Abstract
It recently has been shown that epithelial Na+ channels are controlled by a receptor for intracellular Na+, a G protein (Go), and a ubiquitin-protein ligase (Nedd4). Furthermore, mutations in the epithelial Na+ channel that underlie the autosomal dominant form of hypertension known as Liddle’s syndrome inhibit feedback control of Na+ channels by intracellular Na+. Because all epithelia, including those such as secretory epithelia, which do not express Na+ channels, need to maintain a stable cytosolic Na+ concentration ([Na+]i) despite fluctuating rates of transepithelial Na+ transport, these discoveries raise the question of whether other Na+ transporting systems in epithelia also may be regulated by this feedback pathway. Here we show in mouse mandibular secretory (endpiece) cells that the Na+-H+ exchanger, NHE1, which provides a major pathway for Na+ transport in salivary secretory cells, is inhibited by raised [Na+]i acting via a Na+ receptor and Go. This inhibition involves ubiquitination, but does not involve the ubiquitin protein ligase, Nedd4. We conclude that control of membrane transport systems by intracellular Na+ receptors may provide a general mechanism for regulating intracellular Na+ concentration.
Keywords: salivary gland, NHE1, G protein, Nedd4, ubiquitin
Since the pioneering studies of MacRobbie and Ussing (1), epithelial cells have been known to regulate the activity of the ion transporters in their plasma membranes to maintain their cytosolic composition relatively constant despite rapid fluctuations in the rate of transepithelial ion transport. The mechanisms that underlie this so-called homocellular regulation have been the subject of controversy (2–5), but recent experiments examining the mechanisms by which epithelial Na+ channels are controlled in the absorptive cells of salivary ducts have revealed a previously unsuspected mechanism in which cytosolic Na+ is sensed by an intracellular receptor (6). This receptor activates the G protein, Go (7), the α-subunit of which (6) then causes the ubiquitin-protein ligase, Nedd4 (8, 9), to ubiquitinate and inactivate the Na+ channels (10, 11). This receptor for intracellular Na+ is blocked by amiloride and amiloride analogs such as dimethylamiloride and benzimidazole guanidinium (6), thus explaining the previously puzzling ability of these agents to stimulate Na+ channel activity (4). It also has been reported that intracellular Na+ regulates the activity of Na+ channels expressed in the renal MDCK cell line (12) and in frog skin (2) and that feedback regulation by intracellular Na+ of epithelial Na+ channels expressed in Xenopus oocytes is lost when the expressed channels contain mutations known to cause the autosomal dominant form of hypertension, Liddle’s syndrome (13). The mechanisms by which intracellular Na+ acts in these systems are, however, not yet known (12, 14). These findings suggest that feedback control by intracellular Na+ of epithelial Na+ channels may be a phenomenon of general physiological significance in absorptive epithelia, and they raise the question of whether other epithelial Na+ transport systems also might be controlled by a similar mechanism.
One Na+-dependent transporter that could be expected to be subject to feedback regulation by intracellular Na+ is the Na+-H+ exchanger in the secretory (endpiece) cells of salivary glands. The endpieces of salivary glands secrete Na+, Cl−, and HCO3− by a mechanism relying on the transport of Na+ across the basolateral membrane by transporters such as Na+-H+ exchangers and Na+-K+-2Cl− and Na+-HCO3− cotransporters (15–18). The onset of secretion by salivary endpiece cells is accompanied by a dramatic increase in the activity of these transporters (15–17, 19), and at maximum secretory rates the intracellular Na+ content in the secretory cells can be calculated to turn over every 15 sec (18). It is clear that to maintain a relatively stable intracellular composition during secretion requires that these basolateral Na+-dependent transporters be subject to feedback regulation, and, in fact, intracellular Na+ concentration has been observed to oscillate during secretion in a manner suggestive of the presence of such a feedback mechanism (15). Nevertheless, despite the considerable work that has been done on the mechanisms that activate the basolateral transporters at the onset of salivary secretion (19–25), no work has been done on these hypothetical inhibitory feedback systems. In the present paper we investigate whether the Na+-H+ exchanger in the secretory cells of the mouse mandibular gland is subject to feedback regulation by intracellular Na+.
