFSE-labeled 3A9 CD4 T cells. CFSE dilution was examined 72 h later by FACS analysis. Addition of T cells to anti-CD3/CD28-coated plates confirmed that each T cell population was capable of stimulation. (B) CFSE-labeled primed 3A9 CD4 T cells were incubated alone, together with exosomes obtained from activated spleen B cells cultured in medium alone (mock exosomes) or medium containing 1 mg/ml HEL (HEL exosomes), or on anti-CD3/CD28-coated plates. The percentage of CD4 T cells that had divided more than once in 48 h was determined by measuring CFSE dilution by FACS. (C) Supernatants were harvested from each culture from (B) and each was assayed to determine the amount of IFNγ released from the T cells.
Discussion
There is little information available regarding the fate of mature pMHC-II on the surface of B cells upon activation. While it is clear that pMHC-II efficiently recycle through the early endocytic pathway from the plasma membrane (Salamero et al, 1990; Pinet et al, 1995; Pathak et al, 2001), the half-life of antigenic pMHC-II complexes or the mechanisms by which these complexes persist or disappear from the B cell surface have remained largely undetermined. Since CD4 T cells require constant stimulation of their TCR to divide and differentiate into cytokine-secreting effector cells that assist in T cell-dependent B cell activation (Bird et al, 1998; Bajenoff et al, 2002; Schrum and Turka, 2002; Obst et al, 2005), identifying the mechanisms regulating persistence of mature pMHC-II is critical for a complete understanding of the factors that influence the development of an immune response.
Our observation that nearly half of all pMHC-II complexes disappear from an activated B cell in 1 day was unexpected. Previous studies have shown that more than half of the total pool of newly synthesized MHC-II is lost from B cells in one day (Germain and Hendrix, 1991), however this result was attributed to the selective degradation or shedding of newly synthesized MHC-II that failed to bind antigenic peptide. The kinetics of disappearance of total MHC-II and specific I-Ak-HEL46−61 complexes from the surface of activated B cells triggered our interest to analyze pMHC-II complex secretion on APC-derived exosomes. We have shown that a significant proportion of an antigenic pMHC-II is secreted on exosomes derived from B cells, and that ‘basal' exosome secretion is modulated by B cell activation. Most significantly, we have found that exosome release can be stimulated by the encounter of antigen-loaded B cells with antigen-specific CD4 T cells. While it is intriguing to speculate that T cell-induced secretion of antigenic pMHC-II on exosomes is a mechanism to allow those functionally relevant complexes to escape degradation in the APC, the fact that we observe I-E release upon I-A crosslinking indicates that the induced exocytosis affects the entire pool of surface pMHC-II. Such a mechanism could allow the release of pMHC-II containing different epitopes derived from the same antigen.
It has been reported that MHC-II αβ dimers interact with the peptide editor H2-M only on the intralumenal vesicles of MVB, suggesting that intralumenal vesicles serve as the site of binding of antigenic peptides to newly synthesized MHC-II (Zwart et al, 2005). Since intralumenal vesicles of MVB are thought to be the organelles that give rise to exosomes (Raposo et al, 1996; Denzer et al, 2000a), we had expected that newly synthesized MHC-II would be the primary source of MHC-II present on exosomes. The preferential secretion of surface-exposed/recycling versus recently loaded pMHC-II on exosomes could be explained if pMHC-II internalized from the cell surface remain exclusively on the intralumenal vesicles of the MVB, while recently loaded MHC-II are rapidly retrieved by back-fusion of the intralumenal vesicle with the MVB limiting membrane (Kleijmeer et al, 2001). Recent studies have shown that surface MHC-II expression in DCs is regulated by ubiquitination (Shin et al, 2006; van Niel et al, 2006b; Matsuki et al, 2007), and that Ii-associated MHC-II is not a substrate for ubiquitination (van Niel et al, 2006b), the extent to which ubiquitination regulates MHC-II sorting into the intralumenal vesicle of MVB is still a matter of dispute. Since ubiquitination of many proteins regulates their sorting into the intralumenal vesicles of MVB (Longva et al, 2002), such a finding could provide a mechanism to discriminate between pMHC-II (ubiquitinated) and MHC-II–Ii complex (non-ubiquitinated) sorting into MVBs. It is also possible that distinct subpopulations of MVBs exist in cells, with one population being enriched in recycling pMHC-II and another population being enriched in newly generated pMHC-II. Support for this latter hypothesis comes from data describing the existence of distinct MVB subtypes that share morphological appearance but differ in their protein and lipid composition (White et al, 2006), and from the fact that lipids that preferentially accumulate in intralumenal vesicles of some MVBs are absent on exosomes released from B cells (Mobius et al, 2003; Wubbolts et al, 2003). It is possible that both MVB subpopulations could coexist in B cells, or that one or the other could preferentially develop depending on the differentiation stage and/or trigger received by the B cell.
