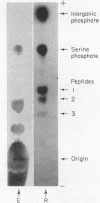
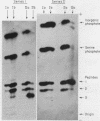
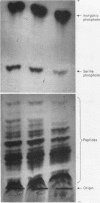
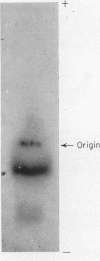
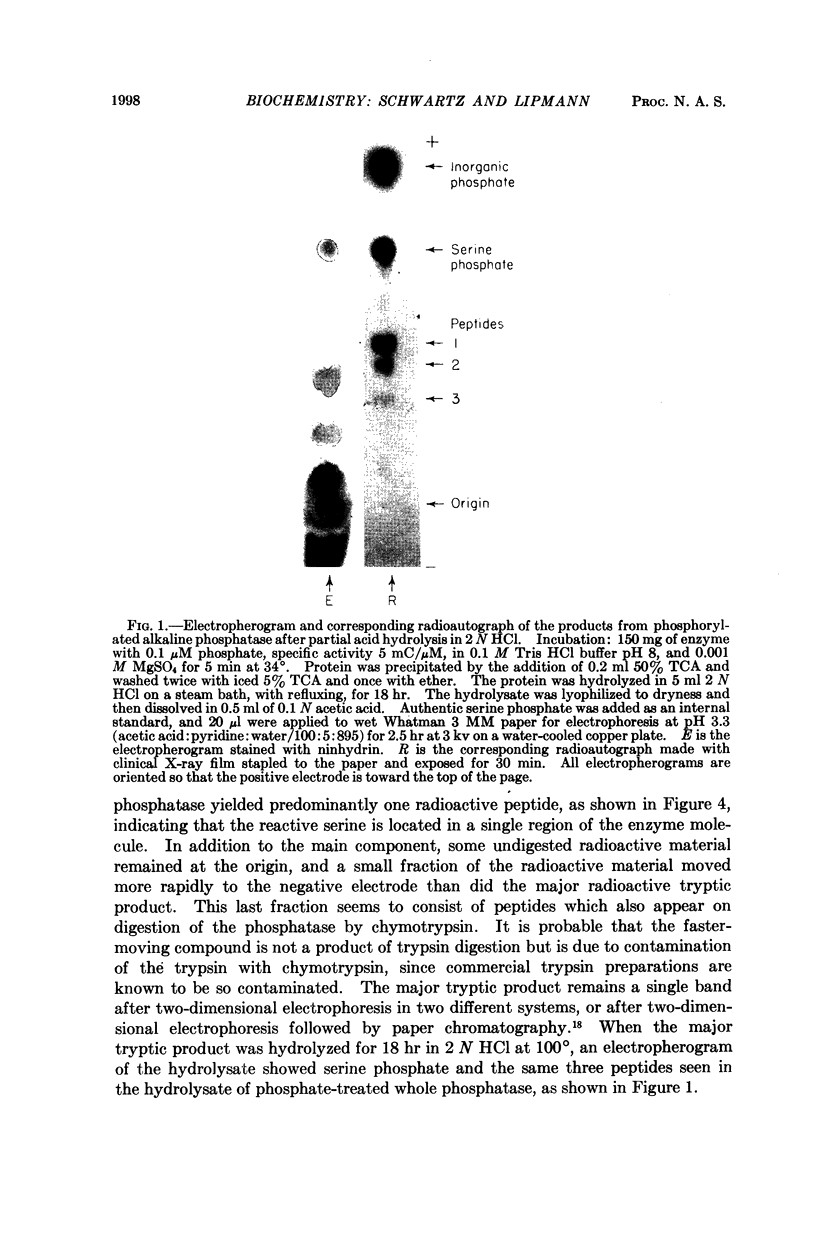
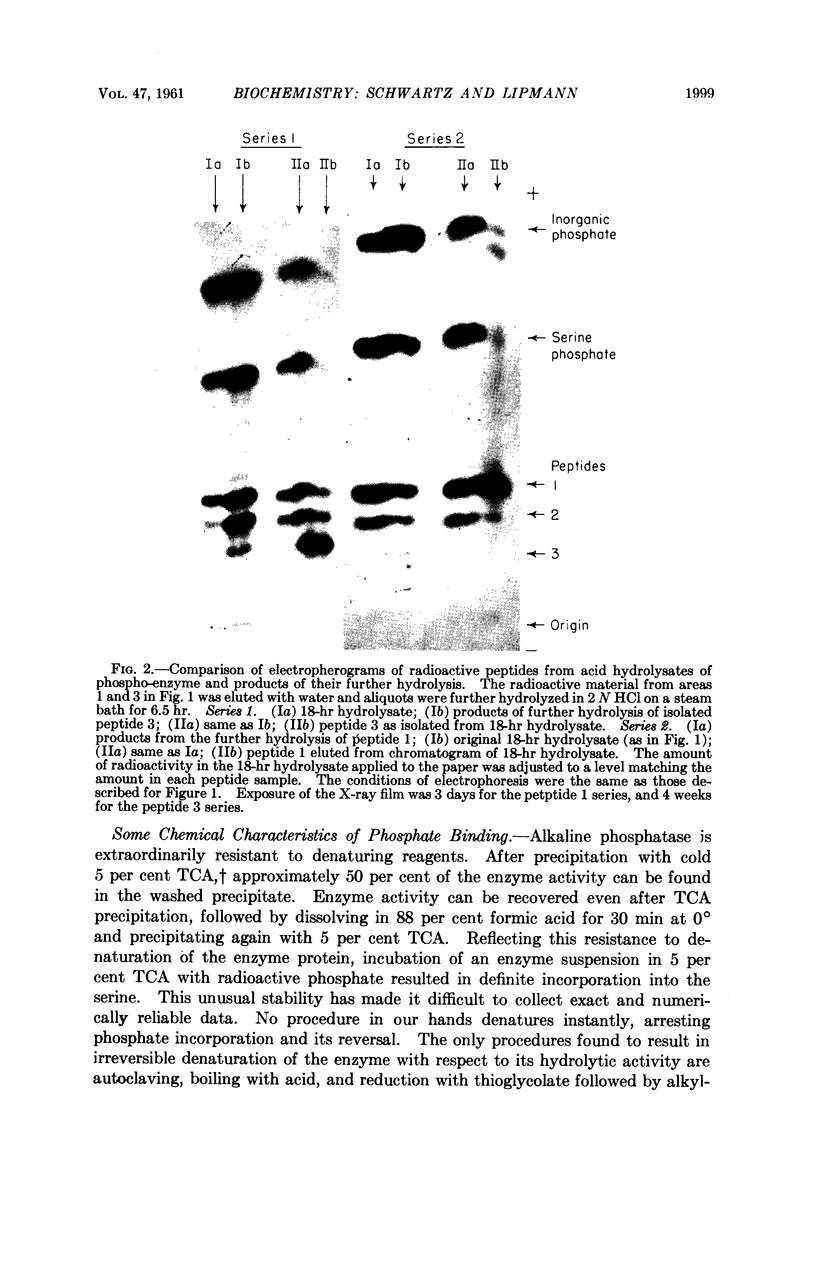
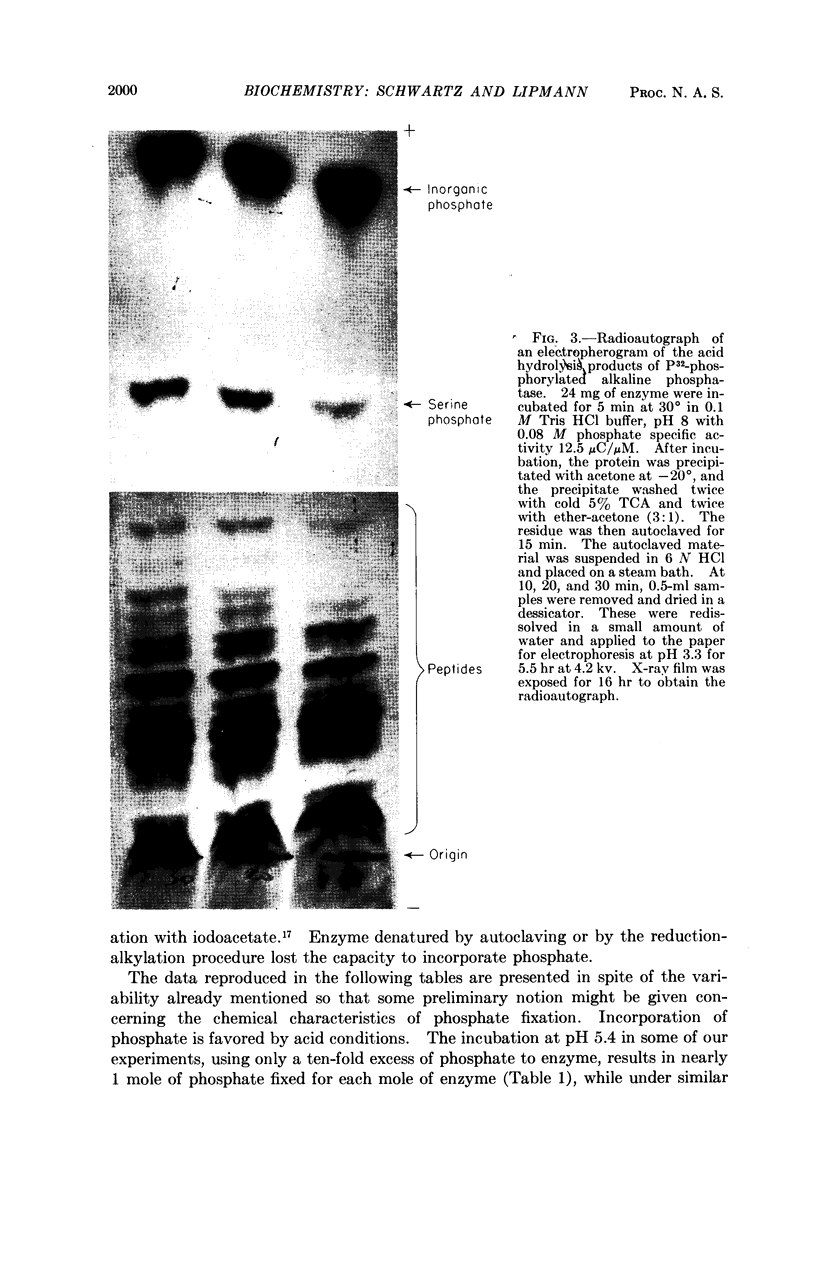
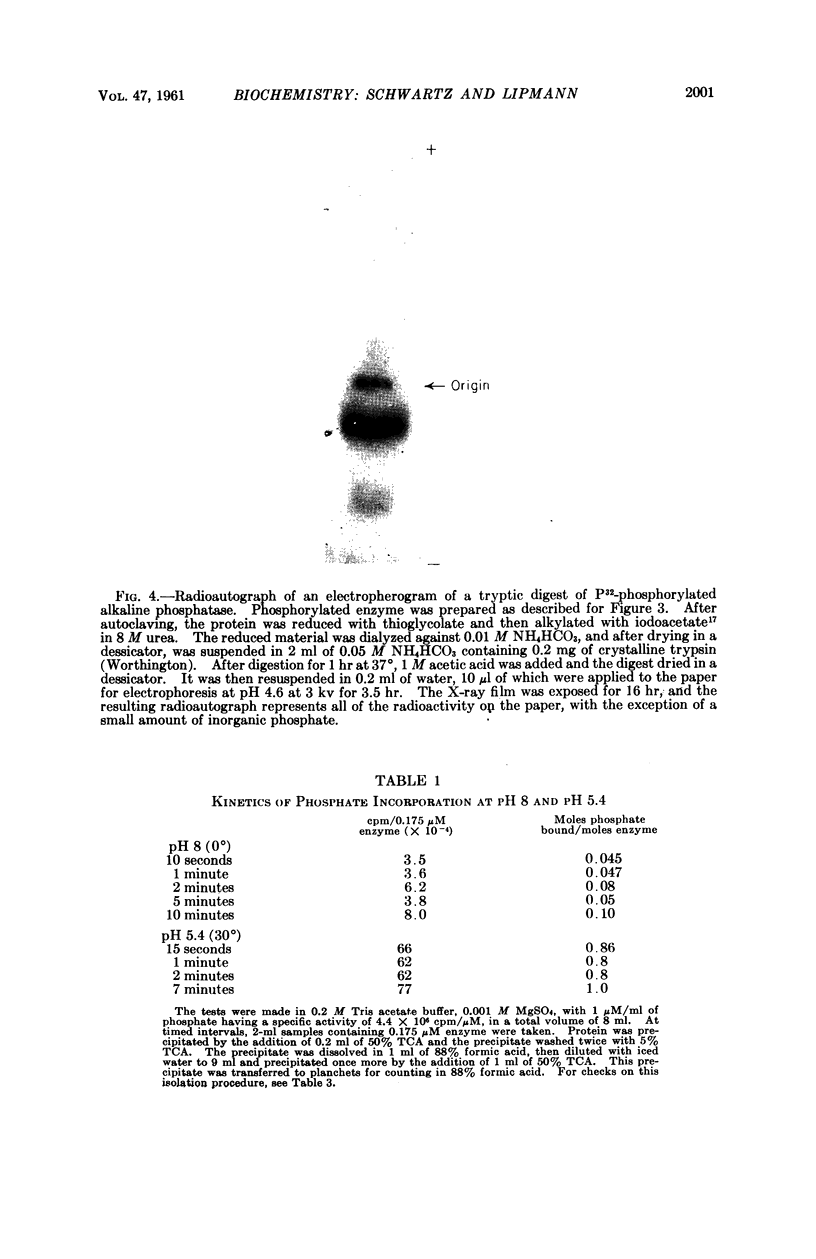
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN J. A., OOSTERBAAN R. A., JANSZ H. S., BERENDS F. The active site of esterases. J Cell Comp Physiol. 