Abstract
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive, and usually fatal pulmonary disease for which there are no proven or approved drug therapies. Anti-inflammatory and immunosuppressive agents have been largely ineffective. The precise relationship of IPF to other idiopathic interstitial pneumonias (IIPs) is not known, despite the observation that different histopathological patterns of IIP may co-exist in the same patient. We propose that these different histopathological “reaction” patterns may be determined by complex interactions between host and environmental factors that alter the local alveolar milieu. Recent paradigms in IPF pathogenesis have focused on dysregulated epithelial-mesenchymal interactions, an imbalance in TH1/TH2 cytokines and potential roles for aberrant angiogenesis. In this review, we discuss these evolving concepts in disease pathogenesis and emerging therapies designed to target pro-fibrogenic pathways in IPF.
CLINICAL EVALUATION AND DIAGNOSTIC APPROACH TO IPF
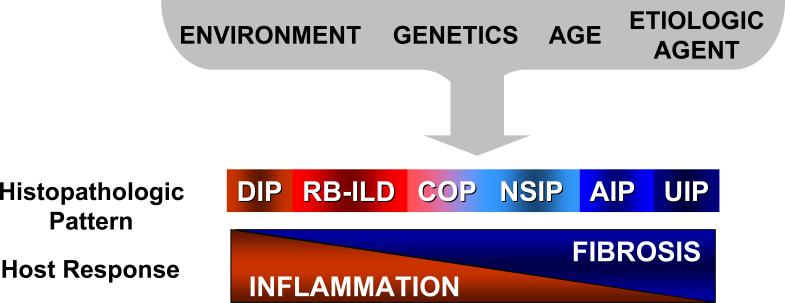
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive parenchymal lung disease with a median survival of less than three years following diagnosis, although the clinical course can be highly variable (1, 2). No pharmacologic therapies have proven effective for this disorder (3). IPF is the most common of the idiopathic interstitial pneumonias (IIPs, Figure 1) with a prevalence of 13−20 per 100,000 people in the general population (3, 4). It is more common in men than women and its prevalence increases with age (3, 4). Predictors of a worse outcome include progressive dyspnea, oxygen desaturation during the 6-minute walk (5), worsening pulmonary function and gas-exchange (6, 7), the presence and extent of honeycombing on high-resolution computed tomography (HRCT) (8), and the presence of pulmonary hypertension (2, 9).
Figure 1.
Idiopathic interstitial pneumonias (IIPs) represent an overlapping spectrum of inflammatory and fibrotic tissue reactions or histopathologic patterns in response to an “unknown” injury. At one end of the spectrum are IIPs marked predominantly by diffuse inflammatory cell infiltration; these IIPs tend to be more responsive to anti-inflammatory and immunosuppressive drug therapy. At the other end of the spectrum are IIPs characterized by fibrosis with minimal inflammation; these disease processes tend to have poor responses to currently available pharmacologic agents. Host factors (such as age and genetic polymorphisms) in combination with environmental factors (such as exposure to infectious agents and cigarette smoke) likely determine the resulting histopathological reaction patterns. DIP, desquamative interstitial pneumonia; RB-ILD, respiratory bronchiolitis associated-interstitial lung disease; COP, cryptogenic organizing pneumonia; NSIP, non-specific interstitial pneumonia; AIP, acute interstitial pneumonia; UIP, usual interstitial pneumonia.
The diagnosis of IPF is based on clinical, radiographic and histopathologic evaluations (3). Common clinical features consist of progressive dyspnea, dry cough and the presence of basilar “velcro-like” rales on examination. Digital clubbing and clinical signs of cor-pulmonale may be present. Extrapulmonary signs/symptoms are usually absent, while constitutional symptoms such as fatigue and malaise may be noted. Secondary causes of pulmonary fibrosis such as collagen-vascular disease, chronic hypersensitivity pneumonitis, adverse drug reactions, granulomatous diseases and pneumoconiosis must be excluded. More recently, HRCT has taken a more prominent role in the diagnosis of IPF and can help distinguish IPF from other IIPs (10). A patchy pattern of peripheral, subpleural and predominantly lower lobe reticular opacities combined with honeycombing, traction bronchiectasis and the absence of significant ground glass opacities together constitute the classic radiographic features of IPF. The presence of these features on HRCT, when reported by an experienced chest radiologist, correlates well with the histologic pattern of usual interstitial pneumonia (UIP) on surgical lung biopsy (11, 12). Thus, classic radiographic findings in the context of an appropriate clinical presentation may abrogate the need for a surgical lung biopsy; however, a bronchoscopy with transbronchial biopsy may be advisable in this setting, primarily to exclude infection and malignancy.
In the absence of typical clinical and radiographic features, a surgical lung biopsy is recommended for the definitive diagnosis of IPF. Diagnostic accuracy may be improved if biopsies are obtained from multiple lobes, as recent studies have shown that several distinct histopathologic patterns may co-exist in the same patient and the presence of UIP on any biopsy confer a worse prognosis (13). Histopathologic features of UIP include patchy areas of fibrosis in association with areas of normal lung architecture, the so-called “temporal” heterogeneity of UIP. Mild inflammatory cell infiltration may be present in UIP, but is not a prominent feature. Fibroblastic foci, consisting of aggregates of myofibroblasts underlying “injured,” reparative epithelium, are key histologic features of IPF (14). The presence and extent of fibroblastic foci, while not pathognomonic, are of prognostic value in IPF as the profusion of these lesions correlates with a worse prognosis (15).
PATHOGENESIS OF IPF
The etiopathogenesis of IPF remains enigmatic. Phenotypic changes in alveolar epithelial cells are an early and consistent features of IPF, suggesting that alveolar epithelial cell injury and apoptosis are key to the pathogenesis of IPF (14, 16-18). The cause(s) of alveolar epithelial cell injury associated with IPF is unknown, and host responses to tissue injury are likely to involve a combination of host-specific, genetic and environmental factors (Figure 1). Certain latent viral infections have been associated with IPF suggesting a possible infectious etiology (19-23). Other environmental factors, including cigarette smoke, have also been associated with the development of IPF (24, 25). Genetic factors seen with increased frequency in patients with IPF include mutations in surfactant protein C and polymorphisms of tumor necrosis factor-alpha (TNF-α) (26-29). Additionally, polymorphisms in transforming growth factor-β1 (TGF-β1) are associated with more rapid progression of IPF (30).
The relationship between IPF and other IIPs, such as nonspecific interstitial pneumonia (NSIP) and desquamative interstitial pneumonia (DIP) are not well defined. Recent studies have identified UIP, NSIP and even DIP in biopsy specimens from the same patient, suggesting that host responses may produce different histopathologic tissue reactions to the same stimulus/injury or that these histopathologic patterns represent a continuum that varies temporally and spatially within the same lung (13, 31, 32). Current prevailing hypotheses in the pathogenesis of IPF have focused on dysregulated interactions between epithelial and mesenchymal cells, a shift from TH1 to TH2-polarized host immune response, and aberrant angiogenesis. Additionally, recent data have demonstrated that acute exacerbations, marked by rapid clinical deterioration in previously stable patients with IPF, contribute to the increased mortality (33). Interventions to reduce the frequency and severity of these acute exacerbations may improve outcomes in IPF. These evolving concepts of disease pathogenesis and the natural history of IPF are the bases for emerging pharmacotherapies for the treatment of this complex disease.
Epithelial-Mesenchymal Interactions in IPF
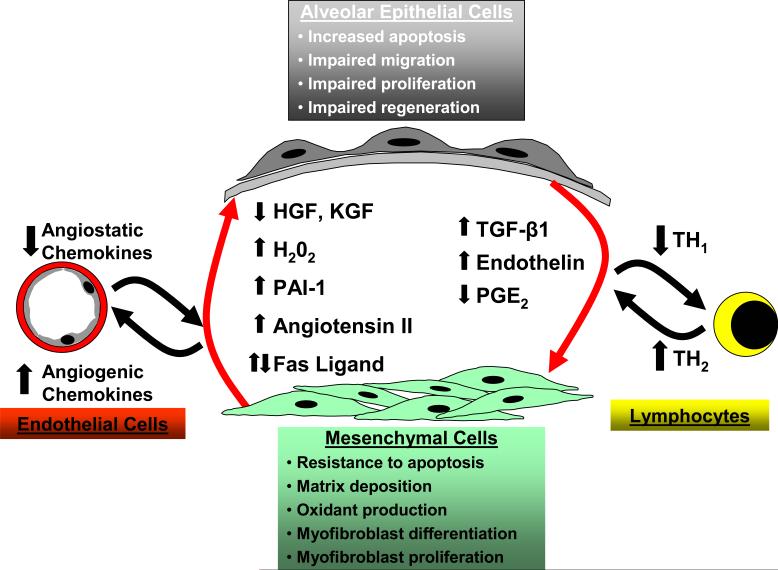
Dysregulated interactions between epithelial and mesenchymal cells within the context of a damaged basement membrane and the surrounding extracellular matrix are increasingly recognized as a key control point in the pathogenesis of fibrotic disorders (Figure 2). Morphologic changes suggestive of epithelial cell injury and apoptosis are early features in the histopathology of IPF (14, 16-18). Several studies have described phenotypic alterations in type II alveolar epithelial cells (AECs) including proliferation, (16), bronchiolarization (34, 35), apoptosis and regenerative hyperplasia (36).
Figure 2.
The pathogenesis of IPF is complex and three major hypotheses or paradigms for the pathogenesis of IPF have emerged: dysregulated epithelial-mesenchymal interactions, aberrant angiogenesis and the TH1/TH2 cytokine imbalance. Epithelial-mesenchymal interactions between altered epithelial and mesenchymal phenotypes results in dysregulated interactions between these cellular compartments. In response to an unknown stimulus, alveolar epithelial cells in IPF develop a phenotype characterized by increased apoptosis, dysregulated proliferation, impaired regeneration/differentiation and perhaps impaired migration. Alveolar epithelial cells elaborate increased TGF-β1 and endothelin-1 along with decreased levels of PGE2. Mesenchymal cells in this alveolar microenvironment acquire a contractile, synthetically active myofibroblast phenotype. Myofibroblasts retain the capacity to proliferate and are resistant to apoptosis while secreting large amounts of extracellular matrix proteins, soluble growth factors/cytokines and extracellular oxidants. Soluble mediators and the insoluble matrix elaborated by these myofibroblasts may, in turn, lead to aberrant reepithelialization that perpetuates a feed-forward cycle. IPF fibroblasts/myofibroblasts and alveolar epithelial cells contribute to an imbalance in angiogenic chemokines that may promote neovascularization and aberrant angiogenesis in IPF. A “shift” in the host immune response, favoring a TH2 cytokine profile, has been postulated to contribute to initiation and/or progression of IPF.
Underlying areas of atypical and apoptotic epithelial cells are fibroblastic foci containing activated contractile myofibroblasts that secrete abundant extracellular matrix (ECM) proteins (14). These myofibroblasts are closely associated with areas of epithelial cell death and regeneration (37). In IPF, dysregulated interactions between epithelial and mesenchymal cells may promote a “vicious cycle” of epithelial injury/apoptosis and mesenchymal cell activation leading to progressive fibrosis.
Epithelial Cell Phenotypes
Reestablishing an intact epithelium following injury is a key component of normal wound healing. This may require orchestrated epithelial cell responses that include proliferation and expansion of progenitor/stem cells, migration and differentiation. The homeostatic function of an intact epithelium may be necessary to suppress mesenchymal cell activation. Altered epithelial cell phenotypes may contribute to fibrosis through excess elaboration of soluble factors, such as TGF-β1, that activate the underlying mesenchyme (38). In addition to secretion of fibrogenic cytokines, altered epithelial cell phenotypes contributing to the pathogenesis of IPF may include exaggerated or inappropriate epithelial cell apoptosis, dysregulated proliferation/differentiation and impaired migration (Figure 2).
There is abundant histopathologic and ultrastructural evidence of ongoing epithelial cell apoptosis in patients with IPF (39-43). Moreover, several studies in animal models have shown that inhibition of apoptosis attenuates pulmonary fibrosis (44) (45). The specific mediator(s) responsible for epithelial cell apoptosis in pulmonary fibrosis remain unclear and several different mechanisms, including TGF-β1, oxidative stress, Fas activation, and angiotensin II have been proposed. In epithelial cells, TGF-β1 typically functions as a tumor suppressor through growth-inhibition and induction of apoptosis (46). In an animal model, conditional activation of TGF-β1 in murine lung epithelium caused marked epithelial cell apoptosis and pulmonary fibrosis (47). Moreover, inhibition of epithelial cell apoptosis, both by pharmacologic caspase inhibition and genetic inactivation of the early growth response gene, blocked pulmonary fibrosis induced by TGF-β1 overexpression (47). Oxidative stress resulting from the release of reactive oxygen species (ROS) by activated phagocytic cells has also been proposed as a mechanism for epithelial cell injury in IPF (48). Although ROS are well recognized to induce cell injury, they are also important regulators of intra- and inter-cellular signaling (49). TGF-β1 is a strong inducer of the sustained production of extracellular oxidants in human lung fibroblasts (50, 51) and extracellular hydrogen peroxide (H2O2) generated by IPF myofibroblasts in response to TGF-β1 has been shown to mediate paracrine induction of epithelial cell apoptosis/death (52). Upregulation of the Fas signaling pathway has been demonstrated in human IPF (42) and a number of studies in animal models have explored the role of the Fas-Fas ligand cascade in epithelial cell apoptosis, sometimes with conflicting results (53-55). Angiotensin II has also been proposed as a soluble mediator of epithelial cell apoptosis in the pathogenesis of IPF. Inhibition of angiotensin II or the angiotensin receptor has been shown to block Fas-mediated epithelial cell apoptosis (56-58). Moreover, IPF-derived fibroblasts can induce paracrine epithelial cell apoptosis through generation of angiotensin II (56). In animal models, inhibition of angiotensin II activation and blockade of its receptor abrogates bleomycin-induced fibrosis (44, 59, 60).