The fact that T cell-induced secretion of B cell exosomes can be mimicked by crosslinking of pMHC-II, that different alleles of MHC-II are released on these exosomes, and that herbimycin inhibits stimulated exosome release strongly argues that signaling through MHC-II molecules is the trigger for regulated exosome release from B cells. BCR crosslinking increases the number of MVBs present in B cells (Lankar et al, 2002) and we have found that B cell activation dramatically augments the release of MHC-II-bearing exosomes. The data presented here demonstrate that pMHC-II crosslinking is also a trigger for remodeling the exocytic pathway of the B cell, however in this case the consequence of MHC-II signaling is the release of exosomes from the intralumenal vesicles of the MVBs. To our knowledge, this is the first evidence for an immunological consequence for the well-documented phenomenon of MHC-II signaling. It is interesting to note that exosome release can also be stimulated by crosslinking FcɛRI on mast cells (Vincent-Schneider et al, 2002), highlighting potential similarities between ‘receptor' crosslinking and signaling-dependent exosome release from B cells and mast cells. While our herbimycin experiments suggest that stimulated release of MHC-II on exosomes is dependent on signaling within the B cell, the precise signaling machinery leading to secretion of exosomes from B cells remains to be determined. It is interesting to note that Igα and Igβ, the heterodimer responsible for signal transduction through the BCR, has been reported to associate with MHC-II after B cell priming (Lang et al, 2001), leading to the possibility that signaling through Igα/β, after MHC-II ligation, could modulate the endocytic pathway and exosome release from B cells.
Although exosomes are released from many different cell types (Denzer et al, 2000a; van Niel et al, 2006a), are abundant in tissues (Hoorn et al, 2005; Taylor et al, 2006), are present on germinal center DCs (Denzer et al, 2000b), and are capable of inducing tumor rejection in vivo (Zitvogel et al, 1998), the physiological function of APC-derived exosomes remains unknown. However, our data showing the preferential activation of primed T cells by B cell-derived exosomes can give us a hint to their immunological relevance. DCs are the primary initiators of naïve T cell activation (Banchereau and Steinman, 1998) and for this reason it is unlikely that B cell-derived exosomes play an important role in this earliest stage of the immune response. However, the fact that pMHC-II on exosomes secreted from activated B cells efficiently stimulate primed antigen-specific T cells, suggests a role for B cell-derived exosomes as modulators of an ongoing immune response or maintaining antigen-specific memory T cells.
Materials and methods
Cells, antibodies, and reagents
Murine LK35.2 B cells (IgG2a; H2k,d; Kappler et al, 1982) were maintained in RPMI-1640 containing 10% fetal bovine serum (FBS), 50 μM β-mercaptoethanol, 10 mM HEPES, and 50 μg/ml gentamycin at 37°C. Spleen B cells and lymph-node CD4 T cells were cultured in RPMI-1640 containing 10% FBS, 10 mM sodium pyruvate, 100 mM non-essential amino acids, 50 μM β-mercaptoethanol, 10 mM HEPES, and 100 μg/ml penicillin/streptomycin. The name, source, and clone number of the various antibodies used in this study are listed in the Supplementary data. Streptavidin-PE-Cy5 and CFSE were purchased from Molecular Probes (Eugene, OR). HEL and PCC proteins were purchased from Sigma-Aldrich (St Louis, MO). Recombinant BAFF was purchased from Alexis Biochemicals (San Diego, CA).
