Abstract
ATP-gated non-selective cation channels from the rat vas deferens (P2X1 receptors) were stably expressed in HEK 293 cells, assayed by patch clamp on the first day after passage of the culture, and found to have whole-cell current kinetics markedly faster in both activation and desensitization than those found in the native vas deferens tissue, in agreement with previous reports.
By the second day after passage of the culture, however, the whole-cell current kinetics of the expressed receptors shifted, slowing in both activation and desensitization. The kinetic change correlated with a change in phenotype of the host cells from round to flat, and the slower kinetics were similar to native P2X1 currents recorded from dissociated rat vas deferens smooth muscle cells. Two point mutations in a pore-like domain near or within the second transmembrane domain of the P2X1 receptor appeared to confer on the receptor the inability to effect this change in kinetics over time.
Treatment of cells on day 3 after passage with cytochalasins B or D caused a reversion to the rapid kinetics phenotype, implicating the actin cytoskeleton in the development of the native kinetics. P2X1 receptors may therefore require interaction with an intact actin cytoskeleton for native kinetics, and the mutants may be defective either in interaction with the actin skeleton or in coupling the interaction to gating.
ATP-gated non-selective cation channels (P2X receptors) are in many tissues of the body. They are known to serve as neurotransmitter receptors and as sensors of local tissue damage and ischaemia (Burnstock & Wood, 1996). The P2X1 subclass of receptor is found primarily on visceral and vascular smooth muscle where it may mediate changes in smooth muscle tone through sympathetic innervation (Longhurst et al. 1996; Vulchanova et al. 1996), but is also present in large quantity in platelets (Clifford et al. 1998), and can be found in tissue culture cells, for example, rat basophilic leukaemia cells (Vulchanova et al. 1996).
The first reported P2X1 receptor was expression cloned from a rat vas deferens cDNA library (Valera et al. 1994). The cDNA encodes a membrane protein with two putative transmembrane domains and a large putative extracellular domain. The P2X1 variant also contains a sequence either within or immediately preceding the second transmembrane domain that bears a striking similarity to a pore-forming domain from other ion channels, being particularly similar to the P-domain of the voltage-gated potassium channel Kv2.1.
Other P2X receptor classes also contain pore-like sequences at similar positions, with the exception of the P2X7 class, including a conserved GXG motif. Recent results of structure-function experiments reported by Egan et al. (1998) illustrating the participation of the second transmembrane domain in forming the P2X2 pore confirm a similarity to potassium channel pore structure: in potassium channels, one of which has recently been crystallized, the pore loop and an adjacent transmembrane helix from each of four monomers come together to form the lining of the pore (Doyle et al. 1998).
In P2X2, interpreting the results of scanning cysteine mutagenesis requires a topology that strongly resembles a pore loop - the channel protein must loop through the membrane from the outside to an inaccessible region within the membrane, back to the outside at the GXG motif, and then back again across the membrane to the cytoplasmic side (Egan et al. 1998). Several other laboratories are also investigating this topic through chimeras and mutational analyses of the various P2X subclasses (Werner et al. 1996).
Isolated rat vas deferens smooth muscle cells assayed by whole-cell patch clamp develop a desensitizing non-selective inward current at negative holding potentials in response to application of external ATP. The concentration that elicits half-activation is between 1 and 10 μM, with maximal current response between 10 and 30 μM (Nakazawa & Matsuki, 1987; Friel, 1988). The P2X1 receptor cloned from that tissue expressed in heterologous expression systems develops a desensitizing non-selective inward current at negative holding potentials with a half-activating concentration of ATP of about 1 μM. The rate of desensitization of the heterologously expressed channel has been reported to be 50-150 ms, but ATP-evoked currents recorded from Xenopus oocytes expressing P2X1 have half-times of desensitization of up to 5 or 10 s (Evans et al. 1995; Werner et al. 1996). The half-time of desensitization of the channel in the native smooth muscle cell is several seconds (Friel, 1988).
Ion channels can be modulated by many cellular processes and factors, including but not limited to: phosphorylation or dephosphorylation (Drain et al. 1994), assembly with other subunits (Balser et al. 1996), association with G-proteins (Reuveny et al. 1994), and association with components of the cytoskeleton (Johnson & Byerly, 1993). It is therefore not surprising that a heterologously expressed channel should differ in kinetics from its native counterpart, or vary between expression systems. The faster rate of desensitization of the P2X1 receptor in several of the most convenient expression systems has made it more difficult to study permeation and pharmacology, and the results of kinetic experiments on the channel have been difficult to compare with native P2X1 activities.
For this study the P2X1 receptor from rat vas deferens has been stably transfected into human embryonic kidney cells (HEK 293). Cells that show the faster rate of desensitization are easier to find and to assay, but cells can also be obtained that show a much slower, more nearly native rate of desensitization. Evidence is provided that the slower kinetics requires an intact actin cytoskeleton, and mutants in the P-like domain that are unable to develop the slower kinetics have been obtained.
METHODS
P2X1 constructs and cell lines
Dr Gary Buell kindly supplied the rat vas deferens P2X1 clone in the mammalian expression vector pBKCMV and it was recloned into the mammalian expression vector pCDNA3 (Invitrogen Corp., San Diego, CA, USA). Both constructs have been stably transfected into HEK 293 cells and used in all experiments, with identical results. For negative control experiments a cell line has been constructed that is stably transfected with the pCDNA3 vector alone. Overlap-extension polymerase chain reaction (PCR) was used to generate point mutants in P2X1. Mutant P2X1 fragments confirmed by DNA sequencing were spliced back into the wild-type gene in the pCDNA3 vector using standard techniques.
HEK 293 cells were maintained in Dulbecco's minimal essential medium supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin and 10 % iron-supplemented neonatal calf serum (Hyclone, Logan, UT, USA). Cells were transfected using Lipofectamine according to the manufacturer's protocol and selected with 500 μg ml−1 active G418 (Gibco-BRL, Grand Island, NY, USA). The resultant G418-resistant foci (approximately 500 per transfection) were pooled and maintained in 500 μg ml−1 G418. The author has made stable cell lines from the mutant constructs twice, and the cell lines have consistent and reproducible electrophysiological characteristics. Cell lines expressing mutant and wild-type receptors are not visually distinguishable from each other.
It is necessary periodically to split cultures and replate the dissociated cells into new dishes. A trypsin-EDTA solution was used to separate the cells for passage. On the first day after passage most of the cells were still rounded and were very easy to seal to patch-clamp electrodes. A high proportion of the cells on day 1 had very low levels of leak current, and a high proportion showed robust whole-cell current responses to 30 or 100 μM extracellular ATP. Cells on days 2-4 were almost always unstable to applications of ATP longer than 1 or 2 s, for unknown reasons, so the standard protocol was to use a 1 s puff. Cells with minimal leak currents had no detectable responses to puffs of external solution lacking ATP.
Electrophysiology solutions and apparatus
The external solution contained (mM): 140 sodium chloride, 10 Hepes, 10 glucose, 5 potassium chloride, 2.5 calcium chloride and 0.5 magnesium chloride; pH 7.4. The internal solution contained (mM): 120 potassium chloride, 20 tetraethyl ammonium chloride (TEA-Cl), 10 EGTA, 10 Hepes and 3 magnesium chloride; pH 7.3. Apyrase (0.5 U ml−1; Sigma grades I or III), a crude enzyme extract with a balanced ratio of ATPase and ADPase activities, was added to the external solution to scavenge agonist and so facilitate recovery from desensitization.
Cells to be assayed were incubated in calcium- and magnesium-free Hank's buffered salt solution with 0.5 U ml−1 apyrase for at least 30 min. In this solution they detached and became rounded. In some experiments cytochalasins B or D were added to this solution at 5 μM and the cells were incubated for at least 2 h. Cells were then diluted into external solution and allowed to settle for a few minutes in the patching chamber. Cells were sealed to borosilicate glass electrodes of between 2 and 10 MΩ and the plasma membrane broken by suction to achieve the whole-cell configuration. Cells were perfused internally for several minutes before the first experiment, during which time they became round. Data were recorded by computer using the pCLAMP series of programs and an Axopatch-1D patch clamp with a TL-1 DMA interface (Axon Instruments).
Saturating concentrations of agonist, 30 or 100 μM ATP, were applied with a puffer pipette against a background of constant flow of external solution containing apyrase. Puffs were commanded by the acquisition software and timed with a stimulator, triggering a pinch valve to open and close. The half-time of agonist application was approximately 50 ms after a lag time of 50-75 ms (Parker & Scarpa, 1995). In experiments in which the same cell was given 30 or 100 μM ATP in alternation the resulting current responses when scaled were superimposable for both the wild-type and mutant receptors, as has been reported before (Evans et al. 1995). 30 μM ATP and 100 μM ATP were therefore used interchangeably.
Current isolation
About one-third of the HEK 293 cells transfected with P2X1 constructs or with the pCDNA3 vector alone showed an ATP-activated, inwardly rectifying current at voltages lower than −40 mV (data not shown). About one-third of the cells also had a variable, highly outwardly rectifying current above 0 mV. To observe rapid ATP-evoked responses in the cell lines transfected with P2X1 constructs the cells were therefore held at −40 mV to isolate the current resulting from P2X1 activity. Untransfected HEK 293 cells and those transfected with the pCNDA3 vector alone did not show rapidly activating current responses to 30 or 100 μM ATP.
RESULTS
Figure 1 shows ATP responses recorded from cells expressing wild-type P2X1 on the first day after passage of the stably transfected culture. They have an extremely rapid time course of activation and desensitization compared with the responses recorded from rat vas deferens smooth muscle cells, as described before (Evans et al. 1995). The rate of desensitization does not vary in any obvious way with the peak current response, over the range 8 to approximately 800 pA, and is well fitted by a single exponential. Because of the relatively slow time course of application of agonist with the puffer system it can only be said that activation is fast, being complete within 10-20 ms. If single exponentials are fitted to the decay of current in the presence of ATP, the average time constant of desensitization (τdesens) is 63 ± 20 ms (± s.d., n = 19).
Figure 1. Wild-type P2X1 responses to ATP: activation.
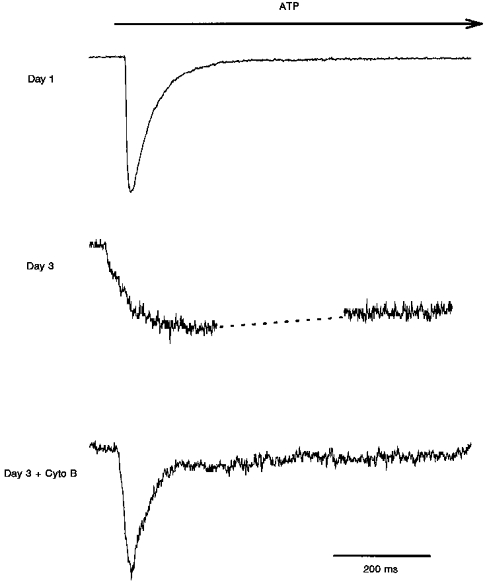
Changes in the rates of activation and desensitization of the wild-type P2X1 in response to application of ATP on the first and third days after passage of the stably transfected HEK 293 cell culture, and after cytochalasin B (Cyto B) treatment on the third day. Cells were assayed in the whole-cell configuration and the holding potential was −40 mV. 1 s applications of agonist were given as indicated. Peak current responses: day 1, 500 pA; day 3, 35 pA; day 3 plus cytochalasin B, 90 pA. The dashed line in the day 3 trace indicates where the response was interrupted to perform a voltage ramp.
By the second day after passage, and through the third or fourth days when the cells must again be split, HEK 293 cells expressing wild-type P2X1 constructs are flat and invariably have much slower ATP responses. Of twenty-three responses to ATP observed in twelve cells on days 2-4, all had current activation and desensitization time courses dramatically slower than those observed on the first day, although there was a great deal of variability in the responses. When single exponentials were fitted to the rising phases of the current responses, the time constant of activation (τact) was 193 ± 126 ms (± s.d., n = 6, ranging from 35 to 450 ms) for peak current responses ranging from 10 to approximately 500 pA. With such short ATP applications, it was not possible to fit exponentials to the current decay, but assuming a linear decrease in the current, the half-desensitization times appear to range from 1 to 5 s. Leak-subtracted voltage ramps performed on these slower currents, in a cell that lacked any apparent contaminating currents, revealed an inwardly rectifying current with a reversal potential of 9 ± 3 mV (± s.d., n = 20, data not shown), matching the predicted reversal potential calculated from published permeability ratios for the heterologously expressed P2X1 receptor with the internal and external solutions described above, using the Goldman-Hodgkin-Katz voltage equation (Hille, 1984).
Cytochalasins B and D (5 μM, 2-4 h treatment) applied on day 3 after passage have a dramatic effect on the time courses of activation and desensitization. After cytochalasin treatment the ATP responses are indistinguishable from the first day responses. No slower responses have yet been observed (3 of 3 responding cells for cytochalasin B, 6 of 6 cells for cytochalasin D). Activation is again complete within 10-20 ms, and the mean τdesens is 60 ± 30 ms (n = 8) for peak currents ranging from 3 to approximately 3000 pA.
Of the cells on days 2-4 expressing wild-type currents, only the cell giving the response shown in Fig. 2 was stable to a prolonged application of ATP solution. The current decay was well fitted by a single exponential, and τdecay was approximately 10 s. In three other cells that were relatively stable during longer ATP applications it was apparent that τdesens would be greater than 1 or 2 s, probably not as long as 10 s. This kinetics of desensitization is similar in magnitude to that estimated from the 1 s applications of agonist, and to that previously reported for ATP responses recorded in oocytes expressing P2X1 receptors and in vas deferens smooth muscle cells (Friel, 1988; Evans et al. 1995).
Figure 2. Wild-type P2X1 responses to ATP: desensitization.
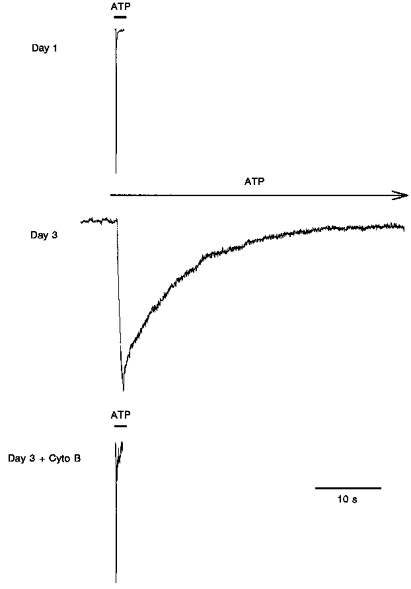
Changes in the rates of activation and desensitization of the wild-type P2X1 in response to application of ATP on the first and third days after passage of the stably transfected cell culture, and after cytochalasin B (Cyto B) treatment on the third day. Cells were assayed in the whole-cell configuration. The holding potential was −40 mV. Peak current responses: day 1, 500 pA; day 3, 360 pA; day 3 plus cytochalasin B, 85 pA.
In this study trypsin was used routinely to separate the cells for passage, so one trivial explanation for this change in kinetics could be that there is a trypsin-sensitive site on the extracellular domain of the ion channel, and that digestion at that site speeds the rates of activation and desensitization. However, when cells were passed without using trypsin, on the first day after passage the cells still had a round phenotype, and the faster rate of desensitization (5 of 5 cells) was still obtained. Passing cells without trypsin is essentially the same procedure that was used for lifting the cells for transfer to the patching chamber. The cytoskeletal rearrangement that the cells seem to undergo during passage, detectable as faster channel kinetics, must therefore occur between 6 and 18 h after passage, and may reflect a proliferative phenotype.
Figure 3 shows whole-cell current responses of cells expressing the wild-type and two mutations of the P2X1 receptor to 1 s applications of 30 μM ATP. The mutations, M332I and T333S, are in the pore-like domain near or within the second transmembrane domain of the receptor. The time course of activation of the M332I mutant was very fast, and the desensitization (62 ± 17 ms, mean ± s.d., n = 8) was indistinguishable from the wild-type on the first day. Activation of the T333S mutant was similarly too fast to characterize meaningfully, and desensitization was somewhat faster than the wild-type even on the first day - τdesens was 26 ± 10 ms (± s.d., n = 15). Most strikingly, and in contrast to the wild-type receptor, these mutants maintained their faster kinetics over time in culture. On days 2-4, τact values were extremely fast and τdesens values were 56 ± 9 ms (± s.d., n = 5) and 29 ± 12 ms (± s.d., n = 13) for the M332T and T333S mutants, respectively.
Figure 3. Wild-type and mutant P2X1 responses to ATP.
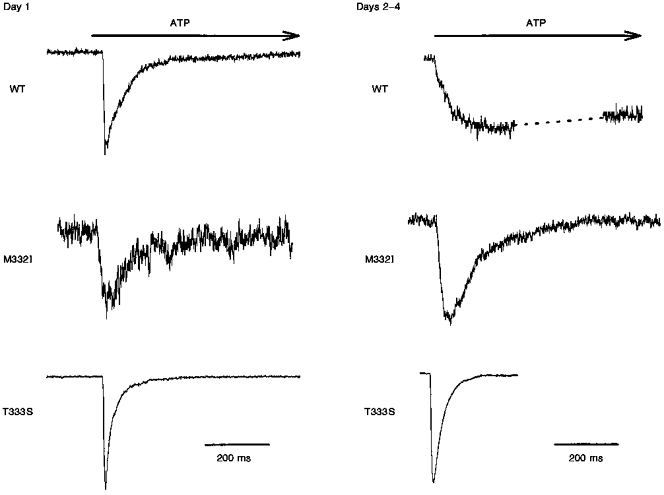
A comparison of the responses of wild-type and mutant P2X1 receptors to 1 s application of 30 μM ATP on the first and subsequent days after passage. The holding potential was −40 mV. The wild-type response shown for days 2-4 is the same shown for day 3 in Fig. 1. Peak current responses were as follows. Day 1: wild-type, 100 pA; M332T, 40 pA; T333S, 310 pA. Days 2-4: wild-type, 35 pA; M332T, 60 pA; T333S, 1075 pA.
DISCUSSION
The simplest conclusions from these experiments are that the actin cytoskeleton is rearranged or disrupted by passage of the cell culture, that full reassembly takes at least 2 days, and that P2X receptors expressed in cells with assembled and unassembled actin cytoskeletons have markedly different activation and desensitization kinetics. P2X1 receptors expressed in HEK 293 cells may directly associate with actin or actin-binding proteins, but may also sense the state of the cytoskeleton through other biochemical modifications in a fashion that is itself sensitive to the state of the actin cytoskeleton.
P2X1 receptor activities recorded from acutely isolated rat vas deferens smooth muscle cells and from Xenopus oocytes expressing P2X1 protein show kinetics of activation and desensitization that are similar to those of the day 3 cells shown in Figs 1 and 2. This is consistent with the above conclusions - in both those cell types the actin cytoskeleton should be intact. It must, however, be noted that other investigators working with P2X1 expressed in Xenopus oocytes report no effect of cytochalasin treatment on desensitization (Werner et al. 1996).
In this study mutants have also been identified in a pore-like domain near or within the second putative transmembrane domain that are unable to change in their kinetics over time in culture, indicating that they may be either unable to sense the state of the cytoskeleton, or that the sensor has become uncoupled from the gating machinery. If this domain indeed forms a pore loop, as the similar domain does in other ion channels and as is probable given the recent report of experiments by Egan et al. (1998), then it may be expected that small changes in this domain could cause large changes in permeation or kinetics or both, since mutations in the similar domain of many other ion channels have both effects (Heginbotham et al. 1992; Balser et al. 1996; Bucossi et al. 1996; Liu et al. 1996; Schneggenburger & Ascher, 1997).
In conclusion, the findings of this study indicate that wild-type P2X1 receptors may sense the state of the actin cytoskeleton, either directly or indirectly, because treatment of cells with cytochalasins dramatically speeds the rates of both activation and desensitization. The structural basis of the cytoskeletal interaction is currently being investigated through biochemistry and through the analysis of mutants that appear to lack the ability to change their rate of desensitization over time in culture.