Epithelial cell regeneration/proliferation and differentiation are critical components of wound healing that may be impaired in fibrotic tissue responses. Exogenous administration of epithelial cell mitogens/motogens appears to be able to attenuate fibrosis in animal models. One of these growth factors, hepatocyte growth factor (HGF), is secreted by myofibroblasts and functions as an epithelial cell mitogen and motogen with actions that tend to oppose those of TGF-β1 (61, 62). Thus, while HGF stimulates epithelial cell proliferation/migration (63), it also promotes myofibroblast apoptosis (64). Fibroblasts/myofibroblasts isolated from patients with IPF secrete lower levels of HGF than normal lung fibroblasts (65). Moreover, HGF has demonstrated anti-fibrotic activity in animal models of renal, hepatic, pulmonary and myocardial fibrosis (64, 66-68). Interestingly, interferon gamma (IFN-γ) has been shown to upregulate the HGF receptor in alveolar epithelial cells (69).
Keratinocyte growth factor (KGF) is another epithelial cell mitogen/motogen that is secreted by mesenchymal cells (70-72). TGF-β1 has been shown to block the mitogenic effects of KGF on type II alveolar epithelial cells (73). In lung injury models, intratacheal instillation of KGF prior to bleomycin injury has been shown to attenuate fibrotic responses (74). In a recent study, IPF-derived fibroblasts generated equivalent amounts of KGF under basal conditions when compared to control fibroblasts; however, IPF-fibroblasts generated significantly lower levels of KGF following IL-1β stimulation (75). Collectively, these studies suggest that impaired regulation of HGF and/or KGF, may impair reepithelialization and contribute to the pathogenesis of pulmonary fibrosis.
Impaired epithelial cell migration may also contribute to the development of fibrosis by delaying or preventing reconstitution of the alveolar epithelium. In animal models of lung injury, growth factors that induce epithelial cell migration, including HGF and GM-CSF, have been shown to attenuate pulmonary fibrosis (67, 76). The plasminogen activation system has been shown to play an important role in the pathogenesis of bleomycin-induced murine pulmonary fibrosis. Overexpression of PAI-1, which inhibits plasminogen activation, promotes fibrosis while suppression of PAI-1 or overexpression of uPA (a plasminogen activator) attenuates fibrosis (77-80). Although the precise mechanism of protection remains unclear, modulation of this proteolytic system can regulate wound healing and reepithlialization (63, 81, 82).
Once reestablished, an intact epithelium functions to suppress fibroblast/myofibroblast activities; the loss of this suppressive effect may contribute to the pathogenesis of pulmonary fibrosis. Intact epithelial cells secrete PGE2, a cyclooxygenase (COX)-dependent arachadonic acid metabolite which suppresses fibroblast proliferation (83, 84), migration (85), differentiation (86), and collagen synthesis (87). Animal models support a role for PGE2, as COX-2 deficient mice develop increased fibrosis and leukotriene-deficient mice are protected from fibrosis following intratracheal bleomycin-induced lung injury (88, 89). Importantly, PGE2 levels in the epithelial lining fluid are significantly reduced while levels of leukotriene B4 and C4 are increased in lung homogenates of patients with IPF, (90, 91).
Recent studies have reported the potential derivation of lung epithelial cells from the bone marrow or systemic circulation. Krause and colleagues showed that transplanted bone marrow-derived stem cells may engraft as type II pneumocytes in the lung (92). Kotton et al. demonstrated increased numbers of bone marrow-derived stem cells in the lung following bleomycin-induced injury (93). More recent studies, however, suggest that some of these earlier findings may have been confounded by technical limitations of in-situ labeling and that true engraftment of the lung by bone marrow-derived stem cells may not occur, even in response to an injury (94). Bone marrow-derived stem cells have been used as a vehicle for transgene expression in the lung epithelium (95). The precise role(s) of circulating bone marrow-derived cells in repair and regeneration of the alveolar epithelium in response to injury is presently unclear.
Finally, there has been recent interest in alternative fates of epithelial cells, particularly with regard to epithelial-mesenchymal transition (EMT). Wnt/beta-catenin, a signaling pathway that is crucial in embryonic development and is implicated in EMT, is activated in epithelial cells at bronchiolo-alveolar junctions in IPF lung (18). Recent evidence suggests that EMT may contribute to the fibrotic responses in lungs of IPF patients (96). Further studies are required to determine the precise role and significance of EMT in the pathogenesis of IPF.
Mesenchymal Cell Phenotypes
Underlying areas of epithelial injury in lungs of patients with IPF are fibroblastic foci composed of aggregates of activated fibroblasts/myofibroblasts, which are the key effector cells in fibrogenesis (14, 97, 98). Fibroblasts have the ability to undergo distinct phenotypic transitions and participate in the stereotypic repair processes of diverse human tissues and organs (99, 100). Myofibroblasts are contractile and highly activated, secretory cells with characteristics intermediate between fibroblasts and smooth muscle cells (100, 101). Following injury, mesenchymal cells infiltrate wounds, differentiate into myofibroblasts and secrete ECM proteins that help constitute a provisional matrix which serves as the “scaffold” for normal tissue repair (101). Contraction of the provisional matrix approximates epithelial cell margins to facilitate reepithelialization while collagen secretion stabilizes the contracted matrix (100). Interestingly, recent studies indicate that lung mesenchymal cells following injury may derive from several sources including resident interstitial fibroblasts (102), circulating fibrocytes (103, 104), bone marrow-derived fibroblast precursors (105) and lung epithelial cells via epithelial-mesenchymal transition (96, 106, 107).
For normal healing to occur, wound myofibroblasts must undergo apoptosis (108); failure of apoptosis leads to myofibroblast accumulation, exuberant ECM production, persistent tissue contraction, and pathologic scar formation (100). TGF-β1 is a critical mediator of the myofibroblast phenotype and has been implicated in the pathogenesis of fibrotic disease in virtually every organ system studied (32, 97, 109, 110). In rodent models, over-expression of TGF-β1 leads to pulmonary fibrosis (111, 112) and blockade of TGF-β signaling abrogates fibrosis (113). In contrast to its effects on epithelial cells, TGF-β1 promotes an anti-apoptotic phenotype in fibroblasts (114, 115).
Multiple other soluble and insoluble substances in the cellular microenvironment have been shown to promote a “pro-fibrotic” mesenchymal cell phenotype. Soluble factors that induce myofibroblast differentiation include endothelin-1 (116), thrombin (117), and thrombospondin-1 (118). Consistent with a pro-fibrotic role, angiotensin II acts as a fibroblast mitogen (119) and the ACE inhibitor, captopril, inhibits fibroblast proliferation (120). Angiotensin II also induces the synthesis of TGF-β1 in several cell systems (121, 122). The ECM itself plays a key role in regulating mesenchymal cell phenotypes, as the composition and biomechanical properties of the ECM may also regulate myofibroblast differentiation, contractile capacity and apoptosis (123-126). Moreover, adhesion-dependent activation of focal adhesion kinase is required for stable induction of myofibroblast differentiation by TGF-β1 (127).
Soluble and insoluble factors secreted by myofibroblasts can contribute to the development of fibrosis through several mechanisms. First, the activated myofibroblast may be a source of soluble mediators that induce epithelial cell injury/apoptosis through paracrine signaling (52, 128). Autocrine secretion of growth factors promotes activation of pro-survival signaling pathways in myofibroblasts (114), and the autocrine induction of TGF-β1 itself may amplify these signaling pathways in fibroblasts while inhibiting epithelial cell growth and/or inducing epithelial cell apoptosis. Additionally, decreased production of “epithelial-protective” factors by myofibroblasts may feed into a state of epithelial-mesechymal dysrepair. For example, fibroblasts/myofibroblasts from IPF patients have a reduced capacity to elaborate the epithelial cell mitogens, HGF and KGF (65, 75). Myofibroblasts are likely the most important source of ECM proteins in the chronically injured lung. TGF-β1-stmulated generation of extracellular hydrogen peroxide has been shown, in certain contexts, to induce oxidative cross-linking reactions in the matrix overlying myofibroblasts (129). Such alterations in the biochemical and biophysical properties of the ECM may perpetuate dysregulated epithelial and mesenchymal phenotypes.
Finally, myofibroblasts may participate in the initiation or propagation of inflammatory responses (130). The myofibroblast has been described as an “inflammatory cell” due to its capacity to secrete multiple cytokines, chemokines and growth factors (130-132). Additionally, IPF-derived myofibroblasts and fibroblasts induced to undergo myofibroblast differentiation in response to TGF-β1 have been shown to generate extracellular oxidants (50, 52).
The Role of Inflammation and the TH1/TH2 Hypothesis
The role of inflammation in the pathogenesis of IPF has been the subject of considerable debate (133). For several decades, chronic inflammation was the prevailing and dominant paradigm in IPF pathogenesis (134, 135). However, the failure of anti-inflammatory strategies to improve outcomes in patients with IPF and the lack of prominent inflammation in IPF lung biopsy specimens have prompted a reevaluation of the role of inflammation in pathogenesis (136, 137). A recent study using microarray analyses comparing lung tissues from patients with IPF and hypersensitivity pneumonitis showed that the IPF gene signature was distinguished by the relative lack of typical inflammation-associated genes (138). While a comprehensive review of the inflammatory mechanisms in fibrogenesis is beyond the scope of this review, the reader is referred to existing reviews on this subject (139, 140). The precise role of inflammation and classical inflammatory cells in the pathogenesis of IPF remains uncertain. Evolving hypotheses regarding the role of inflammation now focus more on the balance between a “pro-inflammatory” TH1 cytokine profile and a “pro-fibrotic” TH2 cytokine profile.
The TH1/TH2 hypothesis proposes that progressive fibrosis results from a maladaptive immune/inflammatory response to a chronic or persistent pathogen/antigen (141, 142). In the typical host, a variety of cells may respond to pathogens/antigens by producing either TH1 cytokines [such as interferon gamma (IFN-γ) and interleukin (IL)-12] which tend to suppress fibrotic responses or TH2 cytokines (such as IL-4, IL-10, IL-13) that promote fibrotic responses. This hypothesis proposes that an inappropriate shift in the TH1/TH2 cytokine balance, favoring the TH2 profile, results in the development of fibrotic disease (recently reviewed in reference #142). Much of our understanding of TH1/TH2 polarization in the pathogenesis pulmonary fibrosis has come from rodent models of fibrosis provoked by intratracheal bleomycin to induce epithelial injury (143, 144). Since the bleomycin animal model is an imperfect model of IPF (145), the significance of these findings to human IPF is unclear. Interestingly, even in the bleomycin animal model, the precise roles of host immune T-cell responses are not entirely clear. One study showed that T-cell depleted mice are capable of developing bleomycin-induced pulmonary fibrosis (146), while another study showed that CD-28, necessary for co-stimulatory T-cell activation, was required for the fibrotic response to injury (147). Despite the imperfections of animal modeling and the controversial role of inflammation, recent studies in human IPF tissues and IPF-derived cells seem to support the TH1/TH2 polarization hypothesis. Increased expression of the receptors for IL-4 and IL-13 was shown in lung tissues from IPF patients compared with those from patients with non-IPF idiopathic interstitial pneumonias (148). Fibroblasts derived from IPF lung similarly demonstrated increased expression of these TH2 cytokine receptors (149). The use of a chimeric protein comprised of human IL-13 and a truncated version of Pseudomonas exotoxin appears to attenuate the proliferation of IPF-derived fibroblasts to a greater extent than non-IPF derived fibroblasts (149).
The Role of Angiogenesis in IPF
Angiogenesis is an important component of wound healing and aberrant angiogenesis has been associated with fibrosis in cutaneous wound models (99, 150). In rodent models, lung injury is followed by marked pulmonary vascular changes including neovascularization (150). In patients with IPF, early studies demonstrated the presence of pulmonary-systemic vascular anastomoses (151). A more recent study showed increased levels of pro-angiogenic chemokines and cytokines in the plasma of patients with IPF compared to normal volunteers (152).
In a series of studies using both in-vivo murine models and lung tissue from IPF patients, Keane and colleagues have shown differences in the expression of “angiogenic” ELR+ CXC chemokines (IL-8/CXCL8, ENA-78/CXCL5, MIP-2/CXCL2) and the “angiostatic” interferon (IFN)-inducible ELR− CXC chemokines (IP10/CXCL10). Patients with IPF have increased expression of angiogenic ELR+ CXC chemokines and decreased expression of the angiostatic chemokines (153, 154). Moreover, both inhibition of the angiogenic chemokines and administration of angiostatic chemokines have been shown to attenuate murine pulmonary fibrosis in the bleomycin model (155-157). Mice deficient in the angiostatic chemokine IP10/CXCL10 develop increased pulmonary fibrosis following bleomycin-induced lung injury (158). Collectively, these studies suggest that an imbalance of angiogenic and angiostatic chemokines contributes to the pathogenesis of pulmonary fibrosis and that intervention targeted at this imbalance may be beneficial in the treatment of IPF.