Mice
3A9 TCR transgenic mice (Ho et al, 1994) and B10.BR mice were obtained from The Jackson Laboratory (Bar Harbor, ME). AND TCR transgenic mice on a C57BL/6 background (Kaye et al, 1989) were maintained by breeding transgene-positive mice with C57BL/6 mice in our colony.
Exosome isolation
Tissue culture supernatants from LK35.2 B cells growing in log phase for 24 h were harvested, centrifuged at 10 000 g to remove cellular debris, and exosomes isolated by subjecting the 10 000 g supernatant to centrifugation at 100 000 g. The exosome pellet was washed once in a large volume of PBS. To avoid contamination with bovine exosomes, FBS was subjected to centrifugation overnight at 100 000 g at 4°C. The total protein content of exosomes was determined by BioRad protein assay (BioRad Laboratories). For additional exosome purification, the 100 000 g exosome pellet was subjected to sucrose density centrifugation. Spleen B cells from B10.BR mice were isolated by negative selection on magnetic beads (Miltenyi Biotech, Auburn, CA). Resting B cells were activated by culturing the cells overnight in media containing anti-CD40 and BAFF. The next morning cells were pulsed with anti-IgM F(ab)2 for 5 h, washed, and placed back in culture in exosome-free media containing BAFF for 24 or 48 h as indicated in each experiment. Exosomes from the culture supernatant were isolated by differential centrifugation as described above and in the Supplementary data.
Electron microscopy
Exosome droplets were directly air-dried on thin carbon film-coated grids and fixed with 2% glutaraldehyde in PBS. Grids were contrasted and embedded in uranyl oxalate (pH 7) for 5 min and transferred to drops of methylcellulose uranyl acetate for 10 min on ice. The samples were dried and examined with a JEOL JEM-1010 transmission electron microscope.
Immunoprecipitation and immunoblotting
Exosomes and cells were lysed on ice for 1 h in 10 mM Tris–HCl/150 mM NaCl, pH 7.4, containing 1% Triton X-100, and protease inhibitors (500 μM PMSF, 100 μM TLCK, 5 mM iodoacetamide, 10 μg/ml aprotinin, 5 μg/ml leupeptin). Proportional fractions of the different preparations were analyzed on 10.5% SDS–PAGE, transferred to PVDF membranes (Bio-Rad, Hercules, CA), and immunoblotted with primary rabbit, mouse, or rat antibodies as described previously (Poloso et al, 2004). Blots were developed with HRP-conjugated anti-mouse IgG, anti-rabbit IgG, or anti-rat IgG (Southern Biotechnology Associates, Birmingham, AL), followed by ECL (Perkin Elmer, Boston, MA). Cells were radiolabeled with [35S]methionine in the presence or absence of cycloheximide, as described in the Supplementary data.
Biotinylation of cell surface proteins
Cell surface proteins were tagged with sulfo-NHS-LC-biotin (Pierce, Rockford, IL) in HBSS buffer for 30 min on ice. Live cells were isolated on a Lympholyte cushion (Cedarlane Laboratories, Burlington, NC), cultured in complete medium at 37°C for various times, and cells/cell supernatants harvested for exosome isolation. Biotinylated proteins were isolated using streptavidin–agarose beads and the proportion of biotinylated (bound) and non-biotinylated (non-bound) proteins present in the exosomes was determined as described in the Supplementary data.
CD4 T cell isolation and CFSE labeling
Lymph node CD4 T cells were isolated from 3A9- or AND-TCR transgenic mice by negative selection using a CD4 T cell purification kit and AutoMacs system (Miltenyi Biotec). To prime T cells, ≈2 × 106 naïve CD4 T cells were cultured on immobilized anti-CD3ɛ mAb (145-2C11; 5 μg/ml) and anti-CD28 mAb (37.51; 1 μg/ml), in the presence of 20 U/ml recombinant IL-2 for 48 h. The cells were then transferred to uncoated plates containing fresh media for 5 days to rest. Naïve and primed T cells (107 cells/ml) were labeled with CFSE (5 μM) in PBS for 8 min at room temperature and washed twice in fresh medium before use.
