Abstract
Epithelia lining the nasal passages and descending colon of wild-type and cystic fibrosis (CF) mice were examined by the short-circuit current technique. Additionally, intracellular Ca2+ ion determinations were made in nasal epithelial cells. Forskolin produced anion secretory currents in wild-type and CF nasal epithelia. It produced similar effects in wild-type colonic epithelia, but not in colonic epithelia from CF mice.
After electrogenic Na+ transport was blocked with amiloride and electrogenic Cl− secretion was stimulated with forskolin, the ability of K+ channel blockers to inhibit the forskolin-induced Cl− current was determined. The order of efficiency for nasal epithelium was: Ba2+ > clofilium ⋙ TEA = azimilide ⋙ trans-6-cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane (293B) = charybdotoxin, whereas for the colonic epithelium the order was: Ba2+ = 293B ⋙ azimilide = TEA ⋙ clofilium = charybdotoxin.
1-Ethyl-2-benzimdazolinone (1-EBIO) was able to generate large Cl−-secretory currents in colonic epithelia which were partially sensitive to charybdotoxin, with the remaining current being inhibited by 293B. In nasal epithelia 1-EBIO produced only a small transient effect on current.
Forskolin released intracellular Ca2+ in nasal epithelial cells; this activity was attenuated when more powerful Ca2+-releasing agents were applied first.
It is concluded that an action on basolateral cAMP-sensitive K+ channels is an important determinant of the maintained responses to forskolin in nasal and colonic epithelia, in addition to the effects on the cystic fibrosis transmembrane conductance regulator (CFTR) in the apical membrane. In CF nasal epithelia the activation of calcium-activated chloride channels (CACs) substitutes for the effect on CFTR. On the basis of the different orders of potency of the blocking agents and the differential response to 1-EBIO it is concluded that the cAMP-sensitive K+ channels are different in the airways and the gut.
The hyperpolarization of the apical membrane which results from the activation of basolateral K+ channels increases the driving force for the exit of Cl− ions in electrogenic secretory epithelia, as first proposed by Smith & Frizzel in 1984. In situations where Cl− secretion is inadequate, activation of K+ channels may provide a strategy for increasing Cl− secretion. Cystic fibrosis (CF) is an example of a disease condition where, as a result of various genetic mutations, the cystic fibrosis transmembrane conductance regulator, CFTR, is absent from the apical membrane or is present but functionally impaired, and Cl− secretion fails (Riordan et al. 1989; Cheng et al. 1990). Abnormalities in the transporting characteristics of epithelia lining the airways, alimentary canal, hepatobiliary tree and exocrine pancreas are responsible for the morbidity and mortality associated with the disease (Boat, Welsh & Beaudet, 1989). In the airways, calcium-activated chloride channel (CAC) responses are upregulated (Grubb, Vick & Boucher, 1994b), and activation of these channels via apically located purinoceptors has been the basis of a clinical trial to treat the disease (Olivier et al. 1996), but no alternative Cl− channels are found in the gut. Most human CF patients have lost a triplet codon at position 508, resulting in CFTR with a phenylalanine missing in the first nucleotide-binding fold. This aberrant protein fails to be trafficked to the apical membrane and is degraded in the cells (Cheng et al. 1990). Efforts have been made to alter cellular processes so that some ΔF508 CFTR can be diverted to an apical location where it shows reduced Cl− channel activity (Rubenstein, Egan & Zeitlen, 1997). In such situations more effective Cl− secretion might be achieved if basolateral K+ channels were activated simultaneously. Mice carrying the CF mutation lack lung pathology, attributed to a major increase in CACs, and die at an early age from gut pathology leading to peritonitis (Clarke, Grubb, Yankaskas, Cotton, McKenzie & Boucher, 1994). We have shown previously that when murine nasal or tracheal epithelia are pretreated with 2,5-di-(tert-butyl)-1,4-benzohydroquinone (TBHQ)-ionomycin the CAC system becomes desensitized, so that wild-type and CF tissues are easily differentiated (MacVinish et al. 1997a; MacVinish, Goddard, Colledge, Higgins, Evans & Cuthbert, 1997b). Here the nasal and colonic epithelia of wild-type and CF mice have been investigated without this pretreatment. The role played by basolateral K+ channels in supporting and maintaining electrogenic Cl− secretion has been investigated.
METHODS
Animals
Isolated mouse tissues were used in all experiments and taken from animals killed by a slowly increasing CO2 concentration. Wild-type and CF mice from two different outbred genetic backgrounds were used. One group yielded CF null mice (Cftrtm1Cam) (Ratcliff et al. 1993) while the other gave CF ΔF508 mice (Cftrtm2Cam) (Colledge et al. 1995). The mice were aged 1-6 months, the mean age for the wild-type being 91.0 ± 5.9 days (n = 43) and for the CF mice, 64.0 ± 6.3 days (n = 30), the difference indicating the short lifespan of the mutant animals.
Measurement of short-circuit current (SCC) in nasal and colonic epithelia
Nasal epithelial sheets were removed and placed in Krebs-Henseleit Solution (KHS), as was the descending colon. The nasal epithelia were mounted in Ussing chambers (window area, 1.8 mm2) exactly as described previously without further preparation (MacVinish et al. 1997b). The colon was opened lengthwise, cleaned and the outer muscle layers dissected away. The mucosa, too, was mounted in Ussing chambers (window area, 20 mm2). Usually only one distal colon and one nasal tissue were taken from each mouse. If two nasal epithelia were taken the mean result of the pair was used for data analysis. All tissues were bathed on both sides with 20 ml KHS, warmed to 37°C and bubbled with 95 % O2-5 % CO2. Tissues were clamped at zero potential, i.e. short circuited, exactly as described previously (Cuthbert, MacVinish, Hickman, Ratcliff, Colledge & Evans, 1994) and a record of the currents collected on a data acquisition system.