Two recent studies, however, have reported decreased neovascularization in fibroblastic foci of patients with IPF (159, 160). Another recent study reported elevated levels of endostatin, an inhibitor of angiogenesis, in the serum of IPF patients (161). Interestingly, a post-mortem study of lungs from patients with scleroderma-associated lung disease showed early increases in microvascularity with subsequent decreases in later stages of disease (162). Thus, continued investigation into the spatial and temporal regulation of angiogenesis may be necessary to determine the precise role(s) of angiogenic and angiostatic factors in the pathogenesis of human IPF.
Pulmonary Hypertension in IPF
Pulmonary hypertension may be an important risk factor for increased mortality in IPF patients (2). Early studies showed that patients with IPF had elevated mean pulmonary artery pressures compared to patients with non-IPF interstitial lung diseases; pulmonary pressures increased with exercise to a greater extent in IPF patients than in non-IPF patients (163). Another study reported that impaired gas exchange in IPF patients was associated with increases in pulmonary vascular resistance (164). More recent retrospective studies have shed further light on the association between pulmonary hypertension and IPF. In one study, pulmonary hypertension inversely correlated with gas exchange; moreover, IPF patients with pulmonary hypertension had increased mortality (9). In another study of IPF patients during an actue exacerbation, pulmonary hypertension was present in all six patients in whom an echocardiogram was obtained (165).
These potential links between pulmonary hypertension and increased IPF mortality have spurred an interest in vasoactive pharmacologic agents in the treatment of lung fibrosis-associated pulmonary hypertension. A preliminary study examined the effects of various vasodilators - inhaled prostacyclin, intravenous prostacyclin and nitric oxide – administered sequentially to eight patients with pulmonary hypertension associated with fibrotic lung disease; in this study, inhaled prostacyclin decreased pulmonary hypertension without affecting systemic blood pressure, shunt fraction, or ventilation/perfusion matching (166). Similarly, sildenafil, a phosphodiesterase-5 inhibitor approved for the treatment of pulmonary hypertension, was compared with epoprostenol in a randomized trial of 16 patients with pulmonary hypertension associated with fibrotic lung disease. Sildenafil induced pulmonary vasodilatation to the same degree as epoprostenol, but did not adversely affect ventilation/perfusion matching or oxygenation (167). A phase II multicenter trial of the safety and efficacy of inhaled prostacyclin in IPF patients with established pulmonary hypertension is currently underway (www.clinicaltrials.gov/ct/gui/show/NCT00109681).
NOVEL THERAPEUTIC STRATEGIES FOR IPF
Therapeutic strategies targeting inflammation with corticosteroids, azathioprine, cyclophosphamide, cyclosporine A and mycophenalate mofetil have shown no definitive benefit in the treatment of IPF (3, 168, 169). This contrasts with other fibrotic lung diseases in which immunosuppressive therapies appear to have more favorable results (170-174). While one cannot rule out a role for inflammation or the possibility of a dysregulated, ongoing, low-grade TH2-mediated host response to a persistent or recurrent antigen in the pathogenesis of IPF, inflammation is not a prominent feature when patients come to clinical attention (14, 136). Here, we will highlight some of the emerging therapies that are currently in, or nearing, clinical trials for IPF (summarized in Table 1).
TABLE 1.
Emerging pharmacotherapy targeting fibrogenic pathways in IPF
| Name/Class | Mechanism of Action | Clinical trials | Other |
|---|---|---|---|
| Interferon-γ1b | Modulates TH1/TH2 balance; inhibits fibroblast activation | Phase 3 (completed) Phase 3 (ongoing) | |
| Pirfenidone | Suppresses TNF-α; inhibits TGF-β; free radical scavenger | Phase 2 (completed) Phase 3 (planned) | |
| Zileuton | Inhibits 5-lipoxygenase; Modulates eicosanoid imbalance | Phase 2 (ongoing) | Approved for asthma |
| Etanercept | Inhibits TNF-α receptor binding | Phase 2 (completed) | |
| N-acetyl cysteine | Anti-oxidant; glutathione precursor | Phase 3 (completed) | Approved for acetaminophen overdose and contrast nephropathy |
| Tetrathiomolybdate | Inhibits angiogenesis; Inhibits TGF-β, TNF-α production; anti-oxidant | Phase 1/2 (ongoing) | |
| Bosentan | Endothelin-1 receptor antagonist | Phase 2/3 (ongoing) | Approved for pulmonary hypertension |
| Monoclonal Anti-TGF-β Antibodies | Inhibits binding of TGF-β to its receptor | Phase 1/2 in scleroderma and post-trabulectomy scar prevention; initiated in IPF | |
| TGF-β receptor kinase inhibitors | Protein kinase inhibitor; Blocks TGF-β type 1 receptor kinase activity | Planning stages for IPF | |
| Imatinib mesylate | Protein kinase inhibitor; Inhibits c-Abl and PDGF tyrosine kinase | Phase 2 (ongoing) | Approved for CML and GIST |
| AG1879 | Protein kinase inhibitor; Modulates myofibroblast differentiation and survival by inhibiting FAK/Src kinases and Akt. | Pre-clinical | |
| Anti-CTGF | Monoclonal antibody against CTGF | Phase 2 (ongoing) |
Interferon Gamma-1b (IFN-γ1b)
IFN-γ appears to target several pathways in the pathogenesis of fibrosis. First, as a TH1 cytokine, it may modulate the TH2 polarization reported in such chronic disorders (142). IFN-γ may also mediate anti-fibrogenic effects by reducing fibroblast proliferation, chemotaxis and collagen production (175-178). Additionally, IFN-γ induces the expression of angiostatic ELR− CXC chemokines, thereby blocking potential angiogenic signals (179). Finally, IFN-γ may aid in re-epithelialization through upregulation of the HGF receptor in alveolar epithelial cells (69). In contrast to these effects, early studies suggested that IFN-γ can function as a mitogen for fibroblasts (180-182). Furthermore, a recent study reported that IFN-γ failed to inhibit TGF-β1 stimulated collagen synthesis, contraction or myofibroblast differentiation in keloid-derived fibroblasts, suggesting that mesenchymal cells in fibrotic lesions may be resistant to the suppressive effects of IFN-γ (183).
Treatment with IFN-γ inhibits renal, pulmonary and hepatic fibrosis in animal models (184-186). In humans, an early clinical trial showed promising results with stabilization/improvement in the pulmonary functions in patients administered IFN-γ (187). These favorable results, however, were not confirmed in a follow-up multicenter, randomized, double blind, placebo-controlled trial (188). In this large Phase III trial, no significant benefit was seen in the primary endpoint of progression-free survival or in secondary endpoints of pulmonary function or quality of life. A trend towards decreased mortality was seen in the IFN-γ-treated group in retrospective subgroup analysis of patients with less severe disease (FVC > 55% predicted and DLCO > 35%). There is an ongoing prospective clinical trial designed to further evaluate the efficacy of IFN-γ1b in this patient subgroup [International Study of Outcomes in IPF with IFN-γ1b (INSPIRE)].
Pirfenidone
Pirfenidone is a pyridone compound [5-methyl-1-phenyl-2(1H)-pyridone] that inhibits fibroblast proliferation, differentiation and ECM synthesis (189). Several potential mechanisms have been proposed for the anti-fibrotic effects of pirfenidone; one possible mechanism relates to suppression of tumor necrosis factor-alpha (TNF-α), which is thought to be a key cytokine in pathogenesis of IPF. Other potential mechanisms include suppression of TGF-β1 transcription (190) and downregulation of heat shock protein 47 (HSP47), a protein involved in procollagen secretion in rodents treated with bleomycin (191, 192). Additionally, pirfenidone may function as a free radical scavenger to reduce oxidative stress (193, 194). In animal models, pirfenidone attenuates liver (195), peritoneal (196), renal (197), biliary (198) and pulmonary (199, 200) fibrosis.
Several studies have investigated the effects of pirfenidone in humans with pulmonary fibrosis. In a compassionate-use protocol enrolling 54 patients with IPF, pirfenidone treatment was well tolerated, allowed most patients to discontinue conventional therapy and some patients appeared to stabilize lung function (201). In a randomized placebo-controlled trial of 21 patients with pulmonary fibrosis due to Hermansky-Pudlak syndrome, pirfenidone-treated patients had a reduced rate of decline in lung function (202). Recently, a prospective, randomized, placebo-controlled trial of 107 patients with IPF reported no significant change in the primary endpoint [the lowest oxygen saturation (SaO2) during a 6-minute walk at 6 months] (203). Significant improvements were seen in the subgroup of patients with less severe disease (those who maintained SaO2 greater than 80% during their baseline 6-minute walk). Moreover, the treatment group had a significant reduction in the number of acute exacerbations of IPF (203), a relevant clinical endpoint as these episodes are increasingly recognized as a major cause of mortality in patients with IPF (33). Given the lack of benefit in the primary endpoint, further study is required before pirfenidone can be recommended for general use in the treatment of IPF.
Zileuton
An imbalance in eicosanoids, arachadonic acid metabolites that include leukotrienes (e.g. LTB4/LTC4) and prostaglandins (e.g. PGE2), has been implicated in the pathogenesis of IPF; in general, leukotrienes mediate pro-fibrotic effects while cyclooxygenase-2 (COX-2)-dependent PGE2 mediates anti-fibrotic effects (recently reviewed in ref #(204)). Fibroblasts from IPF patients synthesize decreased levels of PGE2 at baseline (65, 91) and following TGF-β1 treatment (88). Moreover, IPF fibroblasts may be resistant to the suppressive effects of PGE2, as exogenous PGE2 has an impaired ability to decrease their proliferative capacity (205).
In animal models, COX-2 deficiency is associated with increased bleomycin-induced fibrosis (88) while leukotriene-deficient mice are protected (89). The chemokine, monocyte chemoattractant protein-1 (MCP-1) inhibits PGE2 synthesis by alveolar epithelial cells resulting in enhanced fibroblast proliferation (206); mice deficient in the MCP-1 receptor, CCR2, are protected from pulmonary fibrosis (207), supporting a role for MCP-1/CCR2 mediated suppression of PGE2 in the pathogenesis of fibrosis. Finally, PGE2 mediates the anti-fibrotic actions of GM-CSF in murine models (208).
Collectively, these data support a role for eicosanoid imbalance favoring increased leukotrienes and decreased prostaglandins in the pathobiology of IPF. Although there are no published data on the use of prostaglandin agonists or leukotriene-inhibitors in humans with IPF, there is an ongoing Phase II clinical trial investigating the safety and efficacy of the 5-lipoxygenase inhibitor, Zileuton, at the University of Michigan.
Etanercept
TNF-α is a pro-inflammatory cytokine that may also have roles in the regulation of the ECM and has been implicated in IPF pathogenesis. Alveolar epithelial cells and macrophages from patients with IPF express elevated TNF-α levels (209, 210). Moreover, several studies have identified associations between TNF-α gene polymorphisms and pulmonary fibrosis (28, 29, 211, 212). Murine models support a role for TNF-α in the pathogenesis of pulmonary fibrosis. Transgenic overexpression of TNF-α induces a form of lymphocytic fibrosing alveolitis (213) and adenoviral-mediated overexpression of TNF-α in rat lungs induces inflammation, patchy fibrosis and myofibroblast accumulation (214). Mice deficient in TNF receptors or treated with a soluble TNF receptor develop decreased bleomycin-induced pulmonary fibrosis(215, 216). Paradoxically, several studies demonstrate that TNF-α may suppress collagen synthesis by fibroblasts (217-220) and activate certain matrix mettaloproteinases (221, 222). TNF-α deficient mice develop an intense and persistent inflammatory response without a decrease in lung hydroxyproline content following intratracheal bleomycin, suggesting that the absence of TNF-α may not, in itself, attenuate the fibrotic response to injury (223). Moreover, TNF-α overexpression actually reduces fibrosis induced by TGF-β1 overexpression (224). A Phase II clinical trial of etanercept (a soluble TNF-R-Fc fusion protein) in IPF has been completed and the results were recently presented in abstract form (225). Eighty-seven patients with mild-moderate IPF were randomized to receive twice-weekly subcutaneous etanercept or placebo. After 48 weeks of therapy, etanercept treatment resulted in no significant differences in the primary endpoints (FVC, DLCO, or the differences in alveolar-arterial oxygen gradient) or in the secondary endopoints (TLC, resting oxygen saturation, and 6-minute walk distance). However, some trends for improvement in physiologic parameters were noted which may yet lead to another trial of this agent.
N-acetyl Cysteine
Oxidative stress is thought to play a critical role in IPF pathogenesis (reviewed in ref #(226)). Localized generation of ROS may induce epithelial cell injury/apoptosis, a key ultrastructural feature of UIP/IPF. Inflammatory cells from patients with IPF generate increased levels of oxidants compared to normal volunteers (48) and the epithelial lining fluid of IPF patients contains both increased concentrations of myeloperoxidase (48) and decreased levels of the anti-oxidant, glutathione (GSH) (227). N-acetyl cysteine (NAC) may mediate anti-oxidant effects through augmentation of GSH synthesis. It is safe, efficacious and approved for the treatment of acetaminophen overdose (228) and in the prevention of contrast nephropathy (229). Both GSH and NAC decrease fibroblast proliferation in-vitro (230). In patients with IPF, aerosolized GSH decreases ROS production by alveolar macrophages in IPF (231). Both oral and intravenous NAC increase GSH levels in bronchoalveolar lavage fluid (232, 233). In a small prospective trial of oral NAC in combination with immunosuppressive therapy in patients with IPF, the NAC-treated group had increased levels of GSH in epithelial lining fluid, decreased markers of oxidative stress and improvements in pulmonary function (234). A preliminary study randomizing 30 IPF patients to inhaled NAC or a control for 12 months reported that inhaled NAC had no effect on quality of life or pulmonary function, but did lead to improvements in the lowest oxygen-saturation during a 6-minute walk (235). A large, prospective Phase III clinical trial of oral NAC combined with prednisone/azathioprine compared to prednisone/azathioprine alone has recently been reported(236). In this study, 182 patients were randomized to receive oral NAC (300 mg, three times daily) or placebo in addition on prednisone and azathioprine. After 12 months of therapy, the addition of NAC resulted in a significant decrease in the primary endpoints of decline in vital capacity and DLCO. No significant difference was observed in the secondary endpoint of mortality. It is not known if the positive effects of NAC are related to modulation of prednisone and azathioprine treatment since NAC monotherapy and placebo arms were not included in this trial (237).