T cell activation
T cell proliferation was analyzed by three-color FACS analysis using CD4 and IG12 (TCR clonotype; Peterson et al, 1999) mAbs for 3A9 T cells and CD4, and TCR Vβ3 mAb for AND T cells. A 1-μg weight of HEL-exosomes or 5 μg of PCC-exosomes diluted in 20 μl PBS were pre-incubated in 96-well round-bottom plates overnight at 4°C. The next morning, 4 × 105 CFSE-labeled CD4 T cells were added to each well in a final volume of 200 μl. The percentage of cells undergoing CFSE dilution was assessed by flow cytometry. Release of IFNγ was assessed by sandwich ELISA (R&D-Systems Inc.; Minneapolis, MN).
T cell culture with B cells and MHC-II crosslinking
Spleen B cells were purified, pulsed overnight with or without 1 mg/ml HEL protein, and activated as described above. Recovered cells were replated at a 1:1 ratio with naïve 3A9 CD4 T cells. After 24 h the cells and co-culture supernatants were harvested. An aliquot of the co-culture was stained with mAb to monitor I-Ak expression on the surface of B220+ cells by FACS analysis. The exosome pellets from each co-culture was purified by differential centrifugation and analyzed as described above. For MHC-II crosslinking studies, the B cells were incubated in the presence or absence of 1 μg/ml rat mAb C4H3 and 10 μg/ml of goat anti-rat F(ab)2 at 37°C. After 48 h, culture supernatants were harvested and exosomes were purified by differential centrifugation.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary data
Acknowledgments
We thank Richard Hodes and Al Singer for many helpful discussions and critical reading of this paper, Bob Wenthold and Ron Petralia for assistance in our Electron microscopy studies, and Karis Faust for technical assistance in exosome characterization. We thank Ron Germain, Melanie Vacchio, Lisa Denzin, who have provided various reagents necessary to carry out this study. This research was supported by the Intramural Research Program of the National Institutes of Health, NIH.
References
- Bajenoff M, Wurtz O, Guerder S (2002) Repeated antigen exposure is necessary for the differentiation, but not the initial proliferation, of naive CD4(+) T cells. J Immunol 168: 1723–1729 [DOI] [PubMed] [Google Scholar]
- Bakke O, Dobberstein B (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell 63: 707–716 [DOI] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM (1998) Dendritic cells and the control of immunity. Nature 392: 245–252 [DOI] [PubMed] [Google Scholar]
- Bird JJ, Brown DR, Mullen AC, Moskowitz NH, Mahowald MA, Sider JR, Gajewski TF, Wang CR, Reiner SL (1998) Helper T cell differentiation is controlled by the cell cycle. Immunity 9: 229–237 [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Parker I (2005) Close encounters of the first and second kind: T–DC and T–B interactions in the lymph node. Semin Immunol 17: 442–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Boniface JJ, Reich Z, Lyons D, Hampl J, Arden B, Chien Y (1998) Ligand recognition by alpha beta T cell receptors. Annu Rev Immunol 16: 523–544 [DOI] [PubMed] [Google Scholar]
- Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ (2000a) Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J Cell Sci 113 (Pt 19): 3365–3374 [DOI] [PubMed] [Google Scholar]
- Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ (2000b) Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol 165: 1259–1265 [DOI] [PubMed] [Google Scholar]
- Germain RN, Hendrix LR (1991) MHC class II structure, occupancy and surface expression determined by post-endoplasmic reticulum antigen binding. Nature 353: 134–139 [DOI] [PubMed] [Google Scholar]
- Gudmundsdottir H, Wells AD, Turka LA (1999) Dynamics and requirements of T cell clonal expansion in vivo at the single-cell level: effector function is linked to proliferative capacity. J Immunol 162: 5212–5223 [PubMed] [Google Scholar]
- Hiltbold EM, Roche PA (2002) Trafficking of MHC class II molecules in the late secretory pathway. Curr Opin Immunol 14: 30–35 [DOI] [PubMed] [Google Scholar]