1959 Dec;54:231–244. doi: 10.1002/jcp.1030540419. [DOI] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- JONES M. E., SPECTOR L. The pathway of carbonate in the biosynthesis of carbamyl phosphate. J Biol Chem. 1960 Oct;235:2897–2901. [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr The active site and enzyme action. Adv Enzymol Relat Subj Biochem. 1960;22:45–97. doi: 10.1002/9780470122679.ch2. [DOI] [PubMed] [Google Scholar]
- LIPMANN F., TUTTLE L. C. Lipase-catalysed condensation of fatty acids with hydroxylamine. Biochim Biophys Acta. 1950 Jan;4(1-3):301–309. doi: 10.1016/0006-3002(50)90036-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILSTEIN C., SANGER F. An amino acid sequence in the active centre of phosphoglucomutase. Biochem J. 1961 Jun;79:456–469. doi: 10.1042/bj0790456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATHANS D., LIPMANN F. Amino acid transfer from aminoacyl-ribonucleic acids to protein on ribosomes of Escherichia coli. Proc Natl Acad Sci U S A. 1961 Apr 15;47:497–504. doi: 10.1073/pnas.47.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER G. R., RYDON H. N., SCHOFIELD J. A. Nature of the reactive serine residue in enzymes inhibited by organo-phosphorus compounds. Nature. 1958 Oct 4;182(4640):927–927. doi: 10.1038/182927a0. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- WILLIAMS J., SANGER F. The grouping of serine phosphate residues in phosvitin and casein. Biochim Biophys Acta. 1959 May;33(1):294–296. doi: 10.1016/0006-3002(59)90545-1. [DOI] [PubMed] [Google Scholar]