Tetrathiomolybdate
Tetrathiomolybdate (TM) is a copper-chelating compound that has been shown to protect from fibrogenic effects of bleomycin in animal models of pulmonary fibrosis (238). The mechanism(s) for the anti-fibrotic effects of TM may be mediated, in part, by its anti-angiogenic effects (239). TM may also suppress the in-vivo expression of pro-fibrotic mediators, SPARC and TGF-β1, in response to lung injury (240, 241). The protective effect of oral TM is observed even when the drug was administered several days after bleomycin injury, suggesting that anti-fibrotic effects cannot be explained by the blockade of the early inflammatory response in this model (238). A Phase I/II clinical trial of TM in IPF patients refractory to previous immunosuppressive therapies is nearing completion at the University of Michigan.
Bosentan
Endothelin-1 (ET-1) is smooth muscle cell and fibroblast mitogen that is secreted by alveolar epithelial cells, vascular endothelial cells and macrophages (242). ET-1 has been linked to activated fibroblastic phenotypes (243-246). ET-1 overexpressing transgenic mice develop chronic inflammation and pulmonary fibrosis (247). Bleomcyin-induced pulmonary fibrosis is attenuated by treatment with an endothelin receptor antagonist (248); although, another study showed no effect on collagen deposition in this model (249). Patients with IPF demonstrate increased expression of ET-1 in proliferating type II epithelial cells, endothelial cells, and inflammatory cells (250, 251).
Bosentan is an oral, non-selective endothelin receptor antagonist that is currently approved for the treatment of pulmonary hypertension (252). A Phase II multi-center, double-blind trial randomizing 158 patients with IPF to treatment with Bosentan or placebo has been completed (BUILD-1). The results have not been presented in a peer-review format; however, a press-release from Actelion reported that there was no significant difference seen in the primary endpoint of improvement in 6-minute walk. They did report a trend for improvement in the secondary endpoint of combined death or treatment failure (www.actelion.com).
Inhibitors of TGF-β1 Signaling
A large body of evidence implicates TGF-β1 as a key mediator of human fibrotic disorders. Animal models have demonstrated that blocking TGF-β1 prevents injury-provoked pulmonary fibrosis (111-113). TGF-β1 is upregulated in fibrotic foci of UIP/IPF (253, 254). Anti-TGF-β1 therapies involving monoclonal antibodies, small molecule inhibitors, and other approaches (anti-sense oligonucleotides, receptor kinase inhibitors) are currently being investigated. Recently, Bonniaud and colleagues used an animal model of pulmonary fibrosis induced by the adenoviral-mediated overexpression of TGF-β1 to show that oral treatment with a small molecule inhibitor of type-1 TGF-β receptor (ALK-5) kinase activity could attenuate the development and progression of pulmonary fibrosis (255).
Despite its pro-fibrotic properties, TGF-β1 also has important homeostatic functions as an immune- and tumor-suppressive cytokine (46). Although inhibition of TGF-β1 signaling protects mice from fibrosis, recent animal studies have shown that an extended loss of TGF-β1 signaling can induce emphysematous changes in the lung (256, 257). These concerns temper enthusiasm for the long-term inhibition of TGF-β1 as a therapeutic strategy for the treatment of IPF. Continued investigation and better understanding of the divergent post-receptor signaling mechanisms of TGF-β1 may allow for more targeted blockade of its pro-fibrotic actions in the future (258).
Protein Kinase Inhibitors
Protein kinases are critical intracellular intermediates in the regulation of cell signaling and phenotype. The successful use of the c-Abl kinase inhibitor, imatinib mesylate (Gleevec®) in oncologic disease (259) provided proof-of-principle that protein kinase inhibitors could be used to target altered (activated) signaling pathways in certain human diseases. Recent investigations suggest that several protein kinases are potential targets of inhibition for the treatment of IPF.
c-Abl and Platelet-Derived Growth Factor Receptor Kinase
Recently, TGF-β1 has been shown to activate the Smad-independent c-Abl tyrosine kinase that appeared to mediate ECM synthesis in fibroblasts (260). Treatment with imatinib mesylate decreased bleomycin-induced pulmonary fibrosis in mice (260). Another group reported that this drug decreases fibroblast proliferation and bleomycin-induced murine pulmonary fibrosis through inhibition of platelet derived growth factor (PDGF)-receptor activation (261). Similarly, a recent study with three different inhibitors of PDGF signaling (including imatinib mesylate) demonstrated a reduction in radiation-induced pulmonary fibrosis (262). A multi-center Phase II clinical trail of imatinib mesylate (Gleevec) in IPF is nearing completion.
Focal Adhesion Kinase
Focal adhesion kinase (FAK) is a tyrosine kinase that regulates cell proliferation, migration and survival (263). This adhesion-dependent FAK pathway is required for differentiation of fibroblasts to the myofibroblast phenotype (127). Inhibition of FAK signaling may also induce fibroblast apoptosis (264), while forced activation of FAK signaling can protect fibroblasts from apoptosis through activation of Akt (265). Recent studies demonstrate that FAK and Akt are expressed in areas of fibrosis following intratracheal bleomycin in mice and that treatment with a protein kinase inhibitor (AG1879) that blocks TGF-β1-induced FAK and Akt in myofibroblasts attenuates fibrogenic responses to bleomycin (266).
p38 MAP Kinase
p38 MAP kinase is activated by a variety of stimuli and participates in multiple cellular processes through the activation of downstream transcription factors (267). Early and delayed activation of p38 MAPK by TGF-β1 have been described (268, 269). Furthermore, p38 MAPK activation is necessary for the activation of Akt by TGF-β1 in human lung fibroblasts, supporting a role for p38 MAPK in the anti-apoptotic phenotype of human lung fibroblasts (114). Finally, p38 MAPK activation has been implicated in proliferative responses of fibroblasts in response to TGF-β1 treatment (270). Inhibition of p38 MAPK has been shown to attenuate bleomycin-induced pulmonary fibrosis in murine models (271, 272).
Other Potential Pharmacologic Agents
We have already discussed the potential roles of angiotensin-II in the pathogenesis of IPF. Inhibitors of both angiotensin-converting enzyme and the angiotensin receptor are widely used for the treatment of hypertension, ischemic cardiomyopathy and diabetic nephropathy. Clinical experience with these drugs in IPF, however, is limited. A recent retrospective analysis from the Mayo clinic found no significant differences in the survival of IPF patients receiving an ACE-I compared to IPF patients who were not receiving an ACE-I (273).
Connective Tissue Growth Factor (CTGF) is a growth factor that is produced by fibroblasts in response to TGF-β1 stimulation and is thought to mediate some of the pro-fibrotic effects of TGF-β1 (274). In murine models, CTGF expression is upregulated by bleomycin (275). Adenoviral-mediated overexpression of CTGF in lungs of mice, however, induces only transient fibrosis (276). Increased expression of CTGF has been demonstrated in alveolar epithelial cells and interstitial fibroblasts in IPF (277). Monoclonal antibodies to CTGF are currently in Phase I/II clinical trials in IPF.
CONCLUSION
Idiopathic pulmonary fibrosis is a progressive, debilitating lung disease for which the etiology remains unclear and for which no proven effective therapies exist. Clinical and laboratory investigations have led to changing paradigms in disease pathogenesis and evolving new targets for therapeutic intervention. Therapeutic strategies targeting fibroblast phenotypes (myofibroblast differentiation, proliferation and survival), epithelial regeneration and apoptosis, angiogenesis, ECM regulation and pulmonary hypertension are supplanting traditional anti-inflammatory/immunosuppressive strategies. Continued investigations into the signaling mechanisms supporting these dysregulated cellular phenotypes are likely to identify future targets for intervention.
Acknowledgements
This work was supported, in part, by National Institutes of Health grants K08 HL081059 (to J.C. H.); R01 HL67967 and P50 HL74024 (to V.J.T).
REFERENCES
- 1.Schwartz DA, Helmers RA, Galvin JR, et al. Determinants of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149(2 Pt 1):450–4. doi: 10.1164/ajrccm.149.2.8306044. [DOI] [PubMed] [Google Scholar]
- 2.King TE, Jr., Tooze JA, Schwarz MI, et al. Predicting survival in idiopathic pulmonary fibrosis: scoring system and survival model. Am J Respir Crit Care Med. 2001;164(7):1171–81. doi: 10.1164/ajrccm.164.7.2003140. [DOI] [PubMed] [Google Scholar]
- 3.American Thoracic Society Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–64. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 4.Coultas DB, Zumwalt RE, Black WC, et al. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150(4):967–72. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 5.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(9):1084–90. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 6.Hanson D, Winterbauer RH, Kirtland SH, et al. Changes in pulmonary function test results after 1 year of therapy as predictors of survival in patients with idiopathic pulmonary fibrosis. Chest. 1995;108(2):305–10. doi: 10.1378/chest.108.2.305. [DOI] [PubMed] [Google Scholar]
- 7.Flaherty KR, Mumford JA, Murray S, et al. Prognostic implications of physiologic and radiographic changes in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2003;168(5):543–8. doi: 10.1164/rccm.200209-1112OC. [DOI] [PubMed] [Google Scholar]
- 8.Gay SE, Kazerooni EA, Toews GB, et al. Idiopathic pulmonary fibrosis: predicting response to therapy and survival. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1063–72. doi: 10.1164/ajrccm.157.4.9703022. [DOI] [PubMed] [Google Scholar]
- 9.Nadrous HF, Pellikka PA, Krowka MJ, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–9. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 10.Hunninghake GW, Lynch DA, Galvin JR, et al. Radiologic findings are strongly associated with a pathologic diagnosis of usual interstitial pneumonia. Chest. 2003;124(4):1215–23. doi: 10.1378/chest.124.4.1215. [DOI] [PubMed] [Google Scholar]
- 11.Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol. 1997;169(4):977–83. doi: 10.2214/ajr.169.4.9308447. [DOI] [PubMed] [Google Scholar]
- 12.Flaherty KR, Thwaite EL, Kazerooni EA, et al. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58(2):143–8. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164(9):1722–7. doi: 10.1164/ajrccm.164.9.2103074. [DOI] [PubMed] [Google Scholar]
- 14.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1301–15. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 15.King TE, Jr., Schwarz MI, Brown K, et al. Idiopathic pulmonary fibrosis: relationship between histopathologic features and mortality. Am J Respir Crit Care Med. 2001;164(6):1025–32. doi: 10.1164/ajrccm.164.6.2001056. [DOI] [PubMed] [Google Scholar]
- 16.Katzenstein AL. Pathogenesis of “fibrosis” in interstitial pneumonia: an electron microscopic study. Hum Pathol. 1985;16(10):1015–24. doi: 10.1016/s0046-8177(85)80279-3. [DOI] [PubMed] [Google Scholar]
- 17.Kasper M, Haroske G. Alterations in the alveolar epithelium after injury leading to pulmonary fibrosis. Histol Histopathol. 1996;11(2):463–83. [PubMed] [Google Scholar]
- 18.Chilosi M, Poletti V, Murer B, et al. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab Invest. 2002;82(10):1335–45. doi: 10.1097/01.lab.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 19.Kuwano K, Nomoto Y, Kunitake R, et al. Detection of adenovirus E1A DNA in pulmonary fibrosis using nested polymerase chain reaction. Eur Respir J. 1997;10(7):1445–9. doi: 10.1183/09031936.97.10071445. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JP, Egan JJ, Ross AJ, et al. The detection of Epstein-Barr virus DNA in lung tissue from patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1336–41. doi: 10.1164/ajrccm.159.4.9807077. [DOI] [PubMed] [Google Scholar]
- 21.Kelly BG, Lok SS, Hasleton PS, et al. A rearranged form of Epstein-Barr virus DNA is associated with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2002;166(4):510–3. doi: 10.1164/rccm.2103058. [DOI] [PubMed] [Google Scholar]
- 22.Tang YW, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol. 2003;41(6):2633–40. doi: 10.1128/JCM.41.6.2633-2640.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Procop GW, Kohn DJ, Johnson JE, et al. BK and JC polyomaviruses are not associated with idiopathic pulmonary fibrosis. J Clin Microbiol. 2005;43(3):1385–6. doi: 10.1128/JCM.43.3.1385-1386.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgartner KB, Samet JM, Stidley CA, et al. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155(1):242–8. doi: 10.1164/ajrccm.155.1.9001319. [DOI] [PubMed] [Google Scholar]