- Ho WY, Cooke MP, Goodnow CC, Davis MM (1994) Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med 179: 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoorn EJ, Pisitkun T, Zietse R, Gross P, Frokiaer J, Wang NS, Gonzales PA, Star RA, Knepper MA (2005) Prospects for urinary proteomics: exosomes as a source of urinary biomarkers. Nephrology (Carlton) 10: 283–290 [DOI] [PubMed] [Google Scholar]
- Kappler J, White J, Wegmann D, Mustain E, Marrack P (1982) Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci USA 79: 3604–3607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzmann DJ, Odorizzi G, Emr SD (2002) Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol 3: 893–905 [DOI] [PubMed] [Google Scholar]
- Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM (1989) Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature 341: 746–749 [DOI] [PubMed] [Google Scholar]
- Kim JV, Latouche JB, Riviere I, Sadelain M (2004) The ABCs of artificial antigen presentation. Nat Biotechnol 22: 403–410 [DOI] [PubMed] [Google Scholar]
- Kleijmeer M, Ramm G, Schuurhuis D, Griffith J, Rescigno M, Ricciardi-Castagnoli P, Rudensky AY, Ossendorp F, Melief CJ, Stoorvogel W, Geuze HJ (2001) Reorganization of multivesicular bodies regulates MHC class II antigen presentation by dendritic cells. J Cell Biol 155: 53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Stolpa JC, Freiberg BA, Crawford F, Kappler J, Kupfer A, Cambier JC (2001) TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers. Science 291: 1537–1540 [DOI] [PubMed] [Google Scholar]
- Lankar D, Vincent-Schneider H, Briken V, Yokozeki T, Raposo G, Bonnerot C (2002) Dynamics of major histocompatibility complex class II compartments during B cell receptor-mediated cell activation. J Exp Med 195: 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH (2002) Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol 156: 843–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuki Y, Ohmura-Hoshino M, Goto E, Aoki M, Mito-Yoshida M, Uematsu M, Hasegawa T, Koseki H, Ohara O, Nakayama M, Toyooka K, Matsuoka K, Hotta H, Yamamoto A, Ishido S (2007) Novel regulation of MHC class II function in B cells. EMBO J 26: 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot G, Roux S, Thery C, Segura E, Zitvogel L (2006) Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med 10: 376–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HF, Slot JW, Geuze HJ (2003) Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 4: 222–231 [DOI] [PubMed] [Google Scholar]
- Obst R, van Santen HM, Mathis D, Benoist C (2005) Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med 201: 1555–1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmura-Hoshino M, Matsuki Y, Aoki M, Goto E, Mito M, Uematsu M, Kakiuchi T, Hotta H, Ishido S (2006) Inhibition of MHC class II expression and immune responses by c-MIR. J Immunol 177: 341–354 [DOI] [PubMed] [Google Scholar]
- Pathak SS, Lich JD, Blum JS (2001) Cutting edge: editing of recycling class II:peptide complexes by HLA-DM. J Immunol 167: 632–635 [DOI] [PubMed] [Google Scholar]
- Peterson DA, DiPaolo RJ, Kanagawa O, Unanue ER (1999) Quantitative analysis of the T cell repertoire that escapes negative selection. Immunity 11: 453–462 [DOI] [PubMed] [Google Scholar]
- Pinet V, Vergelli M, Martin R, Bakke O, Long EO (1995) Antigen presentation mediated by recycling of surface HLA-DR molecules. Nature 375: 603–606 [DOI] [PubMed] [Google Scholar]
- Poloso NJ, Muntasell A, Roche PA (2004) MHC class II molecules traffic into lipid rafts during intracellular transport. J Immunol 173: 4539–4546 [DOI] [PubMed] [Google Scholar]
- Raiborg C, Rusten TE, Stenmark H (2003) Protein sorting into multivesicular endosomes. Curr Opin Cell Biol 15: 446–455 [DOI] [PubMed] [Google Scholar]
- Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183: 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis e Sousa C, Germain RN (1999) Analysis of adjuvant function by direct visualization of antigen presentation in vivo: endotoxin promotes accumulation of antigen-bearing dendritic cells in the T cell areas of lymphoid tissue. J Immunol 162: 6552–6561 [PubMed] [Google Scholar]