Estimation of intracellular Ca2+ ion concentration
Intracellular Ca2+ ion concentration ([Ca2+]i) was measured as the ratio of the intensity of fluorescence emission at 340 nm and 380 nm, when irradiated at 510 nm, in freshly isolated, fura-2-loaded nasal epithelial cells. Total nasal epithelium from two mice was found to provide enough material for a single determination. The tissues were chopped finely with scissors until a milky suspension was obtained, suspended in 0.5 ml Krebs Buffered Solution (KBS) and kept cool on ice. The cell suspension was harvested by centrifugation (300 × g for 2 min) and resuspended in 1.25 ml KBS containing 40 μM fura-2 AM (Molecular Probes; from a 10 mM stock solution in DMSO containing 20 % Pluronic F-127, Sigma) plus 250 μM sulphinpyrazone (Sigma, to inhibit extrusion of fura-2), after which the cells were incubated at 25°C with gentle agitation. After 2 h the cells were washed three times by centrifugation and resuspension in KBS. The final suspension, in 0.75 ml KBS, was placed in a stirred cuvette at 37°C in a Hitachi F-2000 Fluorescence Spectrometer. Drugs were added to the suspension in small volumes using a syringe, and the 340 nm/380 nm ratio displayed. The final drug concentrations were: forskolin, 10 μM; and TBHQ-ionomycin, 12.5 μM and 5 μM, respectively.
Drugs and solutions
KHS had the following composition (mM): NaCl, 118; KCl, 5.3; CaCl2, 2.5; MgSO4, 1.2; KH2PO4, 1.2; NaHCO3 25; and glucose 11.0. Gassed with 95 % O2-5 % CO2 at 37°C, the solution had a pH of 7.4. When Ba2+ was used MgSO4 was replaced with MgCl2. When TEACl, 20 mM, was used it was dissolved in modified KHS with reduced NaCl so that there was no change in cation concentration when added to the tissue bath. KHS was modified to give KBS as follows (mM): NaCl, 137; KCl, 5.3; CaCl2, 1.0; MgSO4, 0.3; KH2PO4, 0.4; Hepes, 10; glucose, 11.1; and bovine serum albumin (BSA), 0.1 % w/v. This solution was adjusted to pH 7.4 and bubbled with 100 % O2.
Below are listed drugs, sources and concentrations used, plus the side of the epithelium to which they were added, where ap is apical and bl is basolateral: amiloride (Sigma), 100 μM ap; azimilide (a gift from Proctor and Gamble), 100 μM bl; Ba2+ (Sigma), 5 mM bl; TEACl (Sigma), 20 mM bl; charybdotoxin (Sigma), 50 nM bl; clofilium (Research Biochemicals International), 100 μM bl; 1-ethyl-2-benzimdazolinone (1-EBIO; Aldrich), 600 μM both sides; forskolin (Calbiochem), 10 μM both sides; trans-6-cyano-4-(N-ethylsulphonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane (293B; a generous gift from R. Greger, Universität Freiburg, Germany), 100 μM bl; 2,5-di-(tert-butyl)-1,4-benzohydroquinone (TBHQ; Aldrich); and ionomycin (Calbiochem).
Forskolin, TBHQ, ionomycin and 1-EBIO were made as 1000 × stock solutions in 95 % alcohol, whereas 293B was made as 1000 × stock solution in DMSO. BSA, 0.1 %, was added to the stock solution of charybdotoxin to prevent adsorption to the glass surfaces of the KHS reservoirs.
Statistical analysis
Mean values ± standard error of the mean (s.e.m.) are given throughout together with the number of observations (n). Tests for significance were made using Student's two-tailed t test or, where it was found that the standard deviations of the populations being compared were significantly different, a non-parametric Mann- Whitney U test was used. A P value less than 0.05 was considered significant.
RESULTS
Responses to amiloride and forskolin in wild-type and cystic fibrosis nasal epithelia
In all experiments in this study the tissues were exposed first to amiloride (100 μM) on the apical face. This allowed an estimate of electrogenic Na+ transport and prevented this transporting activity complicating subsequent measurements. Effects of amiloride followed by forskolin in murine nasal epithelia are shown in Fig. 1. Four sets of results are given, one each for the four types of animals used. The first group was of wild-type animals with the same genetic background as the second group, that of CF null mice (Cftrtm1Cam); indeed they were the littermates of the latter. The third group were wild-type animals with the same genetic background as CF ΔF508 mice (Cftrtm2Cam), the latter forming the fourth group. No distinction was made between the responses in wild-type heterozygotes and homozygotes as in previous studies no differences were discernable (Cuthbert et al. 1994). CF null and CF ΔF508 nasal epithelia had significantly larger amiloride-sensitive currents than the corresponding wild-type tissues, as reported in our earlier study (MacVinish et al. 1997b). However, the responses to forskolin were not significantly different in wild-type or CF nasal epithelia from either genetic background (Fig. 1B and C). These responses represent cAMP-dependent, electrogenic Cl− secretion as shown by the sensitivity to the Na+-K+-2Cl− cotransport inhibitor, furosemide (frusemide; Figs 1A and 6A). Strictly, the cAMP-dependent activity should be described as anion secretion as the inhibition by furosemide is incomplete. However, furosemide addition essentially converts the experiment to a Cl−-free condition, as far as efflux through the apical face is concerned, allowing other ions (HCO3−) to substitute for Cl− (A. W. Cuthbert, unpublished observations).
Figure 1. Effects of forskolin on anion secretion in nasal epithelia.
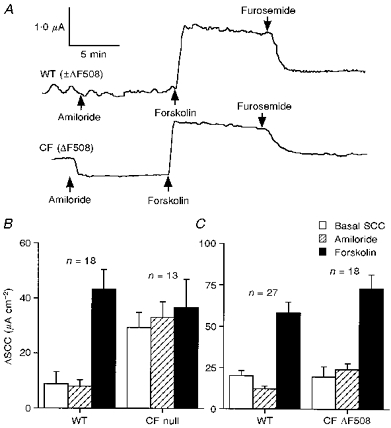
A, typical SCC traces showing the effects of the sequential addition of amiloride (100 μM, apically), forskolin (10 μM, both sides) and furosemide (1 mM, basolaterally) on nasal epithelia from a wild-type (WT, ±ΔF508; upper trace) and a CF (ΔF508; lower trace) mouse. B and C show the basal, amiloride-sensitive and forskolin-sensitive changes in SCC in 4 groups of nasal epithelia. In B, data for wild-type (consisting of 12 heterozygotes and 6 homozygotes) and CF null mouse tissues are shown, all animals being derived from the same outbred genetic background. In C, comparable data are shown for wild-type (11 heterozygotes and 16 homozygotes) and CF ΔF508 mouse tissues. In both B and C the amiloride-sensitive SCC was significantly greater in the CF tissues (P < 0.0003 and 0.005, respectively; Mann-Whitney U test) while the responses to forskolin in each pair were not different.
Figure 6. SCC traces showing effects of K+ channel blockers and 1-EBIO on colonic epithelia.
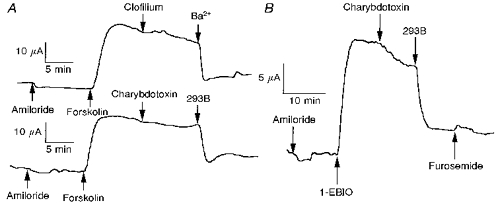
A, lack of effect of clofilium and charybdotoxin and effectiveness of Ba2+ and 293B in blocking the action of forskolin on wild-type colons. B, effect of 1-EBIO on wild-type colon and partial inhibition by charybdotoxin and major inhibition by 293B.
Effects of K+ channel blockers on responses to forskolin in nasal epithelia
Addition of the non-selective K+ channel blocker Ba2+ to the basolateral aspect of nasal epithelia after the SCC had been increased by forskolin produced, in all four experimental groups, a large and significant reduction in SCC, indicating that basolateral K+ channels played a significant role in maintaining Cl− secretion in these conditions (Fig. 2). As the pattern of response was similar in normal and CF tissues, and from either genetic background, no distinction has been made in the rest of this study between wild-type nasal epithelia from either genetic background or between CF null and CF ΔF508 tissues. This was necessary because of the limited supply of transgenic CF animals. Nevertheless, the proportions of the different types of tissue are indicated in the figure captions and legends where appropriate.
Figure 2. Effects of Ba2+ ions on the forskolin-sensitive SCC responses in nasal epithelia.
The figure shows the SCC increases remaining at steady state 10 min after the addition of the drug and the effect Ba2+ (5 mM) had on this value. The 4 groups of tissues are as in Fig. 1. In all instances Ba2+ caused a significant reduction in current (Mann-Whitney paired U test). In 30 % of these measurements another agent (either charybdotoxin or 293B) was added before Ba2+, but had no effect. Numbers of experiments and statistical significance are shown on the figure.
The same protocol as used with Ba2+ was used to further examine K+ channel blockers, i.e. nasal epithelia were treated sequentially with amiloride then forskolin, and after 10 min, when a steady plateau current had been achieved, the K+ channel blocker was added. This new agent was allowed to act for a further 10 min, after which the effects of Ba2+ on any residual current were ascertained. Clofilium produced a significant decrease in SCC following forskolin (Fig. 3), but the effect was relatively slow in onset compared with that of Ba2+ (see for example Fig. 4). A second agent, TEA, used in high concentration always produced a distinct, but small, fall in SCC after forskolin; the reduction, however, never reached statistically significant proportions (Fig. 4). The effects of TEA and clofilium were no different in wild-type or CF tissues.
Figure 3. Effects of clofilium on the responses to forskolin.
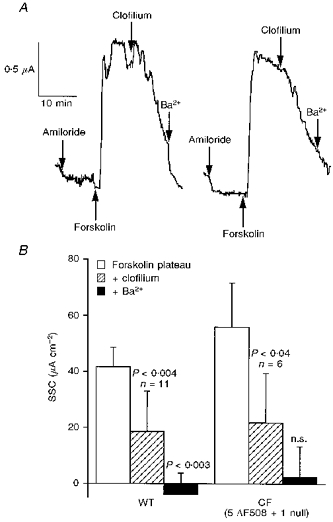
In A, responses in a wild-type (left) and CF (right) nasal epithelium to 100 μM clofilium (applied basolaterally) are shown. Ten minutes after clofilium was added, Ba2+ (5 mM) was added to the same side. Data for 11 wild-type (6 heterozygotes and 5 homozygotes) and 6 CF nasal epithelia are given in B. Mann-Whitney paired U test was used to test for significance. Note, in CF basal epithelia, current was not further reduced by Ba2+ after clofilium.
Figure 4. Effects of TEA on the responses to forskolin.
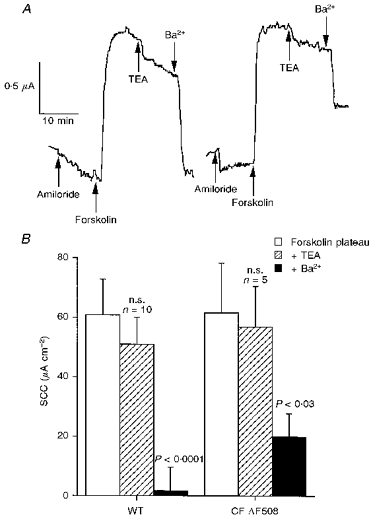
In A, responses of a wild-type (left) and a CF (right) nasal epithelium to 30 mM TEA (applied basolaterally) are shown. Ten minutes after TEA was added, Ba2+ (5 mM) was added to the same side. Data for 10 wild-type (6 heterozygotes and 4 homozygotes) and 5 CF nasal epithelia are given in B. The forskolin-induced current was significantly inhibited by Ba2+, but not TEA (Mann-Whitney paired U test), as indicated on the figure.
Three other K+ channel blockers, charybdotoxin, 293B and azimilide, were either without effect or had only a minimal action on the forskolin-sensitive SCC in nasal epithelia. The data from these experiments are given in Table 1. While the effects of the inhibitors were insignificant or non-existent, subsequent addition of Ba2+ produced highly significant reductions in SCC.
Table 1.
Effects of K+ channel blockers on SCC following activation by forskolin in nasal epithelia
| Genotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blocker | Concentration | Null | ΔF | Basal SCC (μA cm-2) | Amiloride ΔSCC (μA cm-2) | Forskolin (plateau) ΔSCC (μA cm-2) | 1st blocker ΔSCC (μA cm-2) | Ba2+ΔSCC (μA cm-2) | |
| Charybdotoxin | 50 nM | WT | 3 | 3 | 3.8 ± 3.6 | −3.5 ± 1.8 | 31.4 ± 10.5 | 0 ± 0 | −21.8 ± 6.4 |
| Charybdotoxin | 50 nM | CF | — | 3 | 14.9 ± 19.2 | −21.8 ± 15.1 | 64.8 ± 29.6 | 0 ± 0 | −38.7 ± 17.4 |
| 293B | 100 μM | WT | 1 | 4 | 14.3 ± 6.2 | −6.1 ± 2.6 | 51.7 ± 13.0 | 0 ± 0 | −33.1 ± 6.2 |
| 293B | 100 μM | CF | — | 2 | 43.1 | −23.3 | 70.3 | 0 | n.d. |
| Azimilide | 100 μM | WT | 4 | 2 | 5.5 ± 7.6 | −3.5 ± 1.9 | 52.8 ± 15.7 | −7.8 ± 2.7 | −25.8 ± 6.2 |
The genetic background of the wild-type (WT) and CF animals is indicated. 1st blocker refers to the agent given at the left-hand side of the table. Ba2+ was added after the effect of the first blocker had reached equilibrium. n.d., not determined.
Effects of K+ channel blockers on forskolin responses in colonic epithelia
The protocol for investigating the effects of K+ channel blockers on colonic epithelia followed the same pattern as for nasal epithelia. Tissues were exposed sequentially to amiloride then forskolin, followed by the K+ channel blocker once a stable SCC response to forskolin had been achieved. The effects of amiloride and forskolin on murine colonic epithelia have been described previously. In wild-type tissues forskolin produces a Cl− secretory response sensitive to furosemide, while in CF tissues the SCC is decreased following forskolin due to the stimulation of K+ secretion, an effect exposed by the failure to secrete Cl− (Cuthbert et al. 1994).
The non-selective K+ channel blocker Ba2+ was as effective in inhibiting the forskolin-sensitive SCC as it was in nasal epithelia (Fig. 5A). TEA had an insignificant effect on the colon, as it did in nasal epithelia. CF colonic epithelia showed only a transient change in SCC when Ba2+ was applied after forskolin, but the SCC reduction could be reversed by furosemide (Fig. 5B).
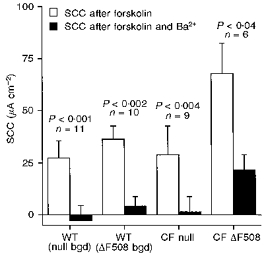
Figure 5. Diagrammatic representations of the effects of Ba2+ and 293B on responses to forskolin in colonic epithelia.
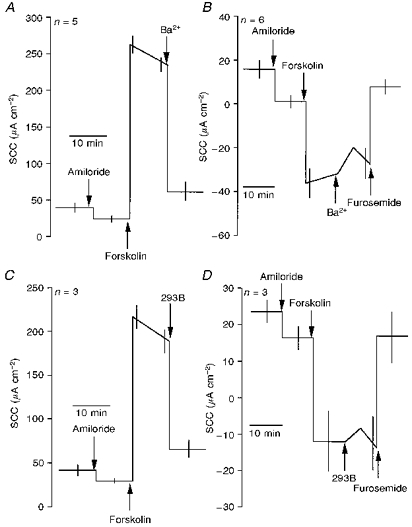
Effects of 5 mM Ba2+ (A and B) and 100 μM 293B (C and D) on responses of wild-type (A and C) and CF (B and D) epithelia are shown. Note that in the CF epithelia both Ba2+ and 293B produced only transient effects, while the response to forskolin was reversed by furosemide. The effects of Ba2+ and 293B on wild-type colonic epithelia were significant (P < 0.0001 and P < 0.001, respectively; Student's t test).
In sharp contrast to the lack of effect on nasal epithelia, 293B proved to be as effective as Ba2+ in blocking the effects of forskolin on colonic epithelia (Fig. 5C), and again only produced a transient effect in CF tissues (Fig. 5D). Clofilium, which was effective in nasal tissue, was without effect on the colon, as was charybdotoxin. Azimilide was only weakly active in the colon, producing 25 % inhibition of the forskolin-stimulated current. In the presence of azimilide, 293B was subsequently able to inhibit virtually all of the remaining current. Data obtained with clofilium, charybdotoxin, TEA and azimilide on colonic epithelia are given in Table 2 and Fig. 6A.
Table 2.
Effects of K+ channel blockers on SCC following activation by forskolin in colonic epithelia
| Genotype | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blocker | Concentration | Null | ΔF | Basal SCC (μA cm-2) | Amiloride ΔSCC (μA cm-2) | Forskolin (plateau) ΔSCC (μA cm-2) | 1st blocker ΔSCC (μA cm-2) | 2nd blocker ΔSCC (μA cm-2) | |
| Clofilium | 100 μM | WT | 2 | 1 | 38.8 ± 4.3 | −13.2 ± 0.9 | 130.5 ± 12 | 0 ± 0 | −85.2 ± 6.5 (Ba2+) |
| Clofilium | 100 μM | CF | 1 | — | 23.7 | −12.0 | −11.3 | 0 | 13.2 (Fr) |
| Charybdotoxin | 50 nM | WT | 3 | 1 | 54.5 ± 8.4 | −8.4 ± 3.2 | 143.2 ± 20.8 | −3.3 ± 3.3 | −106.2 (Ba2+) |
| Azimilide | 100 μM | WT | 2 | 1 | 31.7 ± 7.1 | −2.7 ± 1.4 | 175.2 ± 18.6 | −42.1 ± 21.1 | −114.1 ± 15.8 (293B) |
| Azimilide | 100 μM | CF | 1 | — | 10.7 | −13.6 | −33.7 | 0 | n.d. |
| TEA | 20 mM | WT | 2 | 1 | 22.4 ± 11.6 | −5.4 ± 2.2 | 174.7 ± 79.6 | −20.7 ± 27.8 | −85 ± 29.3 (Ba2+) |
The distribution of genotypes is indicated as for Table 1. The nature of the second blocker is indicated at the right-hand side of the table. Fr, furosemide. n.d., not determined.
Effects of K+ channel blockers on the responses to EBIO in nasal and colonic epithelia
1-EBIO is reported to be a specific Ca2+-sensitive K+ channel opener which also opens CFTR Cl− channels (Devor, Singh, Frizzell & Bridges, 1996a, b). As the thrust thus far has been to examine the effects of K+ channel blockers on cAMP-induced SCCs, presumably by acting on cAMP-dependent K+ channels, it seemed worthwhile to examine their effects on 1-EBIO induced currents.
In wild-type nasal epithelium, 1-EBIO had only a minor effect which was transient and did not prevent subsequent addition of forskolin from causing the usual Cl− secretory current, sensitive to furosemide. In contrast, 1-EBIO was as effective as forskolin in increasing SCC in wild-type colonic epithelium (Fig. 6B). In accordance with prediction, the 1-EBIO-sensitive current was sensitive to charybdotoxin, but only 20 % inhibition was produced, while much of the remaining current was sensitive to 293B. Figure 7 summarizes the effects of 1-EBIO on nasal and colonic epithelia.
Figure 7. Diagrammatic representations of the effects of 1-EBIO on Cl− secretory responses of wild-type nasal and colonic epithelia.
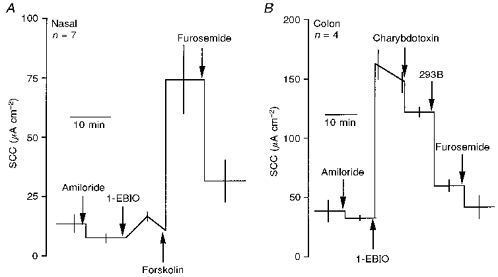
A, the effect of 600 μM 1-EBIO (applied both sides) on nasal epithelia was small and transient. Subsequently, forskolin was able to increase SCC, which was significantly inhibited by furosemide (P < 0.03). B, the current generated by 600 μM 1-EBIO (applied both sides) in the colon was partially sensitive to charybdotoxin (50 nM, P < 0.01) and majorly affected by 293B (100 μM, P < 0.001; Student's t test throughout).
Effect of forskolin on [Ca2+]i in murine nasal epithelium
Tissues from Cftrtm1Cam and Cftrtm2Cam mice lack functional CFTR (Ratcliff et al. 1993; Colledge et al. 1995) and therefore would not be expected to demonstrate cAMP-dependent Cl− secretion. The data of Fig. 5 indicate this is so for colonic epithelia. However, from Fig. 1 both wild-type and CF nasal epithelia show equivalent responses to forskolin. Ca2+-dependent Cl− secretion has been proposed as an explanation for the aberrant responses in murine CF tracheal epithelium (Grubb, Paradiso & Boucher, 1994a), but no demonstration of the Ca2+-releasing action of forskolin has been made in nasal epithelia. Direct evidence for this was sought by measuring the ratio of the intensity of fluorescence emission at 340 nm and 380 nm of fura-2-loaded cells irradiated at 510 nm (the 340 nm/380 nm ratio). Forskolin (10 μM) increased the 340 nm/380 nm ratio in freshly isolated, wild-type, nasal epithelial cells from 3.51 ± 0.39 to 3.81 ± 0.42 (P < 0.001,n = 9, Student's paired t test; i.e. an increase of 0.294 ± 0.059). The Ca2+-releasing agents TBHQ-ionomycin caused a larger increase in the 340 nm/380 nm ratio from 3.28 ± 0.39 to 4.09 ± 0.55 (P < 0.006,n = 7, Student's paired t test; i.e. an increase of 0.817 ± 0.197). Patterns of response to these agents are given in Fig. 8A. Of particular interest was the ratio of the response to forskolin compared with that to TBHQ-ionomycin. The ratio was 1.83 ± 0.11 when forskolin was given first, and 7.16 ± 2.19 when forskolin was given second, these values being significantly different, P < 0.03 (Fig. 8B). Thus, the release of intracellular Ca2+ by TBHQ-ionomycin attenuated the ability of forskolin to cause further release, indicating that at least some of the storage sites are common to both agents. As nasal epithelia from two mice are needed to obtain sufficient cells for a single fura-2 determination it was possible to make only a few observations in CF nasal epithelia, where the pattern of response was as with the wild-type epithelia (Fig. 8C).
Figure 8. Fura-2 fluorescence in nasal epithelial cells.
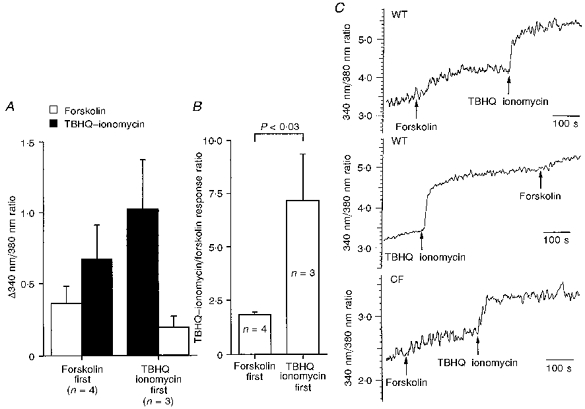
A, effects of forskolin (10 μM) and TBHQ-ionomycin (12.5 μM and 5 μM, respectively) on [Ca2+]i indicated by the 340 nm/380 nm ratio in fura-2-loaded tissues. B, ratio of the response to TBHQ-ionomycin to that for forskolin depends on the order in which the agents are given. The ratio is significantly greater when TBHQ-ionomycin are given first. C shows examples of 340 nm/380 nm ratios for preparations from wild-type nasal epithelia (top and middle traces) and from a CF preparation (bottom trace).
DISCUSSION
It may seem paradoxical that forskolin-dependent anion secretion was not different in wild-type and CF nasal epithelia, a situation which is not so for colon epithelia. Others have reported a difference in the response to forskolin between wild-type and CF nasal epithelia using Cftrtm1UNC mice (Grubb et al. 1994b), but even here the CF tissues still showed a substantial forskolin response. Clearly, CF nasal epithelia from null mice can have no functional CFTR, so the ability to maintain a secretory current must depend on the development or upregulation of alternative mechanisms. One mechanism, involving upregulation of the calcium-activated chloride channel (CAC) response and the ability of forskolin to release intracellular Ca2+ has been proposed (Grubb et al. 1994a). When nasal epithelia are first treated with TBHQ-ionomycin, to release intracellular Ca2+ stores, subsequent addition of forskolin clearly differentiates wild-type and CF tissues (MacVinish et al. 1997b). Nevertheless, CF nasal epithelia retain other characteristics which underlie their genetic status, for example increased electrogenic Na+ absorption, as shown both here and previously (MacVinish et al. 1997b). However, responses to Ca2+-releasing agents in nasal epithelia were small and poorly maintained, quite unlike the large, well-maintained currents to forskolin seen in this study. The conclusion is that other activities, as well as Ca2+ release, are responsible for the behaviour of CF nasal epithelia in response to forskolin. It has been shown here that forskolin can release intracellular Ca2+ in nasal epithelium, but is less effective in this respect after more powerful Ca2+-releasing agents. The main hypothesis tested in this study is that cAMP-dependent K+ channels (Hwang, Suh, Bae, Lee & Jung, 1996; Rufo et al. 1997) are important for the maintenance of forskolin responses, both in the airway epithelium and in the colon. Activation of basolateral K+ channels will hyperpolarize the cell, increasing the electrical driving force for Cl− exit across the apical face (Smith & Frizzell, 1984). To examine this, a variety of K+ channel blockers have been used, paying particular attention to any showing tissue specificity.
In this study, no differences were seen between the responses of wild-type and CF nasal epithelia to K+ channel blockers, unlike the situation in the colon where the blockers have no effect in CF tissues. K+ channel blockers were each used at a single concentration, indicated in the literature as producing maximal effects, as referenced in the following paragraph. While this approach may seem arbitrary, the relative activities of blockers are very different and, more importantly, a different sequence of activity is given by airway epithelium compared with the colon. For the nasal epithelium the sequence is: Ba2+ > clofilium ⋙ azimilide = TEA ⋙ 293B = charybdotoxin, whereas for the colon the sequence is: Ba2+ = 293B ⋙ azimilide = TEA ⋙ clofilium = charybdotoxin.
Ba2+ is not considered to be a selective K+ channel blocker (Mandel, McRoberts, Beuerlein, Foster & Dharmsathaphorn, 1986) and produces a rapid and major reduction in the forskolin-sensitive current in both tissues. In contrast the Ca2+-sensitive K+ channel blocker charybdotoxin (McCann, Matsuda, Garcia, Kaczorowski & Welsh, 1990) had no effect on the forskolin-sensitive current in either tissue, suggesting that Ba2+ action is predominantly on cAMP-sensitive K+ channels. A major interest, however, centres on two other agents, namely clofilium (Folander, Smith, Antanavage, Bennett, Stein & Swanson, 1990) and 293B (Lohrmann et al. 1995). The former is effective in the nasal epithelium without any effect on the colon, while the latter is very effective on the colon without any effect on nasal tissue. Others have shown that 293B, applied basolaterally, is effective in the colon (Lohrmann et al. 1995; Diener, Hug, Strabel & Scharrer, 1996), but we are unaware of any other study with airway tissues. Taken together the results with 293B and clofilium suggest the K+ channels are not the same in the airways and gut. The lead chromanol 293B is considered to block a cAMP-regulated K+ conductance found in the basolateral membranes of the colonic crypt cells (Lohrmann et al. 1995). Originally this was thought to be a IsK channel consisting of the slowly activating voltage-dependent IsK membrane protein, but it now appears that IsK forms a hetero-oligomeric complex with KvLQT1 (Barhanin, Lesage, Guillemare, Fink, Lazdunski &Romey, 1996; Sanguinetti et al. 1996; Loussouarn, Charpentier, Mohammad-Panah, Kunzelmann, Baro & Escande, 1997). Formation of the channel complex radically alters the channel kinetics of KvLQT1, and mutations in these channels are responsible for the long-QT interval syndrome in man (Tyson et al. 1997). Blockers of IsK may be valuable in cardiac arrhythmias, so it is not surprising that two of the drugs investigated here are class III antiarrhythmics, namely clofilium and azimilide. The failure of 293B to affect the cAMP-dependent Cl− current in nasal epithelium may mean that the hetero-oligomeric composition of K+ channels in the colon is different from the airways, or alternatively totally different K+ channels exist in the two tissues. Some evidence for the former view follows from a study in which the cDNA encoding rat kidney IsK was expressed in Xenopus oocytes (Folander et al. 1990), where it is now known that endogenous KvLQT-like proteins exist (Bleich et al. 1997). Delayed rectifier K+ currents were obtained which were sensitive to the antiarrhythmic drug clofilium. This agent was effective on the nasal, but not the colonic, epithelium in the present study. Thus two agents, 293B and clofilium, shown to block IsK-KvLQT-type channels, are exclusively selective on the two epithelial tissues we have explored. Others have reported that the IsK-mediated cAMP-sensitive K+ currents in permeabilized cultured rat tracheal cells are blocked by clofilium (Hwang et al. 1996), consistent with our findings with nasal epithelium. Azimilide, another class III antiarrhythmic, was found to block IsK channels expressed in oocytes (Busch, Herzer, Takumi, Krippeit-Drews, Waldegger & Lang, 1994), while in this study on intact tissues it had only a modest effect, non-selectively on both tissues.