- 25.Baumgartner KB, Samet JM, Coultas DB, et al. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study. Collaborating Centers. Am J Epidemiol. 2000;152(4):307–15. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 26.Lawson WE, Grant SW, Ambrosini V, et al. Genetic mutations in surfactant protein C are a rare cause of sporadic cases of IPF. Thorax. 2004;59(11):977–80. doi: 10.1136/thx.2004.026336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grutters JC, du Bois RM. Genetics of fibrosing lung diseases. Eur Respir J. 2005;25(5):915–27. doi: 10.1183/09031936.05.00133404. [DOI] [PubMed] [Google Scholar]
- 28.Whyte M, Hubbard R, Meliconi R, et al. Increased risk of fibrosing alveolitis associated with interleukin-1 receptor antagonist and tumor necrosis factor-alpha gene polymorphisms. Am J Respir Crit Care Med. 2000;162(2 Pt 1):755–8. doi: 10.1164/ajrccm.162.2.9909053. [DOI] [PubMed] [Google Scholar]
- 29.Pantelidis P, Fanning GC, Wells AU, et al. Analysis of tumor necrosis factor-alpha, lymphotoxin-alpha, tumor necrosis factor receptor II, and interleukin-6 polymorphisms in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;163(6):1432–6. doi: 10.1164/ajrccm.163.6.2006064. [DOI] [PubMed] [Google Scholar]
- 30.Xaubet A, Marin-Arguedas A, Lario S, et al. Transforming growth factor-beta1 gene polymorphisms are associated with disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(4):431–5. doi: 10.1164/rccm.200210-1165OC. [DOI] [PubMed] [Google Scholar]
- 31.Katzenstein AL, Zisman DA, Litzky LA, et al. Usual interstitial pneumonia: histologic study of biopsy and explant specimens. Am J Surg Pathol. 2002;26(12):1567–77. doi: 10.1097/00000478-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Thannickal VJ, Toews GB, White ES, et al. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 33.Martinez FJ, Safrin S, Weycker D, et al. The clinical course of patients with idiopathic pulmonary fibrosis. Ann Intern Med. 2005;142(12 Pt 1):963–7. doi: 10.7326/0003-4819-142-12_part_1-200506210-00005. [DOI] [PubMed] [Google Scholar]
- 34.Sutinen S, Rainio P, Huhti E, et al. Ultrastructure of terminal respiratory epithelium and prognosis in chronic interstitial pneumonia. Eur J Respir Dis. 1980;61(6):325–36. [PubMed] [Google Scholar]
- 35.Kawanami O, Ferrans VJ, Crystal RG. Structure of alveolar epithelial cells in patients with fibrotic lung disorders. Lab Invest. 1982;46(1):39–53. [PubMed] [Google Scholar]
- 36.Corrin B, Dewar A, Rodriguez-Roisin R, et al. Fine structural changes in cryptogenic fibrosing alveolitis and asbestosis. J Pathol. 1985;147(2):107–19. doi: 10.1002/path.1711470206. [DOI] [PubMed] [Google Scholar]
- 37.Coalson JJ. The ultrastructure of human fibrosing alveolitis. Virchows Arch A Pathol Anat Histol. 1982;395(2):181–99. doi: 10.1007/BF00429611. [DOI] [PubMed] [Google Scholar]
- 38.Xu YD, Hua J, Mui A, et al. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285(3):L527–39. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- 39.Kuwano K, Kunitake R, Kawasaki M, et al. P21Waf1/Cip1/Sdi1 and p53 expression in association with DNA strand breaks in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154(2 Pt 1):477–83. doi: 10.1164/ajrccm.154.2.8756825. [DOI] [PubMed] [Google Scholar]
- 40.Uhal BD, Joshi I, Hughes WF, et al. Alveolar epithelial cell death adjacent to underlying myofibroblasts in advanced fibrotic human lung. Am J Physiol. 1998;275(6 Pt 1):L1192–9. doi: 10.1152/ajplung.1998.275.6.L1192. [DOI] [PubMed] [Google Scholar]
- 41.Barbas-Filho JV, Ferreira MA, Sesso A, et al. Evidence of type II pneumocyte apoptosis in the pathogenesis of idiopathic pulmonary fibrosis (IFP)/usual interstitial pneumonia (UIP). J Clin Pathol. 2001;54(2):132–8. doi: 10.1136/jcp.54.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maeyama T, Kuwano K, Kawasaki M, et al. Upregulation of Fas-signalling molecules in lung epithelial cells from patients with idiopathic pulmonary fibrosis. Eur Respir J. 2001;17(2):180–9. doi: 10.1183/09031936.01.17201800. [DOI] [PubMed] [Google Scholar]
- 43.Plataki M, Koutsopoulos AV, Darivianaki K, et al. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127(1):266–74. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 44.Wang R, Ibarra-Sunga O, Verlinski L, et al. Abrogation of bleomycin-induced epithelial apoptosis and lung fibrosis by captopril or by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L143–51. doi: 10.1152/ajplung.2000.279.1.L143. [DOI] [PubMed] [Google Scholar]
- 45.Kuwano K, Kunitake R, Maeyama T, et al. Attenuation of bleomycin-induced pneumopathy in mice by a caspase inhibitor. Am J Physiol Lung Cell Mol Physiol. 2001;280(2):L316–25. doi: 10.1152/ajplung.2001.280.2.L316. [DOI] [PubMed] [Google Scholar]
- 46.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–8. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 47.Lee CG, Cho SJ, Kang MJ, et al. Early growth response gene 1-mediated apoptosis is essential for transforming growth factor beta1-induced pulmonary fibrosis. J Exp Med. 2004;200(3):377–89. doi: 10.1084/jem.20040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cantin AM, North SL, Fells GA, et al. Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest. 1987;79(6):1665–73. doi: 10.1172/JCI113005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1005–28. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 50.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270(51):30334–8. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 51.Thannickal VJ, Aldweib KD, Fanburg BL. Tyrosine phosphorylation regulates H2O2 production in lung fibroblasts stimulated by transforming growth factor beta1. J Biol Chem. 1998;273(36):23611–5. doi: 10.1074/jbc.273.36.23611. [DOI] [PubMed] [Google Scholar]
- 52.Waghray M, Cui Z, Horowitz JC, et al. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005:04–2882fje. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 53.Hagimoto N, Kuwano K, Miyazaki H, et al. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol. 1997;17(3):272–8. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 54.Kuwano K, Hagimoto N, Kawasaki M, et al. Essential roles of the Fas-Fas ligand pathway in the development of pulmonary fibrosis. J Clin Invest. 1999;104(1):13–9. doi: 10.1172/JCI5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aoshiba K, Yasui S, Tamaoki J, et al. The Fas/Fas-ligand system is not required for bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2000;162(2 Pt 1):695–700. doi: 10.1164/ajrccm.162.2.9907012. [DOI] [PubMed] [Google Scholar]
- 56.Uhal BD, Gidea C, Bargout R, et al. Captopril inhibits apoptosis in human lung epithelial cells: a potential antifibrotic mechanism. Am J Physiol. 1998;275(5 Pt 1):L1013–7. doi: 10.1152/ajplung.1998.275.5.L1013. [DOI] [PubMed] [Google Scholar]
- 57.Wang R, Zagariya A, Ang E, et al. Fas-induced apoptosis of alveolar epithelial cells requires ANG II generation and receptor interaction. Am J Physiol. 1999;277(6 Pt 1):L1245–50. doi: 10.1152/ajplung.1999.277.6.L1245. [DOI] [PubMed] [Google Scholar]
- 58.Papp M, Li X, Zhuang J, et al. Angiotensin receptor subtype AT(1) mediates alveolar epithelial cell apoptosis in response to ANG II. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L713–8. doi: 10.1152/ajplung.00103.2001. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Rayford H, Uhal BD. Essential roles for angiotensin receptor AT1a in bleomycin-induced apoptosis and lung fibrosis in mice. Am J Pathol. 2003;163(6):2523–30. doi: 10.1016/S0002-9440(10)63607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otsuka M, Takahashi H, Shiratori M, et al. Reduction of bleomycin induced lung fibrosis by candesartan cilexetil, an angiotensin II type 1 receptor antagonist. Thorax. 2004;59(1):31–8. doi: 10.1136/thx.2003.000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shiratori M, Michalopoulos G, Shinozuka H, et al. Hepatocyte growth factor stimulates DNA synthesis in alveolar epithelial type II cells in vitro. Am J Respir Cell Mol Biol. 1995;12(2):171–80. doi: 10.1165/ajrcmb.12.2.7532419. [DOI] [PubMed] [Google Scholar]
- 62.Inoue T, Okada H, Kobayashi T, et al. Hepatocyte growth factor counteracts transforming growth factor-beta1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. Faseb J. 2003;17(2):268–70. doi: 10.1096/fj.02-0442fje. [DOI] [PubMed] [Google Scholar]
- 63.Lazar MH, Christensen PJ, Du M, et al. Plasminogen activator inhibitor-1 impairs alveolar epithelial repair by binding to vitronectin. Am J Respir Cell Mol Biol. 2004;31(6):672–8. doi: 10.1165/rcmb.2004-0025OC. [DOI] [PubMed] [Google Scholar]
- 64.Mizuno S, Matsumoto K, Li MY, et al. HGF reduces advancing lung fibrosis in mice: a potential role for MMP-dependent myofibroblast apoptosis. Faseb J. 2005;19(6):580–2. doi: 10.1096/fj.04-1535fje. [DOI] [PubMed] [Google Scholar]
- 65.Marchand-Adam S, Marchal J, Cohen M, et al. Defect of hepatocyte growth factor secretion by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168(10):1156–61. doi: 10.1164/rccm.200212-1514OC. [DOI] [PubMed] [Google Scholar]
- 66.Dworkin LD, Gong R, Tolbert E, et al. Hepatocyte growth factor ameliorates progression of interstitial fibrosis in rats with established renal injury. Kidney Int. 2004;65(2):409–19. doi: 10.1111/j.1523-1755.2004.00417.x. [DOI] [PubMed] [Google Scholar]
- 67.Dohi M, Hasegawa T, Yamamoto K, et al. Hepatocyte growth factor attenuates collagen accumulation in a murine model of pulmonary fibrosis. Am J Respir Crit Care Med. 2000;162(6):2302–7. doi: 10.1164/ajrccm.162.6.9908097. [DOI] [PubMed] [Google Scholar]
- 68.Taniyama Y, Morishita R, Nakagami H, et al. Potential contribution of a novel antifibrotic factor, hepatocyte growth factor, to prevention of myocardial fibrosis by angiotensin II blockade in cardiomyopathic hamsters. Circulation. 2000;102(2):246–52. doi: 10.1161/01.cir.102.2.246. [DOI] [PubMed] [Google Scholar]
- 69.Nagahori T, Dohi M, Matsumoto K, et al. Interferon-gamma upregulates the c-Met/hepatocyte growth factor receptor expression in alveolar epithelial cells. Am J Respir Cell Mol Biol. 1999;21(4):490–7. doi: 10.1165/ajrcmb.21.4.3614. [DOI] [PubMed] [Google Scholar]
- 70.Rubin JS, Osada H, Finch PW, et al. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989;86(3):802–6. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finch PW, Rubin JS, Miki T, et al. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245(4919):752–5. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 72.Deterding RR, Jacoby CR, Shannon JM. Acidic fibroblast growth factor and keratinocyte growth factor stimulate fetal rat pulmonary epithelial growth. Am J Physiol. 1996;271(4 Pt 1):L495–505. doi: 10.1152/ajplung.1996.271.4.L495. [DOI] [PubMed] [Google Scholar]
- 73.Zhang F, Nielsen LD, Lucas JJ, et al. Transforming growth factor-beta antagonizes alveolar type II cell proliferation induced by keratinocyte growth factor. Am J Respir Cell Mol Biol. 2004;31(6):679–86. doi: 10.1165/rcmb.2004-0182OC. [DOI] [PubMed] [Google Scholar]
- 74.Deterding RR, Havill AM, Yano T, et al. Prevention of bleomycin-induced lung injury in rats by keratinocyte growth factor. Proc Assoc Am Physicians. 1997;109(3):254–68. [PubMed] [Google Scholar]
- 75.Marchand-Adam S, Plantier L, Bernuau D, et al. Keratinocyte growth factor expression by fibroblasts in pulmonary fibrosis: poor response to interleukin-1beta. Am J Respir Cell Mol Biol. 2005;32(5):470–7. doi: 10.1165/rcmb.2004-0205OC. [DOI] [PubMed] [Google Scholar]
- 76.Christensen PJ, Bailie MB, Goodman RE, et al. Role of diminished epithelial GM-CSF in the pathogenesis of bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L487–95. doi: 10.1152/ajplung.2000.279.3.L487. [DOI] [PubMed] [Google Scholar]
- 77.Eitzman DT, McCoy RD, Zheng X, et al. Bleomycin-induced pulmonary fibrosis in transgenic mice that either lack or overexpress the murine plasminogen activator inhibitor-1 gene. J Clin Invest. 1996;97(1):232–7. doi: 10.1172/JCI118396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sisson TH, Hattori N, Xu Y, et al. Treatment of bleomycin-induced pulmonary fibrosis by transfer of urokinase-type plasminogen activator genes. Hum Gene Ther. 1999;10(14):2315–23. doi: 10.1089/10430349950016960. [DOI] [PubMed] [Google Scholar]
- 79.Hattori N, Degen JL, Sisson TH, et al. Bleomycin-induced pulmonary fibrosis in fibrinogen-null mice. J Clin Invest. 2000;106(11):1341–50. doi: 10.1172/JCI10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sisson TH, Hanson KE, Subbotina N, et al. Inducible lung-specific urokinase expression reduces fibrosis and mortality after lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2002;283(5):L1023–32. doi: 10.1152/ajplung.00049.2002. [DOI] [PubMed] [Google Scholar]
- 81.Chan JC, Duszczyszyn DA, Castellino FJ, et al. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol. 2001;159(5):1681–8. doi: 10.1016/S0002-9440(10)63015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Legrand C, Polette M, Tournier JM, et al. uPA/plasmin system-mediated MMP-9 activation is implicated in bronchial epithelial cell migration. Exp Cell Res. 2001;264(2):326–36. doi: 10.1006/excr.2000.5125. [DOI] [PubMed] [Google Scholar]