MATERIALS AND METHODS
Cell Preparation.
Male Quackenbush strain mice were killed by cervical dislocation, and the mandibular glands were removed, finely minced, and incubated for 12 min in a physiological salt solution containing 1 mg/ml collagenase (Worthington type IV). The cell suspension then was dispersed by trituration and washed with fresh Na+-rich bath solution containing 145 mM NaCl, 5.5 mM KCl, 1.2 mM MgCl2, 7.5 mM Na-Hepes, 7.5 mM H-Hepes, 1 mM CaCl2, and 10 mM glucose; the pH was adjusted to 7.4 with NaOH. The cells were filtered through a 75-μm nylon mesh and kept on ice until required.
Patch-Clamp Techniques.
We used a technique based on that of Demaurex and coworkers (26) in which the whole-cell patch-clamp technique is used to control cytosolic composition while the pH-sensitive dye, BCECF [2′,7′-bis(carboxyethyl)-5-carboxyfluorescein], is used to measure intracellular pH (pHi). The patch-clamp techniques we used were as described (27), and the cells were loaded with BCECF by including it in the pipette solution. Except for the experiments summarized in Fig. 1C, in which MgSO4 replaced MgATP, pipettes were filled with solutions containing 145 mM K-glutamate and Na-glutamate combined, 5 mM KCl, 5 mM Mes, 10 mM Mg-ATP, 1 mM EGTA, 40 mM sucrose, and 0.2 mM BCECF; the pH was adjusted to 6.0.
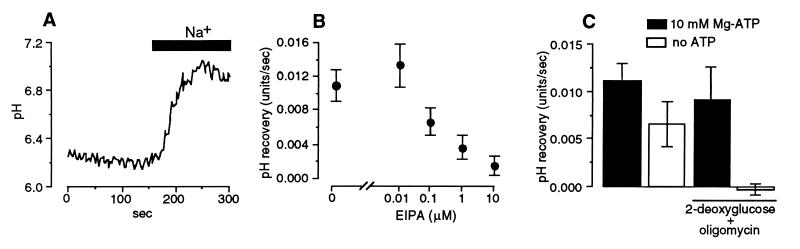
Figure 1.
Features of the Na+-dependent pHi recovery measured with a zero Na+ pipette solution. (A) Representative experiment with 10 mM ATP in the pipette. The bar indicates the period of readmission of 155 mM Na+ solution to the bath. (B) Concentration-response relation for the effect of extracellular ethylisopropylamiloride (EIPA) on the Na+-dependent pHi recovery. (C) The effect of modifying intracellular ATP levels.
Measurement of pHi.
The equipment used to measured pHi was as described (28). The chamber (0.3 ml) was continuously perfused with a Na+-free bath solution containing 145 mM N-methyl-d-glucamine (NMDG)-Cl, 5.5 mM KCl, 15 mM H-Hepes, 1.2 mM MgCl2, 1 mM CaCl2, and 10 mM glucose with a pH of 7.4. Single cells in the whole-cell configuration were voltage-clamped at −30 mV. After 3 min they were illuminated alternately at 490 and 430 nm. Na+-H+ exchange activity was measured by reintroducing Na+ to the bath between 2 and 3 min after the start of illumination. pHi recovery rate was determined by fitting a linear regression to the linear phase of the pHi recovery (i.e., between 20% and 80% of maximum recovery). Calibration of the BCECF signal was by the nigericin high-K+ method (28).
Chemicals.
Sources of chemicals and the methods for activating pertussis toxin and G protein α-subunits were as reported (10, 29). Antibodies directed against the C terminals of the α-subunits of Gi1/Gi2, Gi3 and Gi3/Go were obtained from Calbiochem, and antibodies against the N terminal of the α-subunit of Go were obtained from DuPont-NEN. They were used in the pipette solution at a 1 in 200 (vol/vol) dilution of the solution provided by the manufacturer. Glutathione-S-transferase (GST)-WW (G-W), GST-dominant negative-ubiquitin (K48R), and GST-wild type-ubiquitin fusion proteins were produced as described (10). The anti-Nedd4 antibody (A-Nd4) was purified IgG raised in rabbits against the C-terminal half of the protein (10, 30).