- Salamero J, Humbert M, Cosson P, Davoust J (1990) Mouse B lymphocyte specific endocytosis and recycling of MHC class II molecules. EMBO J 9: 3489–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrum AG, Turka LA (2002) The proliferative capacity of individual naive CD4(+) T cells is amplified by prolonged T cell antigen receptor triggering. J Exp Med 196: 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura E, Amigorena S, Thery C (2005a) Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis 35: 89–93 [DOI] [PubMed] [Google Scholar]
- Segura E, Nicco C, Lombard B, Veron P, Raposo G, Batteux F, Amigorena S, Thery C (2005b) ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T cell priming. Blood 106: 216–223 [DOI] [PubMed] [Google Scholar]
- Shin JS, Ebersold M, Pypaert M, Delamarre L, Hartley A, Mellman I (2006) Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444: 115–118 [DOI] [PubMed] [Google Scholar]
- Simonsen A, Stang E, Bremnes B, Roe M, Prydz K, Bakke O (1997) Sorting of MHC class II molecules and the associated invariant chain (Ii) in polarized MDCK cells. J Cell Sci 110 (Pt 5): 597–609 [DOI] [PubMed] [Google Scholar]
- Taieb J, Chaput N, Zitvogel L (2005) Dendritic cell-derived exosomes as cell-free peptide-based vaccines. Crit Rev Immunol 25: 215–223 [DOI] [PubMed] [Google Scholar]
- Taylor DD, Akyol S, Gercel-Taylor C (2006) Pregnancy-associated exosomes and their modulation of T cell signaling. J Immunol 176: 1534–1542 [DOI] [PubMed] [Google Scholar]
- Thery C, Duban L, Segura E, Veron P, Lantz O, Amigorena S (2002) Indirect activation of naive CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol 3: 1156–1162 [DOI] [PubMed] [Google Scholar]
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G (2006a) Exosomes: a common pathway for a specialized function. J Biochem (Tokyo) 140: 13–21 [DOI] [PubMed] [Google Scholar]
- van Niel G, Wubbolts R, Ten Broeke T, Buschow SI, Ossendorp FA, Melief CJ, Raposo G, van Balkom BW, Stoorvogel W (2006b) Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25: 885–894 [DOI] [PubMed] [Google Scholar]
- Vidal M, Mangeat P, Hoekstra D (1997) Aggregation reroutes molecules from a recycling to a vesicle-mediated secretion pathway during reticulocyte maturation. J Cell Sci 110 (Pt 16): 1867–1877 [DOI] [PubMed] [Google Scholar]
- Vincent-Schneider H, Stumptner-Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C (2002) Exosomes bearing HLA-DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol 14: 713–722 [DOI] [PubMed] [Google Scholar]
- Vinuesa CG, Tangye SG, Moser B, Mackay CR (2005) Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol 5: 853–865 [DOI] [PubMed] [Google Scholar]
- Watts C (1997) Capture and processing of exogenous antigens for presentation on MHC molecules. Annu Rev Immunol 15: 821–850 [DOI] [PubMed] [Google Scholar]
- White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE (2006) EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 25: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, Slot JW, Geuze HJ, Stoorvogel W (2003) Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. J Biol Chem 278: 10963–10972 [DOI] [PubMed] [Google Scholar]
- Zhong G, Reis e Sousa C, Germain RN (1997) Production, specificity, and functionality of monoclonal antibodies to specific peptide–major histocompatibility complex class II complexes formed by processing of exogenous protein. Proc Natl Acad Sci USA 94: 13856–13861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4: 594–600 [DOI] [PubMed] [Google Scholar]
- Zwart W, Griekspoor A, Kuijl C, Marsman M, van Rheenen J, Janssen H, Calafat J, van Ham M, Janssen L, van Lith M, Jalink K, Neefjes J (2005) Spatial separation of HLA-DM/HLA-DR interactions within MIIC and phagosome-induced immune escape. Immunity 22: 221–233 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary data