Abstract
Immunoblot analysis, [3H]ryanodine binding, and planar lipid bilayer techniques were used to identify and characterize the functional properties of ryanodine receptors (RyRs) from Lytechinus pictus and Strongylocentrotus purpuratus sea urchin eggs.
An antibody against mammalian skeletal RyRs identified an ≈400 kDa band in the cortical microsomes of sea urchin eggs while a cardiac-specific RyR antibody failed to recognize this protein. [3H]Ryanodine binding to cortical microsomes revealed the presence of a high-affinity (Kd = 13 nM), saturable (maximal density of receptor sites, Bmax = 1.56 pmol (mg protein)−1) binding site that exhibited a biphasic response to Ca2+.
Upon reconstitution of cortical microsomes into lipid bilayers, only sparse and unstable openings of a high-conductance cation channel were detected. Addition of crude sea urchin egg homogenate to the cytosolic (cis side) of the channel increased the frequency of openings and stabilized channel activity. The homogenate-activated channels were Ca2+ sensitive, selective for Ca2+ over Cs+, and driven by ryanodine into a long-lived subconductance state that represented ≈40 % of the full conductance level. Homogenate dialysed in membranes with a molecular weight cut-off ≤ 2000 lacked the capacity to increase the frequency of RyR openings and to stabilize channel activity.
Direct application of cyclic adenosine diphosphoribose (cADPR) or photolysis of NPE-cADPR (‘caged’ cADPR) by ultraviolet laser pulses produced transient activation of sea urchin egg RyRs. Calmodulin (CaM) failed to activate reconstituted RyRs; however, channel activity was inhibited by the CaM blocker trifluoroperazine, suggesting that CaM was necessary but not sufficient to sustain RyR activity.
These findings suggest that a functional Ca2+ release unit in sea urchin eggs is a complex of several molecules, one of which corresponds to a protein functionally similar to mammalian RyRs.
Cyclic adenosine diphosphoribose (cADPR), an endogenous metabolite of nicotinamide adenine dinucleotide, was first identified as an agent capable of releasing Ca2+ from intracellular stores in sea urchin eggs (Lee, Walseth, Bratt, Hayes & Clapper, 1989). More recently, cADPR has also been shown to mobilize Ca2+ in several mammalian cells, including those from pancreatic islets, intestinal longitudinal muscle, sympathetic neurons, dorsal root ganglion, liver and brain (Galione, 1994). In all of these cells, cADPR either generates or amplifies an intracellular Ca2+ wave that sets in motion a series of events that culminates in egg fertilization, hormone secretion, muscle contraction, neurotransmitter release, etc.
The molecular mechanism by which cADPR mobilizes intracellular Ca2+ has not been clearly established. cADPR-induced Ca2+ release is insensitive to heparin and inositol 1,4,5-trisphosphate but sensitive to caffeine and ryanodine (Galione, Lee & Busa, 1991), two classical modulators of sarcoplasmic reticulum (SR) Ca2+ release channels (ryanodine receptors, RyRs; Meissner, 1994). It would seem therefore that RyRs are the molecular target of cADPR. However, in sea urchin eggs, cADPR crosslinks with ∼140 and 100 kDa proteins (Walseth, Aarhus, Kerr & Lee, 1993), not with the expected ∼500 kDa RyR monomer detected in cardiac and skeletal muscle (Meissner, 1994). Furthermore, calmodulin (CaM), which regulates mammalian RyRs but is not necessary to sustain channel activity (Tripathy, Xu, Mann & Meissner, 1995), is an indispensable component of cADPR-induced Ca2+ release in sea urchin eggs (Lee, Aarhus, Graeff, Gurnack & Walseth, 1994). Conversely, while cADPR is a clear Ca2+ mobilizing agent in sea urchin eggs, it produces little (Sitsapesan, McGarry & Williams, 1994) or no effect (Fruen, Mickelson, Shomer, Velez & Louis, 1994; Guo, Laflamme & Becker, 1996) in cardiac muscle.
We have reconstituted cortical microsomes of sea urchin eggs into lipid bilayers in an attempt to identify the molecular target of cADPR and characterize its mechanism of action. We found that cADPR activates a cation channel that is similar to cardiac and skeletal RyRs in several elementary properties including unitary channel conductance, Ca2+ selectivity, subconductance states and ryanodine sensitivity. However, there was also a strict dependence on accessory components to sustain the activity of this channel, a condition not seen with cardiac and skeletal RyRs. Thus, a functional cADPR-dependent Ca2+ release unit in sea urchin eggs seems to be a complex of several molecules, one of which corresponds to a protein with elementary properties similar to those of mammalian RyRs.
METHODS
Preparation of cortical reticulum membranes and total homogenate from sea urchin eggs
Cortical reticular membranes, a honeycomb network of internal membranes that associates with the plasma membrane, were isolated from unfertilized Lytechinus pictus or Strongylocentrotus purpuratus sea urchin eggs using a modification of the procedure of McPherson, McPherson, Mathews, Campbell & Longo (1992). Briefly, eggs suspended in complete sea water (486 mM NaCl, 10 mM KCl, 26 mM MgCl2, 30 mM MgSO4, 10 mM CaCl2, 2.4 mM NaHCO3, 10 mM Hepes, pH 8.0) were allowed to sediment by gravity and homogenized (1:10, v/v) in iced sea water C (SWC; 500 mM NaCl, 10 mM KCl, 3 mM NaHCO3, 30 mM EGTA, 60 mM NaOH, 200 μM benzamidine, 2 μM leupeptin, pH 8.0). A portion of this total homogenate was supplemented with 5 mM K2ATP and 26 mM CaCl2 to bring [free Mg2+] and [free Ca2+] to 1.5 mM and ∼30 μM, respectively. After titration to pH 7.4, the supplemented total homogenate was stored at -70°C in small aliquots until used. The remaining homogenate was diluted 1:5 (v/v) in SWC and spun in a tabletop centrifuge at 2000 r.p.m. for 2 min. The pellet was resuspended in 0.5 volumes of SWC and centrifuged again until the resuspension volume was 2 ml. The last pellet, corresponding to cortical microsomes, was suspended in 1 ml of modified sea water C (MSWC; same as above except that NaOH was replaced by 30 mM Tris (pH 8.0) and EGTA was decreased to 1 mM) and stored in small aliquots at - 70°C until used.
Dialysis of total homogenate was performed as follows: 2 ml of unsupplemented total homogenate was dialysed for 4 h against 2 l of SWC at 4°C. Dialysis was conducted using a 3 ml dialysis cassette with a cellulose film of molecular weight cut-off (MWCO) ≤ 2000 (Cat. No. 66225, Pierce, Rockford, IL, USA). At the end of the 4 h dialysis, the dialysed homogenate was supplemented with ATP and CaCl2 as described above and stored in small aliquots at −70°C.
[3H]Ryanodine binding
[3H]Ryanodine (60 Ci mmol−1, Dupont NEN) was incubated with cortical microsomes (0.01-1.0 mg ml−1) in medium containing 0.2 M KCl, 20 mM Mops (pH 7.2), 1 mM EGTA, and different amounts of CaCl2 to set [free Ca2+] in the range of 0.08-100 μM. The incubation took place in a volume of 0.1 ml at 36°C for 90 min. After incubation, bound and free [3H]ryanodine were separated by rapid filtration onto Whatman GF/B filters. The filters were washed twice with cold distilled water and placed in a liquid scintillation cocktail to measure radioactivity in a β-counter. Non-specific binding was determined in the presence of 100 μM unlabelled ryanodine and has been subtracted from all reported values. Unless otherwise indicated, data represent the mean ± s.e.m. with n ≥ 3. Mathematical fitting of data was accomplished with the computer program Origin (v4.0, Microcal Inc., Northampton, MA, USA).
Reconstitution of single RyRs in planar lipid bilayers and activation by photolysis of caged cADPR
Single channel recordings of sea urchin egg RyRs were performed by fusing cortical microsomes to a Mueller-Rudin type phospholipid bilayer as described previously (El-Hayek, Lokuta, Arevalo & Valdivia, 1995; Lokuta, Rogers, Lederer & Valdivia, 1995). Cortical microsomes (∼10 μg) were added to an aqueous chamber (the cis chamber) connected to the head stage of a 200A Axopatch amplifier (Axon Instruments). The trans side was held at virtual ground. The cis and trans chambers (0.8 ml each) were initially filled with 50 mM caesium methanesulphonate and 10 mM Mops (pH 7.2). After bilayer formation, an asymmetrical caesium methanesulphonate gradient (300 mM cis/50 mM trans) was established. A Ca2+-EGTA admixture was then added to the cis chamber from a 100-fold stock to reach the [free Ca2+] specified in the text. After addition of cortical microsomes, voltage steps from 0 to −40 mV were applied at random. If channel openings were detected, Cs+ in the trans chamber was raised to 300 mM to collapse the chemical gradient and to avoid further vesicle insertion. Photolysis of caged cADPR was accomplished with a Q-switched, Nd:YAG laser (model GCR-12, Spectra-Physics, Mountain View, CA, USA), as described for caged Ca2+ (Valdivia, Kaplan, Ellis-Davies & Lederer, 1995). The laser beam (at 354 nm wavelength) was focused onto a 400 μm outer diameter, fused-silica fibre optic. The end of the fibre optic was positioned with a micromanipulator ∼400 μm in front of the bilayer aperture to photolyse the caged compound in the region between the end of the fibre optic and the bilayer cup. Therefore, only a small fraction of the caged compound was photolysed with each flash and the procedure could be repeated several times during the course of a single experiment. Channel activity was recorded and analysed with Axon Instruments software and hardware (pCLAMP v6.1, Digidata 1200 AD/DA interface). The probability of a single channel being open (Po) was calculated from current amplitude histograms using the equation: Po = Iopen/(Iopen + Iclosed), where Iopen and Iclosed were the binned currents of the channel in the open and closed states, respectively. Records were filtered at 1.5-2.0 kHz and digitized at 4-5 kHz.
SDS-PAGE and Western blot analysis of RyRs
Rabbit skeletal, sea urchin egg and pig cardiac microsomal proteins were separated by SDS-PAGE in 7 % acrylamide gels, transferred to a nitrocellulose membrane, and probed with either a rabbit monoclonal skeletal RyR antibody (XA7B6, Upstate Biotech Incorporated, Goldey, CO, USA) or a mouse monoclonal cardiac RyR antibody (MA3-916, Affinity Bioreagents, Inc., Goldey, CO, USA). Secondary goat anti-rabbit or goat anti-mouse horseradish peroxidase-conjugated antibodies were then applied and detection of protein-antibody complexes was accomplished via chemiluminescence as described previously (Lokuta, Meyers, Sanders, Fishman & Valdivia, 1997).
RESULTS
Immunoblot analysis and [3H]ryanodine binding to cortical microsomes of sea urchin eggs
Figure 1A shows that a monoclonal antibody raised against a cytosolic segment of the rabbit skeletal RyR recognized an ∼450 kDa protein in rabbit skeletal SR (lane 1) and an ∼400 kDa protein in the cortical microsomes of L. pictus eggs (lane 2). RyR from pig cardiac SR did not cross-react with this antibody (lane 3). Conversely, an antibody against mammalian cardiac RyRs failed to recognize high molecular weight proteins in skeletal SR and in sea urchin eggs. Thus, a high molecular weight protein of sea urchin eggs cross-reacts only with the skeletal RyR antibody. Figure 1B shows that high-affinity (Kd = 13 ± 5 nM) and saturable (maximal density of receptor sites, Bmax = 1.5 ± 0.5 pmol (mg protein)−1) [3H]ryanodine binding was detectable in the cortical microsomes. We therefore used [3H]ryanodine binding to test for the effect of [Ca2+] on sea urchin egg RyRs. All three mammalian RyR isoforms (skeletal or ryr1, cardiac or ryr 2, and brain or ryr 3) possess a high-affinity Ca2+ binding site that activates the channel and a low-affinity Ca2+ binding site that inactivates (or closes) the channel (Meissner, 1994). However, skeletal RyR inactivates at a [Ca2+] (ED50 = ∼280 μM) lower than cardiac and brain RyR (ED50 = ∼4 mM) (El-Hayek et al. 1995; Xu, Mann & Meissner, 1996; Meissner, Rios, Tripathy & Pasek, 1997). Figure 1C shows that the Ca2+ dependence of [3H]ryanodine binding to sea urchin egg microsomes was biphasic with ED50 values for Ca2+ activation and inactivation of 0.2 and 60 μM, respectively. These values are lower than those of any known RyR isoform, but the bell-shaped [3H]ryanodine binding curve bears more resemblance to that exhibited by skeletal RyRs. Taken together, the immunological and binding data suggest that a RyR is present and functional in sea urchin egg cortical microsomes and that it has a Ca2+ dependence more similar to skeletal-type RyRs than to other RyR isoforms.
Figure 1. Immunoblot analysis and [3H]ryanodine binding to cortical microsomes of sea urchin eggs.
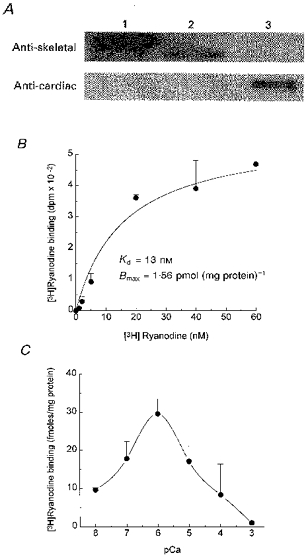
A, Western blot analysis with RyR antibodies. Cortical microsomal proteins and cardiac and skeletal SR microsomes were separated by SDS-PAGE, transferred to a nitrocellulose membrane, and probed with a rabbit monoclonal skeletal RyR antibody and cardiac RyR antibody, as indicated. Lane 1, 30 μg rabbit skeletal microsomes; Lane 2, 50 μg sea urchin egg microsomes; Lane 3, 30 μg pig cardiac microsomes. B, [3H]ryanodine saturation binding curve. Between 1 and 10 μg of L. pictus cortical microsomes were incubated with the indicated concentration of [3H]ryanodine as indicated in Methods. Non-specific binding has been subtracted from each data point. Data were fitted with the equation: B = Bmax [ [3H]ryanodine]/(Kd + [ [3H]ryanodine]), where B corresponds to specific binding of [3H]ryanodine, Bmax is the maximal density of receptor sites and Kd is the apparent dissociation constant of the [3H]ryanodine-RyR complex. C, Ca2+ dependence of [3H]ryanodine binding to sea urchin egg microsomes. Binding conditions were as in B except that 1 mM EGTA and varying concentrations of CaCl2 were added to the medium to bring [free Ca2+] to the specified level. [3H]Ryanodine concentration was 7 nM.
Single channel activity in sea urchin egg cortical microsomes
We next attempted to record single RyR channel activity from sea urchin eggs by fusing cortical microsomes to planar lipid bilayers. We used recording conditions considered standard for studies of cardiac or skeletal RyRs (El-Hayek et al. 1995; Lokuta et al. 1995). Microsomes were added to the cis side and channel activity recorded with symmetrical ionic composition in the cis and trans sides (300 mM caesium methanesulphonate, 10 mM Mops, pH 7.2). Cs+ was chosen as charge carrier instead of Ca2+ to avoid inactivation caused by large Ca2+ gradients, to increase the signal-to-noise ratio (Cs+/Ca2+ conductance ratio, GCs/GCa ≈ 2), and to block K+ channels (Smith, Imagawa, Ma, Fill, Campbell & Coronado, 1988). Methanesulphonate was used to block Cl− channels. Figure 2A shows traces of a Cs+-conducting sea urchin egg channel. Under voltage clamp conditions, only brief and sporadic channel openings were detected (traces labelled Control), which occurred most frequently after abrupt voltage steps from positive to negative holding potentials. However, addition to the cis (cytosolic) chamber of total egg homogenate, supplemented with 5 mM ATP as described in Methods, elicited a remarkable increase of channel activity (second row of traces). The combined addition of ATP and total homogenate stabilized channel openings and allowed steady-state recordings for relatively long times (20-40 min).
Figure 2. Reconstitution of sea urchin egg RyR channels in planar lipid bilayers.
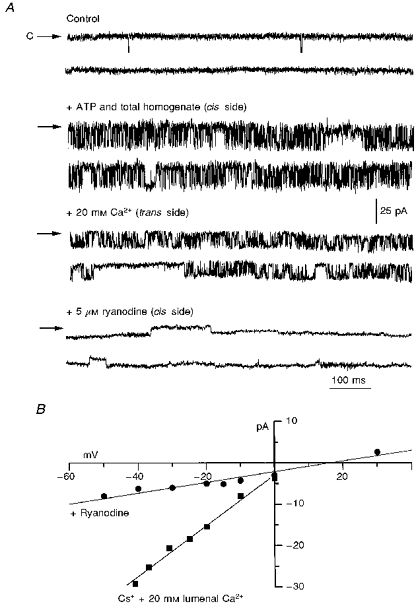
All traces were recorded at a holding potential of −25 mV. At this voltage, Cs+ flows from the trans (lumenal) to the cis (cytosolic) chamber and channel openings correspond to downward deflections of the baseline current. A, traces labelled Control: only brief and sparse openings were observed under our standard recording conditions (symmetrical 300 mM caesium methanesulphonate, 10 mM Mops, pH 7.2). C indicates the closed state. + ATP and total homogenate (cis side): the same channel after addition of 10 μl of sea urchin egg total homogenate (0.1 mg protein ml−1) supplemented with 5 mM ATP. For this particular channel, Po increased from ≤ 0.01 to ≈0.6. This is one of the most dramatic responses. Typical and consistent responses to homogenate addition were an increase in Po from ≤ 0.01 to 0.2-0.5 (n = 9). + 20 mM Ca2+ (trans side): addition of 20 mM CaCl2 to the trans (lumenal) side decreased single channel conductance; + 5 μM ryanodine: ryanodine induced the appearance of a long-lived subconductance state. The transition to this modified state was not reversible within the duration of the experiment (≈20 min). B, current-voltage relation for the RyR channel before and after addition of ryanodine.
The following four crucial observations strongly suggested that the homogenate-activated channels of sea urchin eggs corresponded to functional counterparts of mammalian RyRs. (1) Addition of 20 mM CaCl2 to the trans (lumenal) solution decreased the amplitude of single channel openings (Fig. 2A, third row of traces) and shifted Erev, the reversal potential, from 0 to ∼+10 mV (Fig. 2B). The change in current amplitude was expected from the higher affinity and longer dwelling time of Ca2+ in the channel's pore, while the shift in Erev indicated that the channel was selective for Ca2+ over Cs+ (Smith et al. 1988). (2) The slope conductance for this channel in 300 mM symmetrical Cs+ plus 20 mM Ca2+ (Fig. 2B) was ∼600 pS, similar to that of skeletal and cardiac RyRs under comparable conditions (Smith et al. 1988; Meissner, 1994). (3) Addition of 5 μM ryanodine to the cis solution profoundly modified the amplitude and kinetics of the channel (Fig. 2A, bottom row of traces). Ryanodine ‘locked’ the channel into a long-lived conductance state that represented ∼40 % of the full-conductance openings (Fig. 2B). These kinetic modifications are the signature effects of ryanodine on skeletal and cardiac RyRs (Rousseau, Smith & Meissner, 1987; Lindsay, Tinker & Williams, 1994). (4) Lowering [Ca2+] at the cytosolic (cis) side of the channel markedly decreased channel activity (Fig. 4), as expected from the Ca2+ dependence of open probability (Po) for cardiac and skeletal RyRs (Chu, Fill, Stefani & Entman, 1993) and from the Ca2+ dependence of [3H]ryanodine binding in sea urchin eggs (Fig. 1C). Thus, several elementary properties observed in cardiac and skeletal RyRs are also present in, and may be used as markers for detection of, sea urchin egg RyRs.
Figure 4. Activation of RyRs by photolysis of caged cADPR.
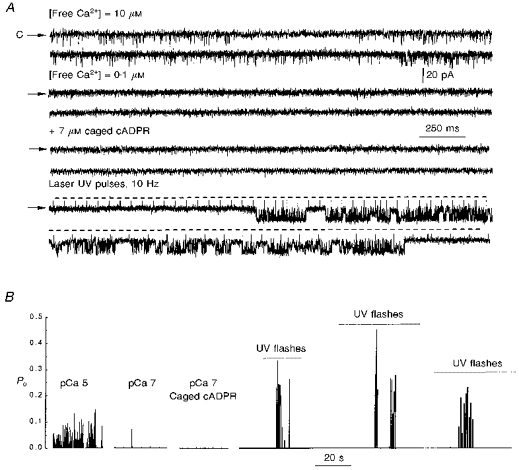
Sea urchin egg RyRs were reconstituted in lipid bilayers as described in Fig. 2, except that Ca2+ in the trans side was omitted and 1 mM EGTA and varying amounts of CaCl2 were added to the cis side to set cytosolic [free Ca2+] to 10 μM (top traces in A) or 0.1 μM (second row of traces in A). A, in the presence of sea urchin egg homogenate, 10 μM [free Ca2+] in the cytosolic (cis) side elicited bursts of channel activity (first row of traces), which disappeared when cis [free Ca2+] was lowered to 0.1 μM (second row of traces). After a period of recording at [free Ca2+] = 0.1 μM, 7 μM caged cADPR was added to the cis side (third row of traces). No activity was detected during this period. A train of UV laser flashes was applied at 10 Hz frequency, shown by the horizontal dashed lines (bottom traces). The upward deflections of the baseline current are electrical artifacts created by the laser pulse. B, diary of activity of the RyR. Continuous records in control and after additions were divided into intervals of 30 s; Po in each interval is plotted as a bar of length 0-1 with level 0 corresponding to no activity. The dotted line underneath the label UV flashes indicates the time in which laser UV flashes were applied at a frequency of 10 Hz. Three representative trains of channel activity elicited by UV pulses are shown (n = 6).
Conductance states in sea urchin egg RyRs
In addition to the predominant ∼600 pS conductance level, two subconducting states were occasionally observed in the homogenate-activated sea urchin egg RyR (Fig. 3). In the standard recording solution, these substates had chord conductances of ∼300 pS (Fig. 3B) and ∼150 pS (Fig. 3C), which corresponded to approximately ½ and ¼ of the main conducting level, respectively (Fig. 3A). The relative fractional conductance of the substates (labelled F¼ and F½ in Fig. 3) were conserved in Cs+- and Ca2+-conducting channels. Direct transitions between full and subconducting states (Fig. 3D) surpassed transitions between closed and subconducting states (first segment of Fig. 3C). Po of the fully open and subconducting levels fluctuated under stationary conditions. Particularly, Po [F½], the open probability of the ∼300 pS conductance state, differed markedly from (Po [F]/2)2, the probability of two independent channels opening to the fully conducting state simultaneously. This argued against F½ being the unitary current of an independently gated channel and suggested instead that it corresponded to a true substate of the sea urchin egg RyR. These current amplitude transitions are reminiscent of the subconducting states observed in cardiac and skeletal RyRs (Liu, Lai, Rousseau, Jones & Meissner, 1989; Ding & Kasai, 1996).
Figure 3. Conductance states in homogenate-activated sea urchin egg RyRs.
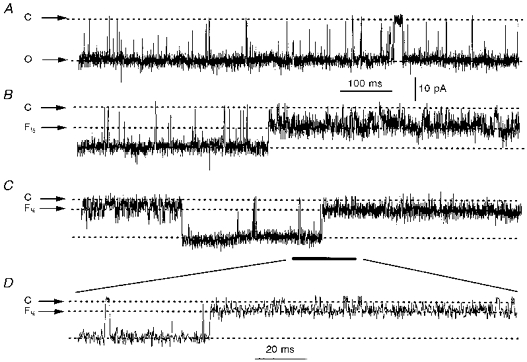
Single channel openings to the full (≈600 pS), most frequently encountered conducting state of the sea urchin egg RyR (a), or to a conducting state with current amplitude value corresponding to ½ (F½; B) or to ¼ (F¼; C) of the fully open channel. D, expansion of the recording segment in C indicated by bar. Holding potential for all traces = −25 mV. The dashed lines indicate the current levels of the various conducting states; C, closed; O, open. Traces were digitized at 4 kHz and filtered at 1.5 kHz.
Effect of cADPR on sea urchin egg RyRs
In sea urchin egg microsomes, the response to cADPR appears to be conditioned to the presence of accessory proteins (Lee et al. 1994). We therefore investigated whether reconstituted RyRs retained their capacity to respond to cADPR. Addition of up to 10 μM cADPR to the cytosolic (cis) side was unable to activate sea urchin egg RyRs (Po = ≤ 0.01 before and after addition, n = 3) (results not shown). We thus resorted again to adding total homogenate to produce a steady level of activity on which to test the effect of cADPR. The top traces in Fig. 4A show a homogenate-activated RyR in the presence of [free Ca2+] of 10 μM at the cytosolic side (cis side) of the channel. Ca2+ elicited a level of activity that was constant over time (lower trace). Upon lowering [free Ca2+] to 0.1 μM, Po decreased to ≤ 0.01, indicating that Ca2+ is a central regulatory element of this channel, as it is for cardiac and skeletal RyRs (Smith et al. 1988; Chu et al. 1993; Meissner, 1994). Addition of 7 μM caged cADPR to the cytosolic side of the channel did not produce any noticeable effect on channel activity. Similarly, sham flashes (flashes of high energy in the absence of caged cADPR) did not elicit channel openings (not shown). However, trains of ultraviolet laser flashes in the presence of caged cADPR elicited bursts of channel activity, the appearance of which correlated with the photolytic stimulus (bottom traces and panel B). Thus, whole homogenate supplements the RyR with accessory components that render the channel functional and responsive to cADPR.
Effect of calmodulin blockers on RyR channel activity
Since CaM has been reported as an indispensable component for activation of sea urchin egg RyRs (Lee et al. 1994; Tanaka & Tashjian, 1995) we tested whether CaM could replace total homogenate in its ability to activate RyRs. Under control conditions, only sporadic channel openings were detected (Fig. 5). Addition of CaM (10 μM) to the cis solution failed to increase channel activity (+ Calmodulin). In contrast, the frequency of channel openings increased remarkably when total homogenate was added, making apparent the presence of two channels in the bilayer (+ Total homogenate). Addition of the CaM blocker trifluoperazine (Meissner, 1986) to homogenate-activated channels reduced channel activity considerably (bottom traces). For the experiment presented in Fig. 5, nPo, the open probability of a single channel multiplied by the number of observable channels (n), was < 0.01 both in control and after CaM, 0.81 after total homogenate, and 0.08 after trifluoperazine. These results suggest that CaM is necessary but not sufficient to elicit channel activity of the sea urchin egg RyR in planar lipid bilayers.
Figure 5. Effect of CaM and a CaM blocker on sea urchin egg RyRs.
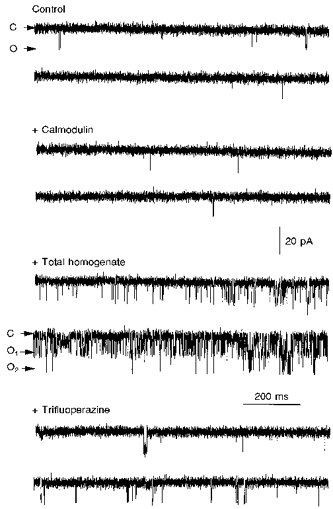
The [free Ca2+] in the cis (cytosolic) side of the channel was 10 μM (1 mM EGTA and 0.997 mM CaCl2). Sporadic openings elicited by voltage steps from 0 to −30 mV were obtained in the absence (Control) and the presence of 10 μM CaM added to the cis side (+ Calmodulin). No change in channel activity was detected following addition of CaM. In contrast, channel activation occurred after addition of 10 μl of sea urchin egg total homogenate (+ Total homogenate), which revealed the presence of two channels in this experiment (open states are labelled O1 and O2). Channel activation was partially reversed by addition of 20 μM trifluoperazine to the cis side (+ Trifluoperazine). All traces were taken from the same experiment, which was repeated twice with essentially qualitatively identical results.