Results are presented as means ± SEM. At least five cells were tested in each experimental group. Statistical significance was assessed by using Student’s unpaired t test. All experiments were performed at 22°C.
RESULTS AND DISCUSSION
We used a technique described by Demaurex and coworkers (26) in which the whole-cell configuration of the patch-clamp technique is used to control cytosolic composition while the pH-sensitive dye, BCECF, is used to measure pHi. The cells were bathed initially in a zero Na+ solution so that they would be unable to oppose the acid load imposed by the pipette solution. The bath solution then was changed to one containing 155 mM Na+ so as to activate the Na+-H+ exchanger and cause pHi to recover toward normal levels (Fig. 1A). We used the rate of this Na+-dependent pHi recovery to estimate Na+-H+ exchange activity. We validated the technique by demonstrating that Na+-dependent pHi recovery has features consistent with its being the result of the NHE1 isoform of Na+-H+ exchanger, which predominates in salivary secretory cells (21, 31, 32). We found that the Na+-dependent pHi recovery was highly sensitive to the amiloride analog, ethylisopropylamiloride (Fig. 1B), and that the recovery depended on the presence of ATP (26), being inactivated when intracellular ATP was depleted by treatment with 2-deoxy-d-glucose (5 mM) and oligomycin (5 μg/ml; Fig. 1C).
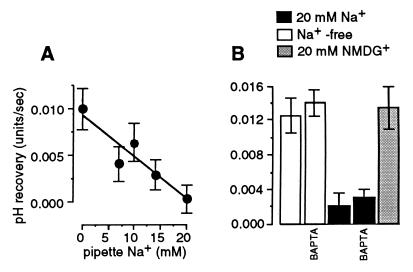
We then demonstrated that the rate of the Na+-dependent pHi recovery declined with increasing pipette Na+ concentration (Fig. 2A) in a manner similar to that described in sheep Purkinje fibers (33). This inhibition evidently was caused by increased [Na+]i, because it could not be reproduced by the large organic cation, NMDG+ (Fig. 2B). Because intracellular free Ca2+ is known to regulate Na+-H+ exchangers (34), we investigated whether a change in free intracellular Ca2+ concentration could mediate this phenomenon. We found that buffering cytosolic and extracellular Ca2+ to nominal zero did not alter the effect of increased [Na+]i (Fig. 2B).
Figure 2.
Inhibition of Na+-dependent pHi recovery by cytosolic Na+. (A) Dependency of the Na+-dependent pHi recovery on pipette Na+. (B) The effects of inclusion of 20 mM NMDG+ in the Na+-free pipette solution, or of buffering intracellular and extracellular Ca2+ to zero by the inclusion of 20 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) in the pipette solution and 1 mM EGTA in the bath solution. No Ca2+ was added to either solution.
We then investigated the mechanism by which [Na+]i controls the activity of the Na+-H+ exchanger. We found that inclusion in the pipette solution of 100 μM GDP-β-S (which competitively inhibits the binding of GTP by G proteins; ref. 35) or of 500 ng/ml activated pertussis toxin (which ADP ribosylates G proteins of the Gi and Go classes so as to prevent their interaction with receptors; ref. 36), reversed the inhibitory effect of 20 mM Na+ (Fig. 3 A and B). The ability of these agents to overcome the inhibitory effect of raised intracellular Na+ completely without altering the electrochemical gradient for Na+ indicates that the inhibition is not caused by a decreased electrochemical driving force for Na+-H+ exchange. Rather, it must be caused by a G protein-mediated feedback pathway. We further found that inclusion in the pipette solution of antibodies directed against the α-subunit of the Go protein, which is known to be expressed in salivary endpiece cells (37), abolished the inhibitory effect of 20 mM Na+ whereas antibodies directed against the α-subunits of Gi1, Gi2, and Gi3 were without effect (Fig. 3C).
Figure 3.
Na+ feedback inhibition is mediated by a G protein. (A) The effect of the addition of 100 μM GDP-β-S to the pipette solution. (B) The effect of the addition of 500 ng/ml activated pertussis toxin to the pipette solution. (C) The effect of the addition to the pipette solution of antibodies directed against various G protein α-subunits [AbGi1,2 = against C terminals of Gαi1 and Gαi2; AbGo, i3 = against C terminals of Gαo and Gαi3; AbGi3 = against C terminal of Gαi3; AbGo = against N terminal of Gαo; all 1 in 200 (vol/vol)].