Useful information can be obtained from the effects of K+ channel blockers on CF colonic epithelia. The exposure of electrogenic K+ secretion by forskolin and its reversal by furosemide is well known (Cuthbert et al. 1994). Neither Ba2+ nor 293B have any substantial effect after forskolin; indeed, it might be expected that they would reduce the electrical gradient for K+ efflux through the apical membrane and transiently they appeared to do this. More importantly, they had no action on the reversal of the K+ current by furosemide, indicating that these two agents do not interfere with the basolateral co-transporter. A similar argument can be made for clofilium (Table 2).
1-EBIO is reported to be a K+ channel opener, specifically of Ca2+-activated K+ channels, but also activates CFTR (Devor et al. 1996a, b). It proved to have a minor effect on nasal epithelium, not unlike the responses to TBHQ- ionomycin, consonant with the idea that the sustained forskolin response in CF nasal epithelium cannot be due to an action on CACs alone or together with Ca2+-activated K+ channels. In contrast, 1-EBIO was as effective on the colon as forskolin, generating a sustained SCC response sensitive to furosemide. Only a fraction of the current generated by 1-EBIO was sensitive to charybdotoxin, indicating some activation of Ca2+-sensitive K+ channels, but a major part of the current was sensitive to 293B. Thus 1-EBIO too differentiates between the K+ channels of the airways and the colon. Mandel et al. (1986) were the first to point out that Cl−-secreting epithelia have two types of basolateral K+ channels, one Ca2+- and the other cAMP-sensitive, both of which could be mobilized to promote anion transport.
Overall, the conclusion from this work suggests that in murine CF airway epithelium forskolin responses are well maintained and not different from those of wild-type tissues, partially because of the upregulation of the CAC response, but also because of the activation of cAMP-dependent K+ channels in the basolateral membrane. This latter effect is likely to be a component of the explanation for the lack of lung pathology in CF mice (Clarke et al. 1994). The absence of alternative Cl− channels in the gut means that even when cAMP-dependent K+ channels are activated there can be no secretory response, and explains why gut pathology is the major cause of morbidity in CF mice. Furthermore, it also indicates that K+ channel openers specifically targeted to airway K+ channels could make other forms of treatment for CF more effective, and some selectivity has already been demonstrated, albeit mainly for blockers. Three examples of such adjuvant therapy might be: the exploitation of CAC channel responses, known to be upregulated in human CF; alongside minimally effective gene therapy for CFTR; and in conjunction with drugs developed to increase the fraction of ΔF508 CFTR reaching the membrane.
Acknowledgments
Support from the Medical Research Council, The Wellcome Trust and the Cystic Fibrosis Trust is gratefully accepted. We thank Professor M. J. Evans and Dr W. H. Colledge for supplying the CF mice used in this study.
References
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Bleich M, Briel M, Busch AE, Lang HJ, Gerlach U, Gogelein H, Greger R, Kunzelmann K. KVLQT1 channels are inhibited by the K+ channel blocker 293B. Pflügers Archiv. 1997;434:499–501. doi: 10.1007/s004240050427. [DOI] [PubMed] [Google Scholar]
- Boat TF, Welsh MJ, Beaudet AL. In: The Metabolic Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, Stansbury JB, Wyngaarden JB, Fredrickson DS, editors. New York: McGraw-Hill; 1989. pp. 2649–2680. [Google Scholar]
- Busch AE, Herzer T, Takumi T, Krippeit-Drews P, Waldegger S, Lang F. Blockade of human IsK channels expressed in Xenopus oocytes by the novel class III antiarrhythmic NE-10064. European Journal of Pharmacology. 1994;264:33–37. doi: 10.1016/0014-2999(94)90632-7. [DOI] [PubMed] [Google Scholar]
- Cheng SH, Gregory RJ, Marshall J, Paul S, Souza DW, White GA, O'Riordan CR, Smith AE. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- Clarke LL, Grubb BR, Yankaskas JR, Cotton CU, McKenzie A, Boucher RC. Relationship of a non-cystic fibrosis transmembrane conductance regulator-mediated chloride conductance to organ level disease in Cftr(-/-) mice. Proceedings of the National Academy of Sciences of the USA. 1994;91:479–483. doi: 10.1073/pnas.91.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, MacVinish LJ, Anderson JR, Cuthbert AW, Evans MJ. Generation and characterisation of a ΔF508 cystic fibrosis mouse model. Nature Genetics. 1995;10:445–452. doi: 10.1038/ng0895-445. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW, MacVinish LJ, Hickman ME, Ratcliff R, Colledge WH, Evans MJ. Ion-transporting activity in the murine colonic epithelium of normal animals and animals with cystic fibrosis. Pflügers Archiv. 1994;428:508–515. doi: 10.1007/BF00374572. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzoimidazolones. I. Direct activation of a Ca2+-dependent K+ channel. American Journal of Physiology. 1996a;271:L775–784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Frizzell RA, Bridges RJ. Modulation of Cl− secretion by benzoimidazolones. II. Coordinate regulation of apical GCl and basolateral GK. American Journal of Physiology. 1996b;271:L785–795. doi: 10.1152/ajplung.1996.271.5.L785. [DOI] [PubMed] [Google Scholar]