Dialysis renders total homogenate ineffective to activate RyRs
The previous experiments suggested that neither cADPR nor CaM were the primary factors in total homogenate that promote and stabilize the activity of sea urchin egg RyRs. To shed light on the nature of the factor(s) in total homogenate which activate RyRs, we subjected total homogenate to 4 h of dialysis in cellulose membranes with molecular weight cut-off ≤ 2000. We reasoned that if the activating factor corresponded to a membranous or cytosolic protein with molecular weight ≥ 2000, then the dialysed homogenate should retain its capacity to activate RyRs. Figure 6A shows that cis addition of 32 μg of undialysed total homogenate evoked numerous channel openings in an otherwise sluggish RyR (Po ≤ 0.01 and 0.05 before and after addition, respectively, n = 3), consistent with the results presented above. In contrast, a similar addition of dialysed homogenate was unable to increase channel activity or to stabilize full and long lasting openings (Fig. 6B). Instead, channel activity in the presence of dialysed homogenate was irregular, and openings fluctuated rapidly between states of different fractional conductance. These results suggested that the activating factor(s) in total homogenate corresponded to molecule(s) with molecular weight ≤ 2000.
Figure 6. RyR activity in the presence of untreated versus dialysed total homogenate.
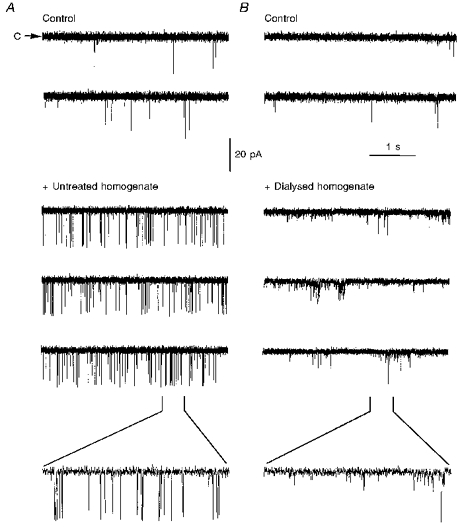
Sea urchin egg RyR activity was recorded before (labelled Control) and after the addition of 32 μg of untreated homogenate (A) or 35 μg of dialysed homogenate (B), prepared as described in Methods. The bottom trace is an expanded 400 ms segment of the trace indicated by the brackets. The results are representative of n = 3 and 6 channels for untreated and dialysed homogenate, respectively.
DISCUSSION
We used immunological, radioligand binding and single channel experiments to provide evidence that a high molecular weight protein of sea urchin eggs, displaying key functional properties of mammalian RyRs (including sensitivity to ryanodine), is part of the Ca2+ release unit sensitive to cADPR. Although extensive data obtained in whole and fragmented cells (Galione, 1994) support RyRs as the effectors of cADPR-induced Ca2+ release, this is the first study that directly links cADPR with single sea urchin egg RyRs.
A skeletal (but not a cardiac) RyR antibody cross-reacted with an ∼400 kDa protein in sea urchin eggs (Fig. 1A). The molecular weight of this protein was similar to that found by McPherson et al. (1992) using a skeletal RyR antibody with cortical microsomes of L. variegatus eggs. Thus, either the sea urchin egg RyR exhibits different mobility in SDS-PAGE, or it represents a new RyR isoform that is smaller than the typical mammalian RyR isoforms. Although immunological cross-reactivity between two proteins is far from guaranteeing similarity in structure/function, the Ca2+ dependence of the [3H]ryanodine binding curve in cortical microsomes (Fig. 1C) was nonetheless bell-shaped like that of skeletal RyRs (Meissner, 1994), or at least more so than that of cardiac or brain RyRs (El-Hayek et al. 1995; Meissner et al. 1997). If [3H]ryanodine binding is to be taken as an index of the activity of RyRs (Meissner, 1994), the bell-shaped curve suggests that Ca2+, over a very narrow range, may act as a trigger and a blocker of cADPR-sensitive Ca2+ stores.
In the presence of cytosolic Ca2+ as the sole agonist, and using Cs+ as the charge carrier, only sparse and unstable channel openings are observed (Figs 2, 5 and 6). Thus, conditions considered standard for recording cardiac and skeletal RyRs do not favour the activity of sea urchin egg RyRs. Addition of total egg homogenate supplemented with ATP (see Methods) brings about robust and sustained channel activity. ATP alone cannot evoke channel activity (not shown). Thus, ATP may be a cofactor for accessory components, or it may overcome the potential inhibitory effect of free Mg2+ (present in the total homogenate since it is a component of the cortical membrane isolation medium). Also, cADPR and CaM, which were added separately and in tandem, could modulate but not evoke the level of sustained activity generated by adding the whole homogenate. Therefore, some unidentified factor(s) of the homogenate seem to be indispensable components of the functional Ca2+ release unit of sea urchin eggs. To characterize the nature of the activating factor(s), we dialysed the sea urchin homogenate in dialysis cassettes with a molecular weight cut-off ≤ 2000 and found that RyRs systematically failed to display the vigorous level of activity elicited by undialysed homogenate (Fig. 6). Because dialysis presumably excluded from the homogenate substances of a molecular weight ≤ 2000, this result alone strongly argues against the activating factor being a single protein. In this context, the role of membranous or cytosolic proteins which by themselves modulate Ca2+ release in sea urchin eggs remains to be determined. These proteins include FK506-binding protein (FKBP12), which restores cADPR sensitivity in pancreatic islets (Noguchi et al. 1997), and a protein akin to the cytosolic protein ‘oscillin’, which induces Ca2+ oscillations in sea urchin eggs (Parrington, Swann, Shevchenko, Sesay & Lai, 1996). Whatever the factor(s), its indispensable presence reinforces the notion that several constituents of sea urchin eggs coexist in close interdependence to form a functional Ca2+ release unit.