- 83.Bitterman PB, Wewers MD, Rennard SI, et al. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986;77(3):700–8. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lama V, Moore BB, Christensen P, et al. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27(6):752–8. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- 85.White ES, Atrasz RG, Dickie EG, et al. Prostaglandin E(2) inhibits fibroblast migration by E-prostanoid 2 receptor-mediated increase in PTEN activity. Am J Respir Cell Mol Biol. 2005;32(2):135–41. doi: 10.1165/rcmb.2004-0126OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kolodsick JE, Peters-Golden M, Larios J, et al. Prostaglandin E2 inhibits fibroblast to myofibroblast transition via E. prostanoid receptor 2 signaling and cyclic adenosine monophosphate elevation. Am J Respir Cell Mol Biol. 2003;29(5):537–44. doi: 10.1165/rcmb.2002-0243OC. [DOI] [PubMed] [Google Scholar]
- 87.Goldstein RH, Polgar P. The effect and interaction of bradykinin and prostaglandins on protein and collagen production by lung fibroblasts. J Biol Chem. 1982;257(15):8630–3. [PubMed] [Google Scholar]
- 88.Keerthisingam CB, Jenkins RG, Harrison NK, et al. Cyclooxygenase-2 deficiency results in a loss of the anti-proliferative response to transforming growth factor-beta in human fibrotic lung fibroblasts and promotes bleomycin-induced pulmonary fibrosis in mice. Am J Pathol. 2001;158(4):1411–22. doi: 10.1016/s0002-9440(10)64092-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peters-Golden M, Bailie M, Marshall T, et al. Protection from pulmonary fibrosis in leukotriene-deficient mice. Am J Respir Crit Care Med. 2002;165(2):229–35. doi: 10.1164/ajrccm.165.2.2104050. [DOI] [PubMed] [Google Scholar]
- 90.Borok Z, Gillissen A, Buhl R, et al. Augmentation of functional prostaglandin E levels on the respiratory epithelial surface by aerosol administration of prostaglandin E. Am Rev Respir Dis. 1991;144(5):1080–4. doi: 10.1164/ajrccm/144.5.1080. [DOI] [PubMed] [Google Scholar]
- 91.Wilborn J, Bailie M, Coffey M, et al. Constitutive activation of 5-lipoxygenase in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1996;97(8):1827–36. doi: 10.1172/JCI118612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105(3):369–77. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 93.Kotton DN, Ma BY, Cardoso WV, et al. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128(24):5181–8. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- 94.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33(4):328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grove JE, Lutzko C, Priller J, et al. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol. 2002;27(6):645–51. doi: 10.1165/rcmb.2002-0056RC. [DOI] [PubMed] [Google Scholar]
- 96.Willis BC, Liebler JM, Luby-Phelps K, et al. Induction of Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells by Transforming Growth Factor-{beta}1: Potential Role in Idiopathic Pulmonary Fibrosis. Am J Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Desmouliere A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13(1):7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- 98.Kuhn C, McDonald JA. The roles of the myofibroblast in idiopathic pulmonary fibrosis. Ultrastructural and immunohistochemical features of sites of active extracellular matrix synthesis. Am J Pathol. 1991;138(5):1257–65. [PMC free article] [PubMed] [Google Scholar]
- 99.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 100.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 101.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–3. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 102.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122(6 Suppl):286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 103.Phillips RJ, Burdick MD, Hong K, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114(3):438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moore BB, Kolodsick JE, Thannickal VJ, et al. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166(3):675–84. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hashimoto N, Jin H, Liu T, et al. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113(2):243–52. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yao HW, Xie QM, Chen JQ, et al. TGF-beta1 induces alveolar epithelial to mesenchymal transition in vitro. Life Sci. 2004;76(1):29–37. doi: 10.1016/j.lfs.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 107.Kasai H, Allen JT, Mason RM, et al. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT). Respir Res. 2005;6(1):56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Desmouliere A, Redard M, Darby I, et al. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- 109.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 110.Chapman HA. Disorders of lung matrix remodeling. J Clin Invest. 2004;113(2):148–57. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sime PJ, Xing Z, Graham FL, et al. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100(4):768–76. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kolb M, Bonniaud P, Galt T, et al. Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of “fibrosis-prone” and “fibrosis-resistant” mouse strains. Am J Respir Cell Mol Biol. 2002;27(2):141–50. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]
- 113.Kolb M, Margetts PJ, Galt T, et al. Transient transgene expression of decorin in the lung reduces the fibrotic response to bleomycin. Am J Respir Crit Care Med. 2001;163(3 Pt 1):770–7. doi: 10.1164/ajrccm.163.3.2006084. [DOI] [PubMed] [Google Scholar]
- 114.Horowitz JC, Lee DY, Waghray M, et al. Activation of the pro-survival phosphatidylinositol 3-kinase/AKT pathway by transforming growth factor-beta1 in mesenchymal cells is mediated by p38 MAPK-dependent induction of an autocrine growth factor. J Biol Chem. 2004;279(2):1359–67. doi: 10.1074/jbc.M306248200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang HY, Phan SH. Inhibition of myofibroblast apoptosis by transforming growth factor beta(1). Am J Respir Cell Mol Biol. 1999;21(6):658–65. doi: 10.1165/ajrcmb.21.6.3720. [DOI] [PubMed] [Google Scholar]
- 116.Sun G, Stacey MA, Bellini A, et al. Endothelin-1 induces bronchial myofibroblast differentiation. Peptides. 1997;18(9):1449–51. doi: 10.1016/s0196-9781(97)00194-0. [DOI] [PubMed] [Google Scholar]
- 117.Bogatkevich GS, Tourkina E, Silver RM, et al. Thrombin differentiates normal lung fibroblasts to a myofibroblast phenotype via the proteolytically activated receptor-1 and a protein kinase C-dependent pathway. J Biol Chem. 2001;276(48):45184–92. doi: 10.1074/jbc.M106441200. [DOI] [PubMed] [Google Scholar]
- 118.Morishima Y, Nomura A, Uchida Y, et al. Triggering the induction of myofibroblast and fibrogenesis by airway epithelial shedding. Am J Respir Cell Mol Biol. 2001;24(1):1–11. doi: 10.1165/ajrcmb.24.1.4040. [DOI] [PubMed] [Google Scholar]
- 119.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161(6):1999–2004. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- 120.Nguyen L, Ward WF, Ts'ao CH, et al. Captopril inhibits proliferation of human lung fibroblasts in culture: a potential antifibrotic mechanism. Proc Soc Exp Biol Med. 1994;205(1):80–4. doi: 10.3181/00379727-205-43681. [DOI] [PubMed] [Google Scholar]
- 121.Ketteler M, Noble NA, Border WA. Transforming growth factor-beta and angiotensin II: the missing link from glomerular hyperfiltration to glomerulosclerosis? Annu Rev Physiol. 1995;57:279–95. doi: 10.1146/annurev.ph.57.030195.001431. [DOI] [PubMed] [Google Scholar]
- 122.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29(7):1947–58. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 123.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–81. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hinz B, Mastrangelo D, Iselin CE, et al. Mechanical tension controls granulation tissue contractile activity and myofibroblast differentiation. Am J Pathol. 2001;159(3):1009–20. doi: 10.1016/S0002-9440(10)61776-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Arora PD, Narani N, McCulloch CA. The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol. 1999;154(3):871–82. doi: 10.1016/s0002-9440(10)65334-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhu YK, Umino T, Liu XD, et al. Contraction of fibroblast-containing collagen gels: initial collagen concentration regulates the degree of contraction and cell survival. In Vitro Cell Dev Biol Anim. 2001;37(1):10–6. doi: 10.1290/1071-2690(2001)037<0010:COFCCG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 127.Thannickal VJ, Lee DY, White ES, et al. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278(14):12384–9. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 128.Uhal BD, Joshi I, True AL, et al. Fibroblasts isolated after fibrotic lung injury induce apoptosis of alveolar epithelial cells in vitro. Am J Physiol. 1995;269(6 Pt 1):L819–28. doi: 10.1152/ajplung.1995.269.6.L819. [DOI] [PubMed] [Google Scholar]
- 129.Larios JM, Budhiraja R, Fanburg BL, et al. Oxidative protein cross-linking reactions involving L-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem. 2001;276(20):17437–41. doi: 10.1074/jbc.M100426200. [DOI] [PubMed] [Google Scholar]
- 130.Phan SH, Zhang K, Zhang HY, et al. The myofibroblast as an inflammatory cell in pulmonary fibrosis. Curr Top Pathol. 1999;93:173–82. doi: 10.1007/978-3-642-58456-5_18. [DOI] [PubMed] [Google Scholar]
- 131.Strieter RM, Wiggins R, Phan SH, et al. Monocyte chemotactic protein gene expression by cytokine-treated human fibroblasts and endothelial cells. Biochem Biophys Res Commun. 1989;162(2):694–700. doi: 10.1016/0006-291x(89)92366-8. [DOI] [PubMed] [Google Scholar]
- 132.Rolfe MW, Kunkel SL, Standiford TJ, et al. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991;5(5):493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- 133.Strieter RM. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest. 2005;128(5 Suppl 1):526S–532S. doi: 10.1378/chest.128.5_suppl_1.526S. [DOI] [PubMed] [Google Scholar]
- 134.Crystal RG, Fulmer JD, Roberts WC, et al. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976;85(6):769–88. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- 135.Mason RJ, Schwarz MI, Hunninghake GW, et al. NHLBI Workshop Summary. Pharmacological therapy for idiopathic pulmonary fibrosis. Past, present, and future. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1771–7. doi: 10.1164/ajrccm.160.5.9903009. [DOI] [PubMed] [Google Scholar]
- 136.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 137.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345(7):517–25. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 138.Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99(9):6292–7. doi: 10.1073/pnas.092134099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Agostini C, Siviero M, Semenzato G. Immune effector cells in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 1997;3(5):348–55. doi: 10.1097/00063198-199709000-00006. [DOI] [PubMed] [Google Scholar]
- 140.Kelly M, Kolb M, Bonniaud P, et al. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des. 2003;9(1):39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- 141.Lukacs NW, Hogaboam C, Chensue SW, et al. Type 1/type 2 cytokine paradigm and the progression of pulmonary fibrosis. Chest. 2001;120(1 Suppl):5S–8S. doi: 10.1378/chest.120.1_suppl.s5. [DOI] [PubMed] [Google Scholar]
- 142.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jakubzick C, Choi ES, Joshi BH, et al. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol. 2003;171(5):2684–93. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- 144.Cavarra E, Carraro F, Fineschi S, et al. Early response to bleomycin is characterized by different cytokine and cytokine receptor profiles in lungs. Am J Physiol Lung Cell Mol Physiol. 2004;287(6):L1186–92. doi: 10.1152/ajplung.00170.2004. [DOI] [PubMed] [Google Scholar]
- 145.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33(1):9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 146.Helene M, Lake-Bullock V, Zhu J, et al. T cell independence of bleomycin-induced pulmonary fibrosis. J Leukoc Biol. 1999;65(2):187–95. doi: 10.1002/jlb.65.2.187. [DOI] [PubMed] [Google Scholar]
- 147.Okazaki T, Nakao A, Nakano H, et al. Impairment of bleomycin-induced lung fibrosis in CD28-deficient mice. J Immunol. 2001;167(4):1977–81. doi: 10.4049/jimmunol.167.4.1977. [DOI] [PubMed] [Google Scholar]
- 148.Jakubzick C, Choi ES, Kunkel SL, et al. Augmented pulmonary IL-4 and IL-13 receptor subunit expression in idiopathic interstitial pneumonia. J Clin Pathol. 2004;57(5):477–86. doi: 10.1136/jcp.2003.012799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jakubzick C, Choi ES, Carpenter KJ, et al. Human pulmonary fibroblasts exhibit altered interleukin-4 and interleukin-13 receptor subunit expression in idiopathic interstitial pneumonia. Am J Pathol. 2004;164(6):1989–2001. doi: 10.1016/S0002-9440(10)63759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Peao MN, Aguas AP, de Sa CM, et al. Neoformation of blood vessels in association with rat lung fibrosis induced by bleomycin. Anat Rec. 1994;238(1):57–67. doi: 10.1002/ar.1092380108. [DOI] [PubMed] [Google Scholar]
- 151.Turner-Warwick M. Precapillary Systemic-Pulmonary Anastomoses. Thorax. 1963;18:225–37. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Simler NR, Brenchley PE, Horrocks AW, et al. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax. 2004;59(7):581–5. doi: 10.1136/thx.2003.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Keane MP, Arenberg DA, Lynch JP, 3rd, et al. The CXC chemokines, IL-8 and IP-10, regulate angiogenic activity in idiopathic pulmonary fibrosis. J Immunol. 1997;159(3):1437–43. [PubMed] [Google Scholar]