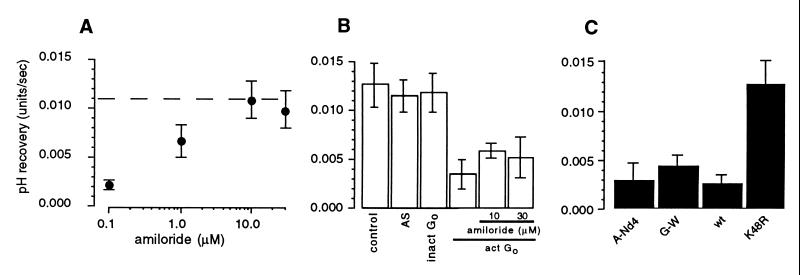
In the absorptive cells of the salivary duct, [Na+]i is sensed by a receptor the effect of which is mediated by Go (6). This receptor is blocked by amiloride and amiloride analogs such as dimethylamiloride and benzimidazolyl guanidinium (6), thus explaining the ability of these agents to stimulate Na+ channel activity (4). In the present study, we found that the inclusion of amiloride in the pipette solution reversed the inhibitory effect of 20 mM Na+ (Fig. 4A). We further found that the inclusion of the activated α-subunit of Go in the zero Na+ pipette solution (Fig. 4B) inhibited the Na+-H+ exchanger and that the inclusion of as much as 30 μM amiloride in the pipette solution was unable to overcome this inhibition (Fig. 4B). Thus, amiloride exerts its inhibitory action upstream of Go, presumably at the putative receptor for intracellular Na+.
Figure 4.
Inhibition of Na+ feedback by intracellular amiloride. (A) Concentration-dependency of the effect of intracellular amiloride when included in 20 mM Na+ solution. (B) The effect of the inclusion of 0.2 μM activated recombinant α-subunit of Go (act Go) and amiloride (10 and 30 μM) in the zero Na+ pipette solution. AS and inact Go denote controls in which activation solution or inactive Gαo, respectively, were added to the pipette solution. (C) The effect of the inclusion of anti-Nedd4 antibody (A-Nd4; 1 μg purified IgG/ml), GST-WW fusion protein (G-W; 0.3 mg/ml), GST-wild type-ubiquitin (wt; 0.3 mg/ml) or GST-dominant negative-ubiquitin (K48R) fusion protein (dn; 0.3 mg/ml) in the 20 mM Na+ pipette solution. In A the broken line indicates the mean rate of pHi recovery observed with zero Na+ pipette solution.
We previously have shown that [Na+]i and the G protein, Go, regulate the activity of the epithelial Na+ channel in the duct cells of the mouse mandibular gland via Nedd4 (10), a ubiquitin-protein ligase that is believed to bind to Na+ channels and regulate their activity by ubiquitinating them (8, 9). In the present study we found that feedback inhibition of the Na+-H+ exchanger was not prevented by inclusion in the pipette solution of an antibody directed against Nedd4 or of a fusion protein composed of GST and the three WW domains of mouse Nedd4 (GST-WW), which acts as a dominant negative mutant of Nedd4 (10) (Fig. 4C). This finding is consistent with the low level of expression of Nedd4 in endpiece cells (10). Feedback inhibition was blocked, however, by inclusion of a dominant negative mutant of ubiquitin (K48R) (10) in the pipette solution (Fig. 4C), indicating that feedback regulation of the exchanger nevertheless is mediated by ubiquitination. Because our preliminary data show that NHE1 expressed in COS cells is ubiquitinated (data not shown), our findings may indicate that feedback regulation of NHE1 is mediated by ubiquitination of the exchanger protein. The control system then would resemble the control of surface expression of epithelial Na+ channels by ubiquitination of the channel protein catalyzed by Nedd4 (9, 10, 38–40). We cannot exclude, however, that the inactivation of NHE1 produced by Na+ feedback is the result of ubiquitination of a protein associated with the exchanger, as recently has been proposed for the control of the growth hormone receptor by ubiquitination (41). Whatever the mechanism, the present findings taken together with the finding that activity of epithelial Na+ channels can be rapidly down-regulated by ubiquitination (10) suggest that ubiquitination may be a general mechanism for the rapid control of membrane transport protein activity.