Similar to cardiac and skeletal RyRs (Liu et al. 1989; Ding & Kasai, 1996), sea urchin egg RyRs exhibited subconducting states which corresponded to approximately ½ and ¼ of the full ∼600 pS conducting level (Fig. 3). The molecular mechanism underlying the appearance of these substates in mammalian RyRs has not been resolved, but an advanced hypothesis is that the fully conducting state represents the simultaneous opening of four RyR monomers, each contributing ¼ of the fully conducting level (Liu et al. 1989; Ding & Kasai, 1996). This hypothesis is consistent with the tetrameric arrangement of the cardiac and skeletal RyR (Wagenknecht, Grassucci, Frank, Saito, Inui & Fleischer, 1989). If applicable to sea urchin egg RyRs, it would suggest that these channels are similarly arranged and, because the full conductance state predominated in the recordings, that the gating of the monomers within the tetramer displays a strong co-operative interaction. Recently, it has been postulated that the co-operative interaction in the gating activities of the skeletal (Brillantes et al. 1994) and cardiac (Xiao, Valdivia, Bogdanov, Valdivia, Lakatta & Cheng, 1997) RyR monomers is enhanced by the immunophilin FKBP12. Given the uncertainty in the molecular components of the Ca2+ release unit in sea urchin eggs, it will be of interest to test whether FKBP12 inhibitors (FK506, rapamycin) affect the gating mechanism of sea urchin egg RyRs.
Photolysis of caged cADPR produced a transient activation of sea urchin egg RyRs (Fig. 4). From the traces of channel activity, it is clear that a latency period exists before the bursts of openings are evident and that the channel closes before the train of flashes is terminated. This is well represented in the three trains of flashes shown in Fig. 4 and was consistently found in other traces. It is unlikely that this phenomenon is a consequence of cADPR diffusion from the microenvironment of the channel because Ca2+, a smaller and more mobile molecule, diffuses from the site of photolysis with a time constant of ∼6 s (Valdivia et al. 1995). Furthermore, in other experiments in which direct additions of cADPR were made, we observed a transient response of sea urchin egg RyRs as well (not shown). Thus, this phenomenon manifests several of the essential characteristics of ‘adaptation’. In receptors that ‘adapt’, there is an attenuation of the response in the presence of a prolonged stimulus (Knox, Devreotes, Goldbeter & Segel, 1986); no further response is detected as long as the stimulus is held constant. Recovery of sensitivity begins when the stimulus is removed (Knox et al. 1986). Since cardiac and skeletal RyRs exhibit adaptation to agonists such as Ca2+ (Györke & Fill, 1993; Valdivia et al. 1995), it is conceivable that the termination of the RyR response to a prolonged cADPR stimulus reflects adaptation of sea urchin egg channels. However, this will not be definitively established until an accurate determination of the magnitude and time course of the cADPR levels produced by photolysis is made, and this phenomenon be discriminated from simple desensitization.
In summary, the behaviour of sea urchin egg RyRs in lipid bilayers is complex and conditional upon the presence of cytosolic constituent(s) of low molecular weight. Once a cortical membrane-embedded RyR is rendered functional by addition of accessory components, it is capable of responding to cADPR and of displaying several of the characteristic properties of its mammalian counterparts. The ability to record sustained channel activity in a system where individual components may be added or subtracted may prove an excellent assay for defining the pharmacological profile of sea urchin egg RyRs and for dissecting the mechanism by which Ca2+, CaM and cADPR induce stimulus-secretion coupling in a variety of cells.
Acknowledgments
This work was supported by NIH grants HL55438 and PO1 HL47053 (to H. H. V.) and by grants from Howard Hughes Medical Institute, DGAPA-UNAM, ICEGB and CONACyT (to A. D.). H. H. V. is an Established Investigator of the American Heart Association.