- 154.Keane MP, Belperio JA, Burdick MD, et al. ENA-78 is an important angiogenic factor in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(12):2239–42. doi: 10.1164/ajrccm.164.12.2104106. [DOI] [PubMed] [Google Scholar]
- 155.Keane MP, Belperio JA, Arenberg DA, et al. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163(10):5686–92. [PubMed] [Google Scholar]
- 156.Keane MP, Belperio JA, Burdick MD, et al. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L92–7. doi: 10.1152/ajplung.2001.281.1.L92. [DOI] [PubMed] [Google Scholar]
- 157.Burdick MD, Murray LA, Keane MP, et al. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171(3):261–8. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- 158.Tager AM, Kradin RL, LaCamera P, et al. Inhibition of pulmonary fibrosis by the chemokine IP-10/CXCL10. Am J Respir Cell Mol Biol. 2004;31(4):395–404. doi: 10.1165/rcmb.2004-0175OC. [DOI] [PubMed] [Google Scholar]
- 159.Renzoni EA, Walsh DA, Salmon M, et al. Interstitial vascularity in fibrosing alveolitis. Am J Respir Crit Care Med. 2003;167(3):438–43. doi: 10.1164/rccm.200202-135OC. [DOI] [PubMed] [Google Scholar]
- 160.Cosgrove GP, Brown KK, Schiemann WP, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170(3):242–51. doi: 10.1164/rccm.200308-1151OC. [DOI] [PubMed] [Google Scholar]
- 161.Sumi M, Satoh H, Kagohashi K, et al. Increased serum levels of endostatin in patients with idiopathic pulmonary fibrosis. J Clin Lab Anal. 2005;19(4):146–9. doi: 10.1002/jcla.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Beer TW, Baldwin HC, Goddard JR, et al. Angiogenesis in pathological and surgical scars. Hum Pathol. 1998;29(11):1273–8. doi: 10.1016/s0046-8177(98)90256-8. [DOI] [PubMed] [Google Scholar]
- 163.Weitzenblum E, Ehrhart M, Rasaholinjanahary J, et al. Pulmonary hemodynamics in idiopathic pulmonary fibrosis and other interstitial pulmonary diseases. Respiration. 1983;44(2):118–27. doi: 10.1159/000194537. [DOI] [PubMed] [Google Scholar]
- 164.Agusti AG, Roca J, Gea J, et al. Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1991;143(2):219–25. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- 165.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest. 2005;128(5):3310–5. doi: 10.1378/chest.128.5.3310. [DOI] [PubMed] [Google Scholar]
- 166.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med. 2002;347(5):322–9. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 167.Ghofrani HA, Wiedemann R, Rose F, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360(9337):895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 168.Douglas WW, Ryu JH, Schroeder DR. Idiopathic pulmonary fibrosis: Impact of oxygen and colchicine, prednisone, or no therapy on survival. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1172–8. doi: 10.1164/ajrccm.161.4.9907002. [DOI] [PubMed] [Google Scholar]
- 169.Lynch JP, 3rd, White E, Flaherty K. Corticosteroids in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2001;7(5):298–308. doi: 10.1097/00063198-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 170.Ryu JH, Myers JL, Capizzi SA, et al. Desquamative interstitial pneumonia and respiratory bronchiolitis-associated interstitial lung disease. Chest. 2005;127(1):178–84. doi: 10.1378/chest.127.1.178. [DOI] [PubMed] [Google Scholar]
- 171.Deheinzelin D, Capelozzi VL, Kairalla RA, et al. Interstitial lung disease in primary Sjogren's syndrome. Clinical-pathological evaluation and response to treatment. Am J Respir Crit Care Med. 1996;154(3 Pt 1):794–9. doi: 10.1164/ajrccm.154.3.8810621. [DOI] [PubMed] [Google Scholar]
- 172.Nicholson AG, Colby TV, du Bois RM, et al. The prognostic significance of the histologic pattern of interstitial pneumonia in patients presenting with the clinical entity of cryptogenic fibrosing alveolitis. Am J Respir Crit Care Med. 2000;162(6):2213–7. doi: 10.1164/ajrccm.162.6.2003049. [DOI] [PubMed] [Google Scholar]
- 173.Bouros D, Wells AU, Nicholson AG, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165(12):1581–6. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 174.Dheda K, Lalloo UG, Cassim B, et al. Experience with azathioprine in systemic sclerosis associated with interstitial lung disease. Clin Rheumatol. 2004;23(4):306–9. doi: 10.1007/s10067-004-0906-7. [DOI] [PubMed] [Google Scholar]
- 175.Clark JG, Dedon TF, Wayner EA, et al. Effects of interferon-gamma on expression of cell surface receptors for collagen and deposition of newly synthesized collagen by cultured human lung fibroblasts. J Clin Invest. 1989;83(5):1505–11. doi: 10.1172/JCI114045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Narayanan AS, Whithey J, Souza A, et al. Effect of gamma-interferon on collagen synthesis by normal and fibrotic human lung fibroblasts. Chest. 1992;101(5):1326–31. doi: 10.1378/chest.101.5.1326. [DOI] [PubMed] [Google Scholar]
- 177.Jaffe HA, Gao Z, Mori Y, et al. Selective inhibition of collagen gene expression in fibroblasts by an interferon-gamma transgene. Exp Lung Res. 1999;25(3):199–215. doi: 10.1080/019021499270268. [DOI] [PubMed] [Google Scholar]
- 178.Adelmann-Grill BC, Hein R, Wach F, et al. Inhibition of fibroblast chemotaxis by recombinant human interferon gamma and interferon alpha. J Cell Physiol. 1987;130(2):270–5. doi: 10.1002/jcp.1041300213. [DOI] [PubMed] [Google Scholar]
- 179.Strieter RM, Belperio JA, Keane MP. CXC chemokines in vascular remodeling related to pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(3 Suppl):S67–9. [PubMed] [Google Scholar]
- 180.Hunninghake GW, Hemken C, Brady M, et al. Immune interferon is a growth factor for human lung fibroblasts. Am Rev Respir Dis. 1986;134(5):1025–8. doi: 10.1164/arrd.1986.134.5.1025. [DOI] [PubMed] [Google Scholar]
- 181.Elias JA, Jimenez SA, Freundlich B. Recombinant gamma, alpha, and beta interferon regulation of human lung fibroblast proliferation. Am Rev Respir Dis. 1987;135(1):62–5. doi: 10.1164/arrd.1987.135.1.62. [DOI] [PubMed] [Google Scholar]
- 182.Moseley PL, Hemken C, Monick M, et al. Interferon and growth factor activity for human lung fibroblasts. Release from bronchoalveolar cells from patients with active sarcoidosis. Chest. 1986;89(5):657–62. doi: 10.1378/chest.89.5.657. [DOI] [PubMed] [Google Scholar]
- 183.Hasegawa T, Nakao A, Sumiyoshi K, et al. IFN-gamma fails to antagonize fibrotic effect of TGF-beta on keloid-derived dermal fibroblasts. J Dermatol Sci. 2003;32(1):19–24. doi: 10.1016/s0923-1811(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 184.Oldroyd SD, Thomas GL, Gabbiani G, et al. Interferon-gamma inhibits experimental renal fibrosis. Kidney Int. 1999;56(6):2116–27. doi: 10.1046/j.1523-1755.1999.00775.x. [DOI] [PubMed] [Google Scholar]
- 185.Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21(5):791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- 186.Weng HL, Cai WM, Liu RH. Animal experiment and clinical study of effect of gamma-interferon on hepatic fibrosis. World J Gastroenterol. 2001;7(1):42–8. doi: 10.3748/wjg.v7.i1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ziesche R, Hofbauer E, Wittmann K, et al. A preliminary study of long-term treatment with interferon gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341(17):1264–9. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- 188.Raghu G, Brown KK, Bradford WZ, et al. A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350(2):125–33. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- 189.Nicod LP. Pirfenidone in idiopathic pulmonary fibrosis. Lancet. 1999;354(9175):268–9. doi: 10.1016/s0140-6736(99)00178-6. [DOI] [PubMed] [Google Scholar]
- 190.Shihab FS, Bennett WM, Yi H, et al. Pirfenidone treatment decreases transforming growth factor-beta1 and matrix proteins and ameliorates fibrosis in chronic cyclosporine nephrotoxicity. Am J Transplant. 2002;2(2):111–9. doi: 10.1034/j.1600-6143.2002.020201.x. [DOI] [PubMed] [Google Scholar]
- 191.Iyer SN, Gurujeyalakshmi G, Giri SN. Effects of pirfenidone on procollagen gene expression at the transcriptional level in bleomycin hamster model of lung fibrosis. J Pharmacol Exp Ther. 1999;289(1):211–8. [PubMed] [Google Scholar]
- 192.Kakugawa T, Mukae H, Hayashi T, et al. Pirfenidone attenuates expression of HSP47 in murine bleomycin-induced pulmonary fibrosis. Eur Respir J. 2004;24(1):57–65. doi: 10.1183/09031936.04.00120803. [DOI] [PubMed] [Google Scholar]
- 193.Giri SN, Leonard S, Shi X, et al. Effects of pirfenidone on the generation of reactive oxygen species in vitro. J Environ Pathol Toxicol Oncol. 1999;18(3):169–77. [PubMed] [Google Scholar]
- 194.Misra HP, Rabideau C. Pirfenidone inhibits NADPH-dependent microsomal lipid peroxidation and scavenges hydroxyl radicals. Mol Cell Biochem. 2000;204(1−2):119–26. doi: 10.1023/a:1007023532508. [DOI] [PubMed] [Google Scholar]
- 195.Garcia L, Hernandez I, Sandoval A, et al. Pirfenidone effectively reverses experimental liver fibrosis. J Hepatol. 2002;37(6):797–805. doi: 10.1016/s0168-8278(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 196.Suga H, Teraoka S, Ota K, et al. Preventive effect of pirfenidone against experimental sclerosing peritonitis in rats. Exp Toxicol Pathol. 1995;47(4):287–91. doi: 10.1016/s0940-2993(11)80261-7. [DOI] [PubMed] [Google Scholar]
- 197.Shimizu T, Fukagawa M, Kuroda T, et al. Pirfenidone prevents collagen accumulation in the remnant kidney in rats with partial nephrectomy. Kidney Int Suppl. 1997;63:S239–43. [PubMed] [Google Scholar]
- 198.Angulo P, MacCarty RL, Sylvestre PB, et al. Pirfenidone in the treatment of primary sclerosing cholangitis. Dig Dis Sci. 2002;47(1):157–61. doi: 10.1023/a:1013240225965. [DOI] [PubMed] [Google Scholar]
- 199.Iyer SN, Margolin SB, Hyde DM, et al. Lung fibrosis is ameliorated by pirfenidone fed in diet after the second dose in a three-dose bleomycin-hamster model. Exp Lung Res. 1998;24(1):119–32. doi: 10.3109/01902149809046058. [DOI] [PubMed] [Google Scholar]
- 200.Kehrer JP, Margolin SB. Pirfenidone diminishes cyclophosphamide-induced lung fibrosis in mice. Toxicol Lett. 1997;90(2−3):125–32. doi: 10.1016/s0378-4274(96)03845-3. [DOI] [PubMed] [Google Scholar]
- 201.Raghu G, Johnson WC, Lockhart D, et al. Treatment of idiopathic pulmonary fibrosis with a new antifibrotic agent, pirfenidone: results of a prospective, open-label Phase II study. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1061–9. doi: 10.1164/ajrccm.159.4.9805017. [DOI] [PubMed] [Google Scholar]
- 202.Gahl WA, Brantly M, Troendle J, et al. Effect of pirfenidone on the pulmonary fibrosis of Hermansky-Pudlak syndrome. Mol Genet Metab. 2002;76(3):234–42. doi: 10.1016/s1096-7192(02)00044-6. [DOI] [PubMed] [Google Scholar]
- 203.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, Placebo-controlled Trial of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2005;171(9):1040–7. doi: 10.1164/rccm.200404-571OC. [DOI] [PubMed] [Google Scholar]
- 204.Charbeneau RP, Peters-Golden M. Eicosanoids: mediators and therapeutic targets in fibrotic lung disease. Clin Sci (Lond) 2005;108(6):479–91. doi: 10.1042/CS20050012. [DOI] [PubMed] [Google Scholar]
- 205.Mio T, Nagai S, Kitaichi M, et al. Proliferative characteristics of fibroblast lines derived from open lung biopsy specimens of patients with IPF (UIP). Chest. 1992;102(3):832–7. doi: 10.1378/chest.102.3.832. [DOI] [PubMed] [Google Scholar]
- 206.Moore BB, Peters-Golden M, Christensen PJ, et al. Alveolar epithelial cell inhibition of fibroblast proliferation is regulated by MCP-1/CCR2 and mediated by PGE2. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L342–9. doi: 10.1152/ajplung.00168.2002. [DOI] [PubMed] [Google Scholar]
- 207.Moore BB, Paine R,, 3rd, Christensen PJ, et al. Protection from pulmonary fibrosis in the absence of CCR2 signaling. J Immunol. 2001;167(8):4368–77. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 208.Moore BB, Coffey MJ, Christensen P, et al. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165(7):4032–9. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- 209.Zhang Y, Lee TC, Guillemin B, et al. Enhanced IL-1 beta and tumor necrosis factor-alpha release and messenger RNA expression in macrophages from idiopathic pulmonary fibrosis or after asbestos exposure. J Immunol. 1993;150(9):4188–96. [PubMed] [Google Scholar]
- 210.Piguet PF, Ribaux C, Karpuz V, et al. Expression and localization of tumor necrosis factor-alpha and its mRNA in idiopathic pulmonary fibrosis. Am J Pathol. 1993;143(3):651–5. [PMC free article] [PubMed] [Google Scholar]
- 211.Libura J, Bettens F, Radkowski A, et al. Risk of chemotherapy-induced pulmonary fibrosis is associated with polymorphic tumour necrosis factor-a2 gene. Eur Respir J. 2002;19(5):912–8. doi: 10.1183/09031936.02.00238102. [DOI] [PubMed] [Google Scholar]
- 212.Riha RL, Yang IA, Rabnott GC, et al. Cytokine gene polymorphisms in idiopathic pulmonary fibrosis. Intern Med J. 2004;34(3):126–9. doi: 10.1111/j.1444-0903.2004.00503.x. [DOI] [PubMed] [Google Scholar]