In this paper we have shown that the Na+-H+ exchanger in salivary endpiece cells is controlled by an intracellular Na+ receptor, the action of which is mediated by a G protein and ubiquitination. This mechanism has not been previously suspected of controlling Na+-H+ exchange, but it is similar to that reported to control epithelial Na+ channels in mouse salivary duct cells (6, 7, 10, 11) (Fig. 5). Under resting conditions, the Na+-H+ exchanger in salivary secretory cells is virtually inactive, but after stimulation its activity increases dramatically (21). This increased activity is apparently the result of the decrease in intracellular Cl− concentration that accompanies activation of the apical membrane anion channels (20) and is not the result of phosphorylation of the exchanger protein (21). The present findings indicate that raised intracellular Na+ should modulate the stimulation in Na+-H+ exchange produced by decreased intracellular Cl− so as to ensure that the cell is not overloaded with Na+.
Figure 5.
The mechanisms of feedback inhibition by intracellular Na+ of epithelial Na+ channels in salivary duct (absorptive) cells (A) and Na+-H+ exchange in salivary endpiece (secretory) cells (B). In each cell model, the apical membrane is on the left and the sodium pump (Na+-K+ ATPase) is shown in the basolateral membrane on the right.
From the present findings, the NHE1 isoform of Na+-H+ exchanger appears to be one of a growing number of membrane proteins that are subject to rapid regulation by ubiquitination. These proteins include growth hormone receptors and the epithelial Na+ channel in mammalian cells as well as uracil permease in yeast (reviewed in ref. 42). Ubiquitination is believed to lead to endocytosis and destruction of the ubiquitinated membrane protein (42), but from the present data it is not clear whether Na+-feedback control of the Na+-H+ exchanger involves proteolysis of the exchanger protein. Epithelial Na+ channels have a half-life of as little as 1 hr (9), consistent with their being controlled by a destructive regulatory system. Clearly for a control system of this type to be viable requires that there be a large intracellular store of the protein from which the plasma membrane can be replenished. Stores of this type have been described for many membrane proteins including the NHE3 isoform of Na+-H+ exchanger and the Na+,K+-ATPase (43, 44).
Growth hormone receptors, however, although regulated by ubiquitination, are not themselves necessarily ubiquitinated (41, 42) and hence may be available to be recycled to the plasma membrane in a manner similar to other endocytosed receptors (45). As mentioned previously, this report has led to the suggestion that an unknown associated regulatory protein is the target for ubiquitination (41, 42). It also raises the possibility that this regulatory protein, once ubiquitinated, may trigger the endocytosis of many growth hormone receptors, thus minimizing the apparent wastefulness of a regulatory system based on proteolysis.
In nonepithelial cells, the Na+-H+ exchanger has been reported to be inhibited by increased [Na+]i (33, 46, 47), but this finding had been explained as the consequence of a decreased electrochemical gradient for Na+ (33) or the presence of a binding site for intracellular Na+ on the exchanger itself (47). The present findings suggest that these explanations require reassessment. More importantly, we show that G protein-mediated receptors for intracellular Na+ not only regulate Na+ channels in an absorptive epithelium, but also regulate Na+-H+ exchangers in a secretory epithelium (Fig. 5). These findings, together with the recent finding that N-methyl-d-aspartate receptors in hippocampal neurons are regulated by [Na+]i (48), suggest that intracellular Na+ receptors form part of a general mechanism for regulating ion transport proteins.
Acknowledgments
This project was supported by the National Health and Medical Research Council of Australia and the National Heart Foundation. H.I. was supported by an Overseas Research Fellowship from Nikon University, Tokyo. K.F.H. is supported by a Dawes Scholarship from the Royal Adelaide Hospital. S.K. is a Wellcome Trust Senior Fellow in Medical Science. D.I.C. is a Fellow of The Medical Foundation of the University of Sydney.