Acknowledgments
My thanks to Pranav Dalal for assistance in the construction of the mutants and to Stephen Jones, Antonio Scarpa and George Dubyak for critical readings of the manuscript. This work was supported by the US National Institutes of Health grant no. HL 41618 to Janice G. Douglas and Antonio Scarpa.
References
- Balser JR, Nuss HB, Chiamvimonvat N, Perez Garcia MT, Marban E, Tomaselli GF. External pore residue mediates slow inactivation in μ1 rat skeletal muscle sodium channels. The Journal of Physiology. 1996;494:431–442. doi: 10.1113/jphysiol.1996.sp021503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucossi G, Eismann E, Sesti F, Nizzari M, Seri M, Kaupp UB, Torre V. Time-dependent current decline in cyclic GMP-gated bovine channels caused by point mutations in the pore region expressed in Xenopus oocytes. The Journal of Physiology. 1996;493:409–418. doi: 10.1113/jphysiol.1996.sp021392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Wood JN. Purinergic receptors: their role in nociception and primary afferent neurotransmission. Current Opinions in Neurobiology. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- Clifford EE, Parker KE, Humphreys BD, Kertesy S, Dubyak GR. The P2x1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leukocytes. Blood. 1998;91:1–10. [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Drain P, Dubin AE, Aldrich RW. Regulation of Shaker K+ channel inactivation gating by the cAMP-dependent protein kinase. Neuron. 1994;12:1097–1109. doi: 10.1016/0896-6273(94)90317-4. 10.1016/0896-6273(94)90317-4. [DOI] [PubMed] [Google Scholar]
- Egan TM, Haines WR, Voigt MM. A domain contributing to the ion channel of ATP-gated P2x2 receptors identified by the substituted cysteine accessibility method. Journal of Neuroscience. 1998;18:2350–2359. doi: 10.1523/JNEUROSCI.18-07-02350.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RJ, Lewis C, Buell G, Valera S, North RA, Surprenant A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Molecular Pharmacology. 1995;48:178–183. [PubMed] [Google Scholar]
- Friel DD. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. The Journal of Physiology. 1988;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Abramson T, MacKinnon R. A functional connection between the pores of distantly related ion channels as revealed by mutant K+ channels. Science. 1992;258:1152–1155. doi: 10.1126/science.1279807. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. Sunderland: Sinauer Associates, Inc.; 1984. [Google Scholar]
- Johnson BD, Byerly L. A cytoskeletal mechanism for calcium channel metabolic dependence and inactivation by intracellular calcium. Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. 10.1016/0896-6273(93)90196-X. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jurman ME, Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. 10.1016/S0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Longhurst PA, Schwegel T, Folander K, Swanson R. The human P2x1 receptor: molecular cloning, tissue distribution, and localization to chromosome 17. Biochimica et Biophysica Acta. 1996;1308:185–188. doi: 10.1016/0167-4781(96)00112-1. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Matsuki N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflügers Archiv. 1987;409:644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- Parker KE, Scarpa A. An ATP-activated nonselective cation channel in guinea pig ventricular myocytes. American Journal of Physiology. 1995;269:H789–797. doi: 10.1152/ajpheart.1995.269.3.H789. [DOI] [PubMed] [Google Scholar]
- Reuveny E, Slesinger PA, Inglese J, Morales JM, Iniguez-Lluhl JA, Lefkowitz RJ, Bourne HR, Jan YN, Jan LY. Activation of the cloned muscarinic potassium channel by G protein βγ subunits. Nature. 1994;370:143–146. doi: 10.1038/370143a0. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Ascher P. Coupling of permeation and gating in an NMDA-channel pore mutant. Neuron. 1997;18:167–177. doi: 10.1016/s0896-6273(01)80055-6. [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Arvidsson U, Riedl M, Wang J, Buell G, Surprenant A, North RA, Elde R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proceedings of the National Academy of Sciences of the USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P, Seward EP, Buell GN, North RA. Domains of P2X receptors involved in desensitization. Proceedings of the National Academy of Sciences of the USA. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]