- Diener M, Hug F, Strabel D, Scharrer E. Cyclic AMP-dependent regulation of K+ transport in the rat distal colon. British Journal of Pharmacology. 1996;118:1477–1478. doi: 10.1111/j.1476-5381.1996.tb15563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folander K, Smith JS, Antanavage J, Bennett C, Stein RB, Swanson R. Cloning and expression of the delayed-rectifier IsK channel from neonatal rat heart and diethylstilbestrol-primed rat uterus. Proceedings of the National Academy of Sciences of the USA. 1990;87:2975–2979. doi: 10.1073/pnas.87.8.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BR, Paradiso AM, Boucher RC. Anomalies in ion transport in CF mouse tracheal epithelium. American Journal of Physiology. 1994a;267:C293–300. doi: 10.1152/ajpcell.1994.267.1.C293. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Vick RN, Boucher RC. Hyperabsorption of Na+ and raised Ca2+-mediated Cl− secretion in nasal epithelia of CF mice. American Journal of Physiology. 1994b;266:C1478–1483. doi: 10.1152/ajpcell.1994.266.5.C1478. [DOI] [PubMed] [Google Scholar]
- Hwang T-H, Suh D-J, Bae H-R, Lee S-H, Jung J-S. Characterisation of K+ channels in the basolateral membrane of rat tracheal epithelia. Journal of Membrane Biology. 1996;154:251–257. doi: 10.1007/s002329900149. [DOI] [PubMed] [Google Scholar]
- Lohrmann E, Burhoff I, Nitchke RB, Lang HJ, Mania D, Englert HC, Hropot M, Warth R, Rohm W, Bleich M, Greger R. A new class of inhibitors of cAMP-mediated Cl− secretion in rabbit colon, acting by the reduction of cAMP-activated K+ conductance. Pflügers Archiv. 1995;429:517–530. doi: 10.1007/BF00704157. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Charpentier F, Mohammad-Panah R, Kunzelmann K, Baro I, Escande D. KvLQT1 potassium channel but not IsK is the molecular target for trans-6-cyano-4-(N-ethylsulfonyl-N-methylamino)-3-hydroxy-2,2-dimethyl-chromane. Molecular Pharmacology. 1997;52:1131–1136. doi: 10.1124/mol.52.6.1131. [DOI] [PubMed] [Google Scholar]
- McCann JD, Matsuda J, Garcia M, Kaczorowski G, Welsh MJ. Basolateral K+ channels in airway epithelia. Regulation by Ca2+ and block by charybdotoxin. American Journal of Physiology. 1990;258:L334–342. doi: 10.1152/ajplung.1990.258.6.L334. [DOI] [PubMed] [Google Scholar]
- MacVinish LJ, Gill DR, Hyde SC, Moffard KA, Evans MJ, Higgins CF, Colledge WH, Huang L, Sorgi F, Ratcliff R, Cuthbert AW. Chloride secretion in the trachea of null cystic fibrosis mice: the effects of transfection with pTrial10-CFTR2. The Journal of Physiology. 1997a;499:677–687. doi: 10.1113/jphysiol.1997.sp021960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVinish LJ, Goddard C, Colledge WH, Higgins CF, Evans MJ, Cuthbert AW. Normalisation of ion transport in murine cystic fibrosis nasal epithelium using gene transfer. American Journal of Physiology. 1997b;273:C734–740. doi: 10.1152/ajpcell.1997.273.2.C734. [DOI] [PubMed] [Google Scholar]
- Mandel KG, McRoberts JA, Beuerlein G, Foster ES, Dharmsathaphorn K. Ba2+ inhibition of VIP- and A23187-stimulated Cl− secretion by T84 monolayers. American Journal of Physiology. 1986;250:C486–494. doi: 10.1152/ajpcell.1986.250.3.C486. [DOI] [PubMed] [Google Scholar]
- Olivier KN, Bennet WD, Hohneker KW, Zemen KL, Edwards LJ, Boucher RC, Knowles MR. Acute safety and effects on mucociliary clearance of aerosolised uridine 5′-triphosphate +/- amiloride in normal human adults. American Journal Respiratory and Critical Care Medicine. 1996;154:217–223. doi: 10.1164/ajrccm.154.1.8680683. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Evans MJ, Cuthbert AW, MacVinish LJ, Foster D, Anderson JR, Colledge WH. Production of a severe cystic fibrosis mutation in mice by gene targeting. Nature Genetics. 1993;4:35–41. doi: 10.1038/ng0593-35. [DOI] [PubMed] [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, Zielenski J, Lok S, Plavsic N, Chou JL, Drumm ML, Iannuzzi MC, Collins FS, Tsui LC. Identification of the cystic fibrosis gene: cloning and characterisation of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rubenstein RC, Egan ME, Zeitlen PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells. Journal of Clinical Investigation. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufo PA, Merlin D, Riegler M, Ferguson-Maltzman MH, Dickinson BL, Brugnara C, Alper SL, Lencer WL. The antifungal antibiotic, clotrimazole, inhibits chloride secretion by human intestinal T84 cells via blockade of distinct basolateral K+ conductances. Demonstration of efficacy in intact rabbit colon and an in vivo mouse model of cholera. Journal of Clinical Investigation. 1997;100:3111–3120. doi: 10.1172/JCI119866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KVLQT1 and minK (IKs) proteins to form cardiac IKs potassium channels. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Smith PL, Frizzell RA. Chloride secretion by canine tracheal epithelium: IV. Basolateral membrane K permeability parallels secretion rate. Journal of Membrane Biology. 1984;77:187–199. doi: 10.1007/BF01870568. [DOI] [PubMed] [Google Scholar]
- Tyson J, Tranebjærg L, Bellman S, Wren C, Taylor JFN, Bathen J, Aslaksen B, Sørland SJ, Lund O, Malcolm S, Pembury M, Bhattacharya S, Bitner-Glindzicz M. IsK and KvLQT1: mutation in either of the two subunits of the slow component of the delayed rectifier potassium channel can cause Jervell and Lange-Nielsen syndrome. Human Molecular Genetics. 1997;6:2179–2185. doi: 10.1093/hmg/6.12.2179. [DOI] [PubMed] [Google Scholar]