ABBREVIATIONS
- pHi,
intracellular pH
- GST
glutathione-S-transferase
- BCECF
2′,7′-bis(carboxyethyl)-5-carboxyfluorescein
- NMDG
N-methyl-d-glucamine
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.MacRobbie E A C, Ussing H H. Acta Physiol Scand. 1961;53:348–365. doi: 10.1111/j.1748-1716.1961.tb02293.x. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert A W, Shum W K. Nature (London) 1977;266:468–469. doi: 10.1038/266468a0. [DOI] [PubMed] [Google Scholar]
- 3.Turnheim K. Physiol Rev. 1991;71:429–445. doi: 10.1152/physrev.1991.71.2.429. [DOI] [PubMed] [Google Scholar]
- 4.Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- 5.Palmer L G, Sackin H, Frindt G. J Physiol (London) 1998;509:151–162. doi: 10.1111/j.1469-7793.1998.151bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Komwatana P, Dinudom A, Young J A, Cook D I. J Membr Biol. 1998;162:225–232. doi: 10.1007/s002329900360. [DOI] [PubMed] [Google Scholar]
- 7.Komwatana P, Dinudom A, Young J A, Cook D I. Proc Natl Acad Sci USA. 1996;93:8107–8111. doi: 10.1073/pnas.93.15.8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staub O, Dho S, Henry P C, Correa J, Ishikawa T, McGlade J, Rotin D. EMBO J. 1996;15:2371–2380. [PMC free article] [PubMed] [Google Scholar]
- 9.Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciecanover A, Schild L, Rotin D. EMBO J. 1997;16:6325–6336. doi: 10.1093/emboj/16.21.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinudom A, Harvey K F, Komwatana P, Young J A, Kumar S, Cook D I. Proc Natl Acad Sci USA. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey K F, Dinudom A, Komwatana P, Joliffe C N, Day M L, Parasivam G, Cook D I, Kumar S. J Biol Chem. 1999;274:12525–12530. doi: 10.1074/jbc.274.18.12525. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa T, Marunaka Y, Rotin D. J Gen Physiol. 1998;111:825–846. doi: 10.1085/jgp.111.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kellenberger S, Gautshci I, Rossier B C, Schild L. J Clin Invest. 1998;101:2741–2750. doi: 10.1172/JCI2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abriel H, Horisberger J D. J Physiol (London) 1999;516:31–43. doi: 10.1111/j.1469-7793.1999.031aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong M M, Foskett J K. Science. 1991;254:1014–1016. doi: 10.1126/science.1948071. [DOI] [PubMed] [Google Scholar]
- 16.Dissing S, Nauntofte B. Am J Physiol. 1994;259:G1044–G1055. doi: 10.1152/ajpgi.1990.259.6.G1044. [DOI] [PubMed] [Google Scholar]
- 17.Poronnik P, Schumann S Y, Cook D I. Pflügers Arch. 1995;429:852–858. doi: 10.1007/BF00374810. [DOI] [PubMed] [Google Scholar]
- 18.Cook D I, Van Lennep E W, Roberts M L, Young J A. In: Physiology of the Gastrointestinal Tract. Johnson L, Christensen J, Jackson M, Jacobson E, Walsh J, editors. Vol. 2. New York: Raven; 1994. 3rd Ed.10611117. [Google Scholar]
- 19.Evans R L, Turner R J. J Physiol (London) 1997;499:351–359. doi: 10.1113/jphysiol.1997.sp021932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robertson M A, Foskett J K. Am J Physiol. 1994;267:C146–C156. doi: 10.1152/ajpcell.1994.267.1.C146. [DOI] [PubMed] [Google Scholar]
- 21.Robertson M A, Woodside M, Foskett J K, Orlowski J, Grinstein S. J Biol Chem. 1997;272:287–294. [PubMed] [Google Scholar]
- 22.Manganel M, Turner R J. J Biol Chem. 1990;265:4284–4289. [PubMed] [Google Scholar]
- 23.Manganel M, Turner R J. J Biol Chem. 1991;266:10182–10188. [PubMed] [Google Scholar]
- 24.Paulais M, Turner R J. J Biol Chem. 1992;267:21558–21563. [PubMed] [Google Scholar]