- 213.Miyazaki Y, Araki K, Vesin C, et al. Expression of a tumor necrosis factor-alpha transgene in murine lung causes lymphocytic and fibrosing alveolitis. A mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96(1):250–9. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Sime PJ, Marr RA, Gauldie D, et al. Transfer of tumor necrosis factor-alpha to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153(3):825–32. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Ortiz LA, Lasky J, Hamilton RF, Jr., et al. Expression of TNF and the necessity of TNF receptors in bleomycin-induced lung injury in mice. Exp Lung Res. 1998;24(6):721–43. doi: 10.3109/01902149809099592. [DOI] [PubMed] [Google Scholar]
- 216.Piguet PF, Vesin C. Treatment by human recombinant soluble TNF receptor of pulmonary fibrosis induced by bleomycin or silica in mice. Eur Respir J. 1994;7(3):515–8. doi: 10.1183/09031936.94.07030515. [DOI] [PubMed] [Google Scholar]
- 217.Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86(12):1259–65. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- 218.Solis-Herruzo JA, Brenner DA, Chojkier M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988;263(12):5841–5. [PubMed] [Google Scholar]
- 219.Kahari VM, Chen YQ, Su MW, et al. Tumor necrosis factor-alpha and interferon-gamma suppress the activation of human type I collagen gene expression by transforming growth factor-beta 1. Evidence for two distinct mechanisms of inhibition at the transcriptional and posttranscriptional levels. J Clin Invest. 1990;86(5):1489–95. doi: 10.1172/JCI114866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Greenwel P, Tanaka S, Penkov D, et al. Tumor necrosis factor alpha inhibits type I collagen synthesis through repressive CCAAT/enhancer-binding proteins. Mol Cell Biol. 2000;20(3):912–8. doi: 10.1128/mcb.20.3.912-918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Han YP, Tuan TL, Hughes M, et al. Transforming growth factor-beta - and tumor necrosis factor-alpha -mediated induction and proteolytic activation of MMP-9 in human skin. J Biol Chem. 2001;276(25):22341–50. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Han YP, Tuan TL, Wu H, et al. TNF-alpha stimulates activation of pro-MMP2 in human skin through NF-(kappa)B mediated induction of MT1-MMP. J Cell Sci. 2001;114(Pt 1):131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kuroki M, Noguchi Y, Shimono M, et al. Repression of bleomycin-induced pneumopathy by TNF. J Immunol. 2003;170(1):567–74. doi: 10.4049/jimmunol.170.1.567. [DOI] [PubMed] [Google Scholar]
- 224.Fujita M, Shannon JM, Morikawa O, et al. Overexpression of tumor necrosis factor-alpha diminishes pulmonary fibrosis induced by bleomycin or transforming growth factor-beta. Am J Respir Cell Mol Biol. 2003;29(6):669–76. doi: 10.1165/rcmb.2002-0046OC. [DOI] [PubMed] [Google Scholar]
- 225.Raghu G, Lasky JA, Costabel U, et al. A RANDOMIZED PLACEBO CONTROLLED TRIAL ASSESSING THE EFFICACY AND SAFETY OF ETANERCEPT IN PATIENTS WITH IDIOPATHIC PULMONARY FIBROSIS (IPF) Chest. 2005;128(4):496S–a-. [Google Scholar]
- 226.Kinnula VL, Fattman CL, Tan RJ, et al. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172(4):417–22. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139(2):370–2. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 228.Smilkstein MJ, Bronstein AC, Linden C, et al. Acetaminophen overdose: a 48-hour intravenous N-acetylcysteine treatment protocol. Ann Emerg Med. 1991;20(10):1058–63. doi: 10.1016/s0196-0644(05)81352-6. [DOI] [PubMed] [Google Scholar]
- 229.Tepel M, van der Giet M, Schwarzfeld C, et al. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343(3):180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 230.Cantin AM, Larivee P, Begin RO. Extracellular glutathione suppresses human lung fibroblast proliferation. Am J Respir Cell Mol Biol. 1990;3(1):79–85. doi: 10.1165/ajrcmb/3.1.79. [DOI] [PubMed] [Google Scholar]
- 231.Borok Z, Buhl R, Grimes GJ, et al. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338(8761):215–6. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 232.Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J. 1994;7(3):431–6. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 233.Meyer A, Buhl R, Kampf S, et al. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals. Am J Respir Crit Care Med. 1995;152(3):1055–60. doi: 10.1164/ajrccm.152.3.7663783. [DOI] [PubMed] [Google Scholar]
- 234.Behr J, Maier K, Degenkolb B, et al. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis. Adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156(6):1897–901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 235.Tomioka H, Kuwata Y, Imanaka K, et al. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005;10(4):449–55. doi: 10.1111/j.1440-1843.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 236.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2229–42. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 237.Hunninghake GW. Antioxidant therapy for idiopathic pulmonary fibrosis. N Engl J Med. 2005;353(21):2285–7. doi: 10.1056/NEJMe058210. [DOI] [PubMed] [Google Scholar]
- 238.Brewer GJ, Ullenbruch MR, Dick R, et al. Tetrathiomolybdate therapy protects against bleomycin-induced pulmonary fibrosis in mice. J Lab Clin Med. 2003;141(3):210–6. doi: 10.1067/mlc.2003.20. [DOI] [PubMed] [Google Scholar]
- 239.Brewer GJ. Copper control as an antiangiogenic anticancer therapy: lessons from treating Wilson's disease. Exp Biol Med (Maywood) 2001;226(7):665–73. doi: 10.1177/153537020222600712. [DOI] [PubMed] [Google Scholar]
- 240.Brewer GJ. Tetrathiomolybdate anticopper therapy for Wilson's disease inhibits angiogenesis, fibrosis and inflammation. J Cell Mol Med. 2003;7(1):11–20. doi: 10.1111/j.1582-4934.2003.tb00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Brewer GJ, Dick R, Ullenbruch MR, et al. Inhibition of key cytokines by tetrathiomolybdate in the bleomycin model of pulmonary fibrosis. J Inorg Biochem. 2004;98(12):2160–7. doi: 10.1016/j.jinorgbio.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 242.Ehrenreich H, Anderson RW, Fox CH, et al. Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med. 1990;172(6):1741–8. doi: 10.1084/jem.172.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Shahar I, Fireman E, Topilsky M, et al. Effect of endothelin-1 on alpha-smooth muscle actin expression and on alveolar fibroblasts proliferation in interstitial lung diseases. Int J Immunopharmacol. 1999;21(11):759–75. doi: 10.1016/s0192-0561(99)00056-9. [DOI] [PubMed] [Google Scholar]
- 244.Goto T, Yanaga F, Ohtsuki I. Studies on the endothelin-1-induced contraction of rat granulation tissue pouch mediated by myofibroblasts. Biochim Biophys Acta. 1998;1405(1−3):55–66. doi: 10.1016/s0167-4889(98)00105-0. [DOI] [PubMed] [Google Scholar]
- 245.Shi-Wen X, Chen Y, Denton CP, et al. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15(6):2707–19. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Xu SW, Howat SL, Renzoni EA, et al. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279(22):23098–103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- 247.Hocher B, Schwarz A, Fagan KA, et al. Pulmonary fibrosis and chronic lung inflammation in ET-1 transgenic mice. Am J Respir Cell Mol Biol. 2000;23(1):19–26. doi: 10.1165/ajrcmb.23.1.4030. [DOI] [PubMed] [Google Scholar]
- 248.Park SH, Saleh D, Giaid A, et al. Increased endothelin-1 in bleomycin-induced pulmonary fibrosis and the effect of an endothelin receptor antagonist. Am J Respir Crit Care Med. 1997;156(2 Pt 1):600–8. doi: 10.1164/ajrccm.156.2.9607123. [DOI] [PubMed] [Google Scholar]
- 249.Mutsaers SE, Marshall RP, Goldsack NR, et al. Effect of endothelin receptor antagonists (BQ-485, Ro 47−0203) on collagen deposition during the development of bleomycin-induced pulmonary fibrosis in rats. Pulm Pharmacol Ther. 1998;11(2−3):221–5. doi: 10.1006/pupt.1998.0142. [DOI] [PubMed] [Google Scholar]
- 250.Giaid A, Michel RP, Stewart DJ, et al. Expression of endothelin-1 in lungs of patients with cryptogenic fibrosing alveolitis. Lancet. 1993;341(8860):1550–4. doi: 10.1016/0140-6736(93)90694-c. [DOI] [PubMed] [Google Scholar]
- 251.Saleh D, Furukawa K, Tsao MS, et al. Elevated expression of endothelin-1 and endothelin-converting enzyme-1 in idiopathic pulmonary fibrosis: possible involvement of proinflammatory cytokines. Am J Respir Cell Mol Biol. 1997;16(2):187–93. doi: 10.1165/ajrcmb.16.2.9032126. [DOI] [PubMed] [Google Scholar]
- 252.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346(12):896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 253.Broekelmann TJ, Limper AH, Colby TV, et al. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991;88(15):6642–6. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Khalil N, O'Connor RN, Unruh HW, et al. Increased production and immunohistochemical localization of transforming growth factor-beta in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 1991;5(2):155–62. doi: 10.1165/ajrcmb/5.2.155. [DOI] [PubMed] [Google Scholar]
- 255.Bonniaud P, Margetts PJ, Kolb M, et al. Progressive Transforming Growth Factor {beta}1-induced Lung Fibrosis Is Blocked by an Orally Active ALK5 Kinase Inhibitor. Am J Respir Crit Care Med. 2005;171(8):889–98. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 256.Bonniaud P, Kolb M, Galt T, et al. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173(3):2099–108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- 257.Chen H, Sun J, Buckley S, et al. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol. 2005;288(4):L683–91. doi: 10.1152/ajplung.00298.2004. [DOI] [PubMed] [Google Scholar]
- 258.Massague J, Blain SW, Lo RS. TGFbeta signaling in growth control, cancer, and heritable disorders. Cell. 2000;103(2):295–309. doi: 10.1016/s0092-8674(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 259.Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1084–6. doi: 10.1056/NEJM200104053441409. [DOI] [PubMed] [Google Scholar]
- 260.Daniels CE, Wilkes MC, Edens M, et al. Imatinib mesylate inhibits the profibrogenic activity of TGF-beta and prevents bleomycin-mediated lung fibrosis. J Clin Invest. 2004;114(9):1308–16. doi: 10.1172/JCI19603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Aono Y, Nishioka Y, Inayama M, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2005;171(11):1279–85. doi: 10.1164/rccm.200404-531OC. [DOI] [PubMed] [Google Scholar]
- 262.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925–35. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Frisch SM, Vuori K, Ruoslahti E, et al. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134(3):793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Hadden HL, Henke CA. Induction of lung fibroblast apoptosis by soluble fibronectin peptides. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1553–60. doi: 10.1164/ajrccm.162.4.2001015. [DOI] [PubMed] [Google Scholar]
- 265.Xia H, Nho RS, Kahm J, et al. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279(31):33024–34. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 266.Vittal R, Horowitz JC, Moore BB, et al. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol. 2005;166(2):367–75. doi: 10.1016/S0002-9440(10)62260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Olson JM, Hallahan AR. p38 MAP kinase: a convergence point in cancer therapy. Trends Mol Med. 2004;10(3):125–9. doi: 10.1016/j.molmed.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 268.Yamaguchi K, Shirakabe K, Shibuya H, et al. Identification of a member of the MAPKKK family as a potential mediator of TGF-beta signal transduction. Science. 1995;270(5244):2008–11. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- 269.Takekawa M, Tatebayashi K, Itoh F, et al. Smad-dependent GADD45beta expression mediates delayed activation of p38 MAP kinase by TGF-beta. Embo J. 2002;21(23):6473–82. doi: 10.1093/emboj/cdf643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Khalil N, Xu YD, O'Connor R, et al. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280(52):43000–9. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 271.Matsuoka H, Arai T, Mori M, et al. A p38 MAPK inhibitor, FR-167653, ameliorates murine bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2002;283(1):L103–12. doi: 10.1152/ajplung.00187.2001. [DOI] [PubMed] [Google Scholar]
- 272.Underwood DC, Osborn RR, Bochnowicz S, et al. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279(5):L895–902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- 273.Nadrous HF, Ryu JH, Douglas WW, et al. Impact of angiotensin-converting enzyme inhibitors and statins on survival in idiopathic pulmonary fibrosis. Chest. 2004;126(2):438–46. doi: 10.1378/chest.126.2.438. [DOI] [PubMed] [Google Scholar]
- 274.Leask A, Holmes A, Black CM, et al. Connective tissue growth factor gene regulation. Requirements for its induction by transforming growth factor-beta 2 in fibroblasts. J Biol Chem. 2003;278(15):13008–15. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- 275.Lasky JA, Ortiz LA, Tonthat B, et al. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998;275(2 Pt 1):L365–71. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 276.Bonniaud P, Margetts PJ, Kolb M, et al. Adenoviral gene transfer of connective tissue growth factor in the lung induces transient fibrosis. Am J Respir Crit Care Med. 2003;168(7):770–8. doi: 10.1164/rccm.200210-1254OC. [DOI] [PubMed] [Google Scholar]
- 277.Pan LH, Yamauchi K, Uzuki M, et al. Type II alveolar epithelial cells and interstitial fibroblasts express connective tissue growth factor in IPF. Eur Respir J. 2001;17(6):1220–7. doi: 10.1183/09031936.01.00074101. [DOI] [PubMed] [Google Scholar]