References
- Brillantes A-M, Ondrias K, Scott A, Kobrinsky E, Ondriasová E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- Chu A, Fill M, Stefani E, Entman ML. Cytosolic Ca2+ does not inhibit the cardiac muscle sarcoplasmic reticulum ryanodine receptor Ca2+ channel, although Ca2+-induced Ca2+ inactivation of Ca2+ release is observed in native vesicles. Journal of Membrane Biology. 1993;135:49–59. doi: 10.1007/BF00234651. [DOI] [PubMed] [Google Scholar]
- Ding J, Kasai M. Analysis of multiple conductance states observed in Ca2+ release channel of sarcoplasmic reticulum. Cell Structure and Function. 1996;21:7–15. doi: 10.1247/csf.21.7. [DOI] [PubMed] [Google Scholar]
- El-Hayek R, Lokuta AJ, Arevalo C, Valdivia HH. Peptide probe of ryanodine receptor function. Journal of Biological Chemistry. 1995;270:28696–28704. doi: 10.1074/jbc.270.48.28696. 10.1074/jbc.270.48.28696. [DOI] [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Shomer NH, Velez P, Louis CF. Cyclic ADP-ribose does not affect cardiac or skeletal muscle ryanodine receptors. FEBS Letters. 1994;352:123–126. doi: 10.1016/0014-5793(94)00931-7. 10.1016/0014-5793(94)00931-7. [DOI] [PubMed] [Google Scholar]
- Galione A. Cyclic ADP-ribose, the ADP-ribosyl cyclase pathway and calcium signalling. Molecular and CellularEndocrinology. 1994;98:125–131. doi: 10.1016/0303-7207(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Galione A, Lee HC, Busa WB. Ca2+-induced Ca2+ release in sea urchin egg homogenates by cyclic ADP-ribose. Science. 1991;253:1143–1146. doi: 10.1126/science.1909457. [DOI] [PubMed] [Google Scholar]
- Guo X, Laflamme MA, Becker PL. Cyclic ADP-ribose does not regulate sarcoplasmic reticulum Ca2+ release in intact cardiac myocytes. Circulation Research. 1996;79:147–151. doi: 10.1161/01.res.79.1.147. [DOI] [PubMed] [Google Scholar]
- Györke S, Fill M. Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science. 1993;260:807–809. doi: 10.1126/science.8387229. [DOI] [PubMed] [Google Scholar]
- Knox BE, Devreotes PN, Goldbeter A, Segel LA. A molecular mechanism for sensory adaptation based on ligand-induced receptor modification. Proceedings of the National Academy of Sciences of the USA. 1986;83:2345–2349. doi: 10.1073/pnas.83.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R, Graeff R, Gurnack ME, Walseth TF. Cyclic ADP-ribose activation of the ryanodine receptor is mediated by calmodulin. Nature. 1994;370:307–309. doi: 10.1038/370307a0. 10.1038/370307a0. [DOI] [PubMed] [Google Scholar]
- Lee HC, Walseth TF, Bratt GT, Hayes RN, Clapper DL. Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. Journal of Biological Chemistry. 1989;264:1608–1615. [PubMed] [Google Scholar]
- Lindsay ARG, Tinker A, Williams AJ. How does ryanodine modify ion handling in the sheep cardiac sarcoplasmic reticulum Ca2+-release channel? Journal of General Physiology. 1994;104:425–447. doi: 10.1085/jgp.104.3.425. 10.1085/jgp.104.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QY, Lai FA, Rousseau E, Jones RV, Meissner G. Multiple conductance states of the purified calcium release channel complex from skeletal sarcoplasmic reticulum. Biophysical Journal. 1989;55:415–424. doi: 10.1016/S0006-3495(89)82835-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuta AJ, Meyers MB, Sanders PR, Fishman GI, Valdivia HH. Modulation of cardiac ryanodine receptors by sorcin. Journal of Biological Chemistry. 1997;272:25333–25338. doi: 10.1074/jbc.272.40.25333. 10.1074/jbc.272.40.25333. [DOI] [PubMed] [Google Scholar]
- Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH. Modulation of cardiac ryanodine receptors by a phosphorylation-dephosphorylation mechanism. The Journal of Physiology. 1995;487:609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson SM, McPherson PS, Mathews L, Campbell KP, Longo FJ. Cortical localization of a calcium release channel in sea urchin eggs. Journal of Cell Biology. 1992;116:1111–1121. doi: 10.1083/jcb.116.5.1111. 10.1083/jcb.116.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G. Evidence of a role for calmodulin in the regulation of calcium release from skeletal muscle sarcoplasmic reticulum. Biochemistry. 1986;25:244–251. doi: 10.1021/bi00349a034. [DOI] [PubMed] [Google Scholar]
- Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annual Review of Physiology. 1994;56:485–508. doi: 10.1146/annurev.ph.56.030194.002413. 10.1146/annurev.ph.56.030194.002413. [DOI] [PubMed] [Google Scholar]
- Meissner G, Rios E, Tripathy A, Pasek DA. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. Journal of Biological Chemistry. 1997;272:1628–1638. doi: 10.1074/jbc.272.3.1628. 10.1074/jbc.272.3.1628. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Takasawa S, Nata K, Tohgo A, Kato I, Ikehata F, Yonekura H, Okamoto H. Cyclic ADP-ribose binds to FK506-binding protein 12.6 to release Ca2+ from islet microsomes. Journal of Biological Chemistry. 1997;272:3133–3136. doi: 10.1074/jbc.272.6.3133. 10.1074/jbc.272.6.3133. [DOI] [PubMed] [Google Scholar]
- Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA. Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature. 1996;379:364–368. doi: 10.1038/379364a0. 10.1038/379364a0. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Smith JS, Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. American Journal of Physiology. 1987;253:C364–368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, McGarry SJ, Williams AJ. Cyclic ADP-ribose competes with ATP for the adenine nucleotide binding site on the cardiac ryanodine receptor Ca2+ release channel. Circulation Research. 1994;75:596–600. doi: 10.1161/01.res.75.3.596. [DOI] [PubMed] [Google Scholar]
- Smith JS, Imagawa T, Ma J, Fill M, Campbell KP, Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. Journal of General Physiology. 1988;92:1–26. doi: 10.1085/jgp.92.1.1. 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tashjian AH. Calmodulin is a selective mediator of Ca2+-induced Ca2+ release via the ryanodine receptor-like Ca2+ channel triggered by cyclic ADP-ribose. Proceedings of the National Academy of Sciences of the USA. 1995;92:3244–3248. doi: 10.1073/pnas.92.8.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A, Xu L, Mann G, Meissner G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor) Biophysical Journal. 1995;69:106–119. doi: 10.1016/S0006-3495(95)79880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis-Davies GCR, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenknecht T, Grassucci R, Frank J, Saito A, Inui M, Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989;338:167–170. doi: 10.1038/338167a0. 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]
- Walseth TF, Aarhus R, Kerr JA, Lee HC. Identification of cyclic ADP-ribose binding proteins by photoaffinity labeling. Journal of Biological Chemistry. 1993;268:26686–26691. [PubMed] [Google Scholar]
- Xiao R-P, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. The Journal of Physiology. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Mann G, Meissner G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circulation Research. 1996;79:1100–1109. doi: 10.1161/01.res.79.6.1100. [DOI] [PubMed] [Google Scholar]