- 25.Tanimura A, Kurihara K, Reshkin S J, Turner R J. J Biol Chem. 1995;270:25252–25258. doi: 10.1074/jbc.270.42.25252. [DOI] [PubMed] [Google Scholar]
- 26.Demaurex N, Romanek R R, Orlowski J, Grinstein S. J Gen Physiol. 1997;109:117–128. doi: 10.1085/jgp.109.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dinudom A, Young J A, Cook D I. J Membr Biol. 1993;135:289–295. doi: 10.1007/BF00211100. [DOI] [PubMed] [Google Scholar]
- 28.Steward M C, Poronnik P, Cook D I. J Physiol (London) 1996;494:819–830. doi: 10.1113/jphysiol.1996.sp021535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dinudom A, Komwatana P, Young J A, Cook D I. J Physiol (London) 1995;487:549–555. doi: 10.1113/jphysiol.1995.sp020899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar S, Harvey K F, Kinoshita M, Copland N G, Noda M, Jenkins N A. Genomics. 1997;40:435–443. doi: 10.1006/geno.1996.4582. [DOI] [PubMed] [Google Scholar]
- 31.Lee M G, Schultheiss P J, Yan M, Shull G E, Bookstein G, Chang E, Tse M, Donowitz M, Park K, Muallem S. J Physiol (London) 1998;513:341–357. doi: 10.1111/j.1469-7793.1998.341bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park K, Olschowska J A, Richardson L, Bookstein C, Chang E B, Melvin J E. Am J Physiol. 1999;276:G470–G478. doi: 10.1152/ajpgi.1999.276.2.G470. [DOI] [PubMed] [Google Scholar]
- 33.Wu M L, Vaughan-Jones R D. J Mol Cell Cardiol. 1997;29:1131–1140. doi: 10.1006/jmcc.1996.0338. [DOI] [PubMed] [Google Scholar]
- 34.Orlowski J, Grinstein S. J Biol Chem. 1997;272:22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 35.Eckstein F, Cassel D, Levkovitz H, Lowe M, Selinger Z. J Biol Chem. 1979;254:9829–9834. [PubMed] [Google Scholar]
- 36.Katada T, Ui M. J Biol Chem. 1982;257:7210–7216. [PubMed] [Google Scholar]
- 37.Watson E L, Olver C, D’Silva N, Belton C M. J Histochem Cytochem. 1994;42:41–47. doi: 10.1177/42.1.7505300. [DOI] [PubMed] [Google Scholar]
- 38.Abriel H, Loffing J, Rebhun J F, Pratt J H, Schild L, Horisberger J D, Rotin D, Staub O. J Clin Invest. 1999;103:667–673. doi: 10.1172/JCI5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimkets R A, Lifton R P, Canessa C M. J Biol Chem. 1997;272:25537–25541. doi: 10.1074/jbc.272.41.25537. [DOI] [PubMed] [Google Scholar]
- 40.Goulet C C, Volk K A, Adams C M, Prince L S, Stokes J B, Snyder P M. J Biol Chem. 1998;273:30012–30017. doi: 10.1074/jbc.273.45.30012. [DOI] [PubMed] [Google Scholar]
- 41.Govers R, ten Broeke T, van Kerkhof P, Schwartz A L, Strous G J. EMBO J. 1999;18:28–36. doi: 10.1093/emboj/18.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hicke L. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/s0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 43.Kurashima K, Szabo E Z, Lukacs G, Orlowski J, Grinstein S. J Biol Chem. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- 44.Lambert R W, Maves C A, Gierow J P, Wood R L, Mircheff A K. Invest Ophthalmol Visual Sci. 1993;34:305–316. [PubMed] [Google Scholar]
- 45.Grady E F, Bohm S K, Bunnett N W. Am J Physiol. 1997;273:G586–G601. doi: 10.1152/ajpgi.1997.273.3.G586. [DOI] [PubMed] [Google Scholar]
- 46.Green J, Yamaguchi D T, Kleeman C R, Muallem S. J Gen Physiol. 1988;92:239–261. doi: 10.1085/jgp.92.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grinstein S, Cohen S, Rothstein A. J Gen Physiol. 1984;83:341–369. doi: 10.1085/jgp.83.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu X M, Salter M W. Nature (London) 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]