Abstract
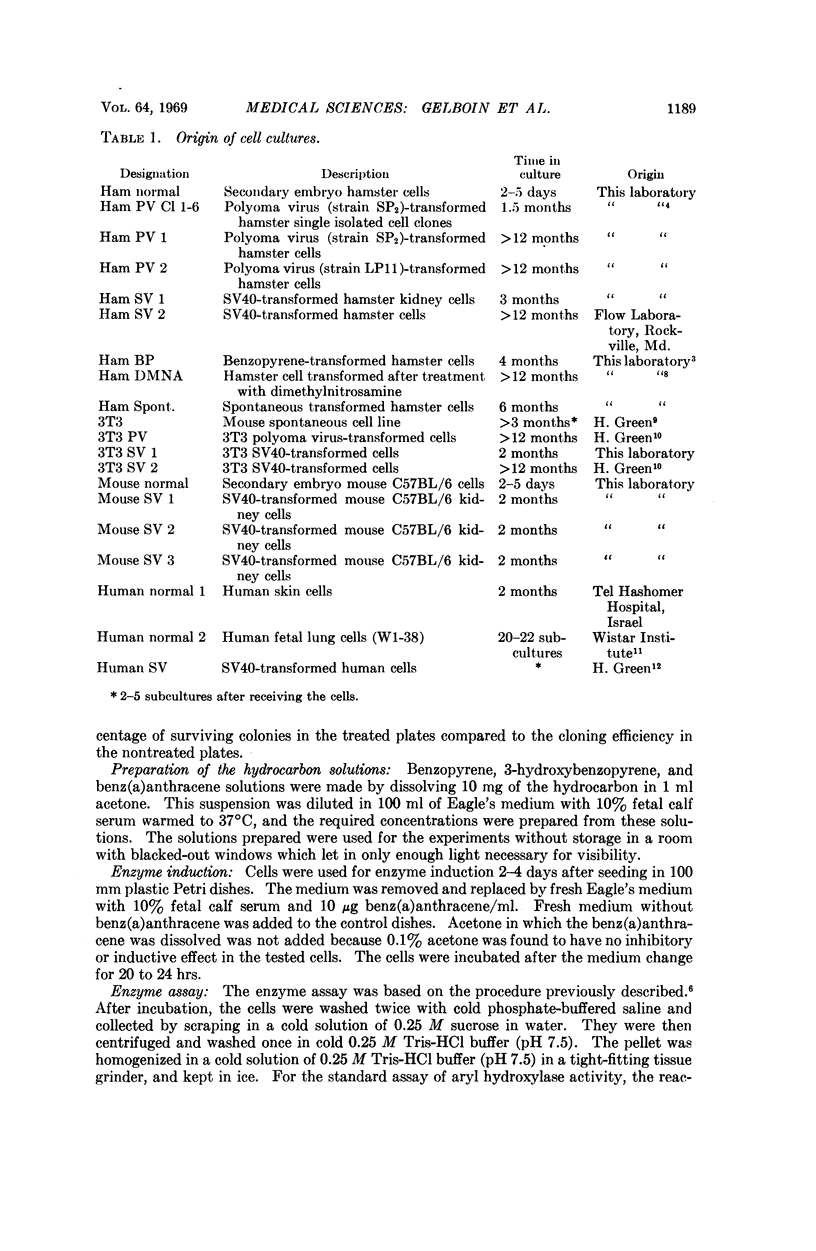
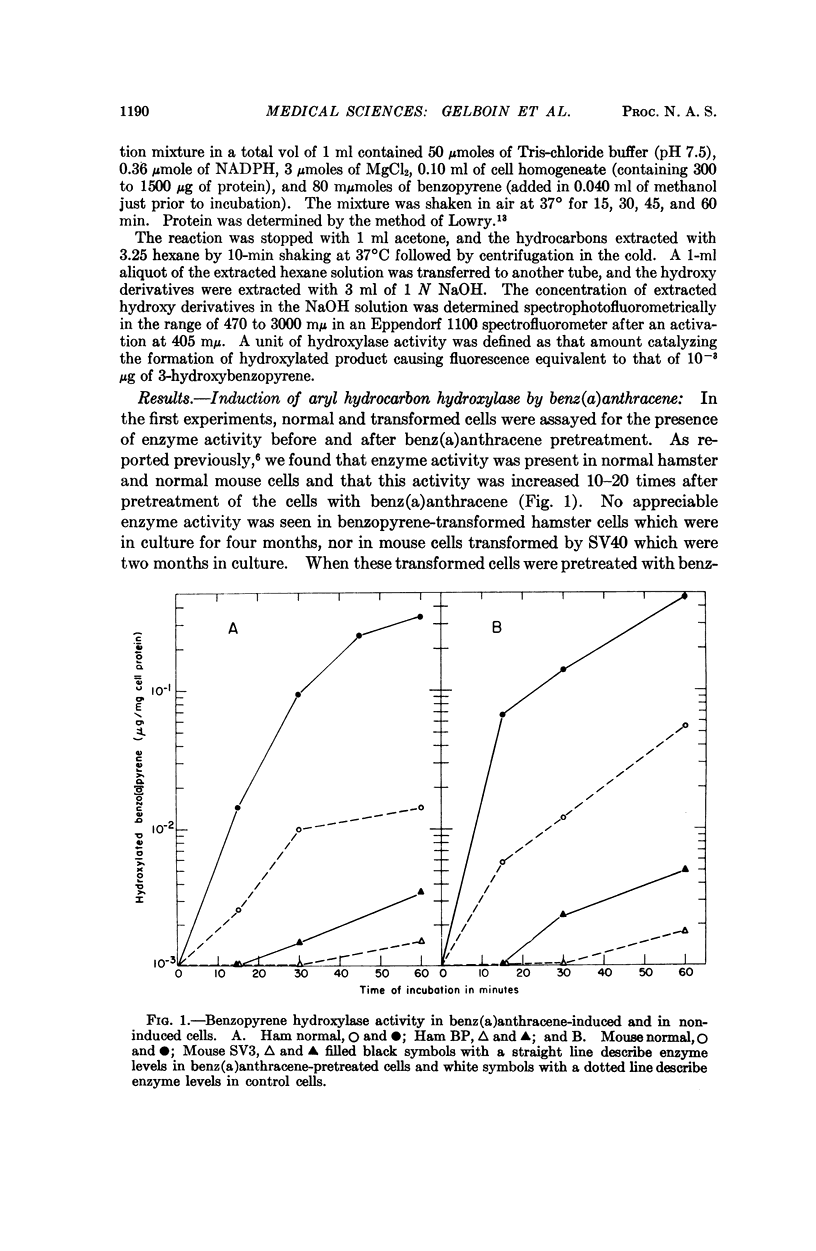
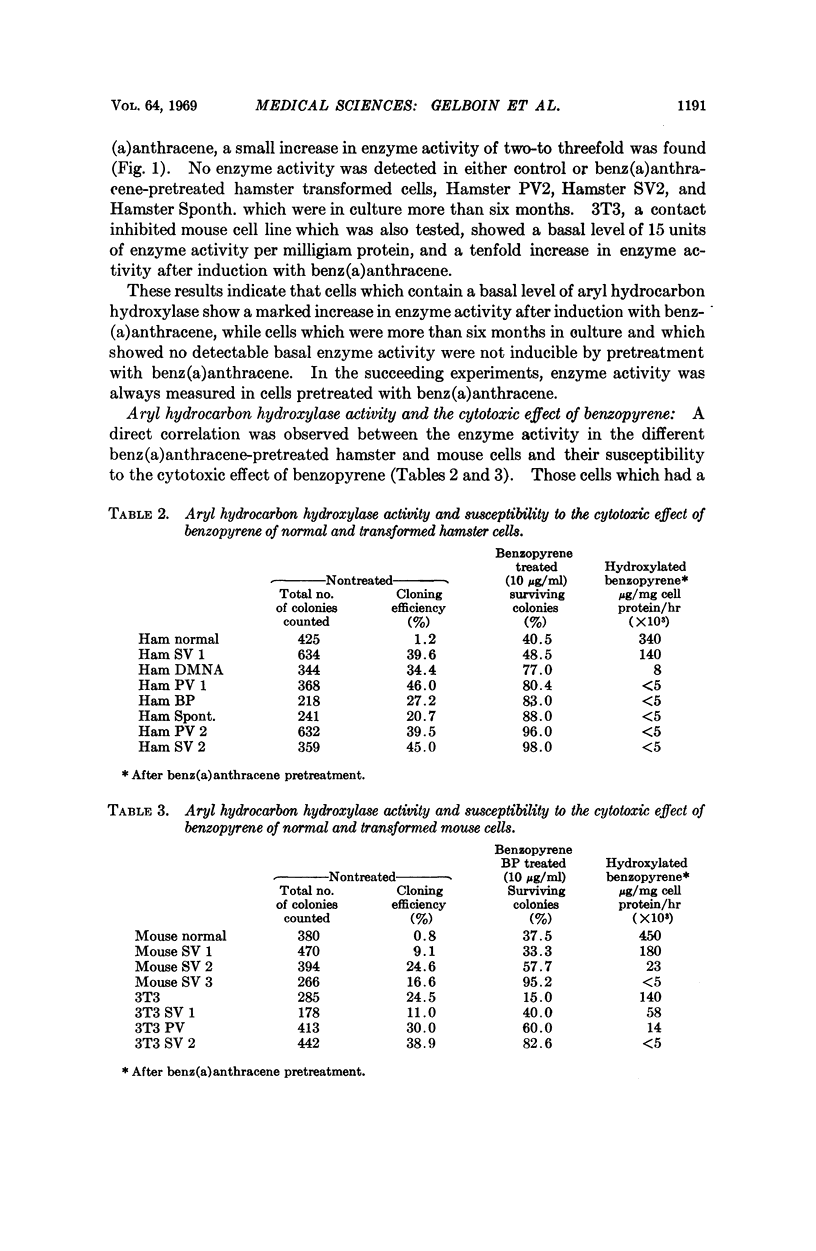
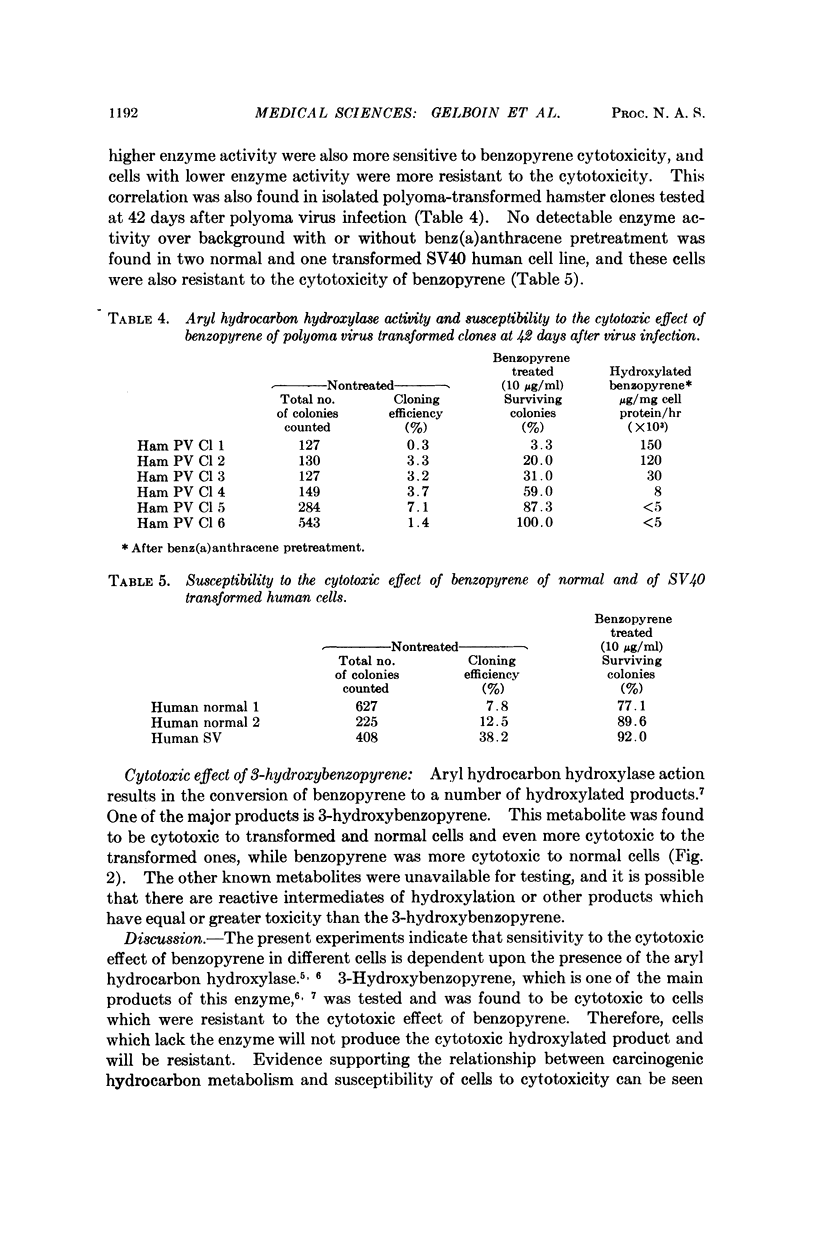
Aryl hydrocarbon hydroxylase, an inducible microsomal enzyme system, has been measured in cultures of normal and transformed hamster, mouse, and human cells. In order to determine the highest level of enzyme, the cells were induced by pretreatment with benz(a)anthracene. A correlation was found between the level of enzyme activity and the susceptibility of the cells to the cytotoxicity produced after treatment with benzopyrene. The results indicate that aryl hydrocarbon hydroxylase is the enzyme system responsible for cell susceptibility to the cytotoxic effect of benzopyrene and the toxic effect of benzopyrene is due to its enzymatic conversion to a cytotoxic metabolite. 3-Hydroxybenzopyrene, one of the products of the enzymatic hydroxylation of benzopyrene, was found to be cytotoxic to cells that were either susceptible or resistant to the cytotoxic effect of benzopyrene.
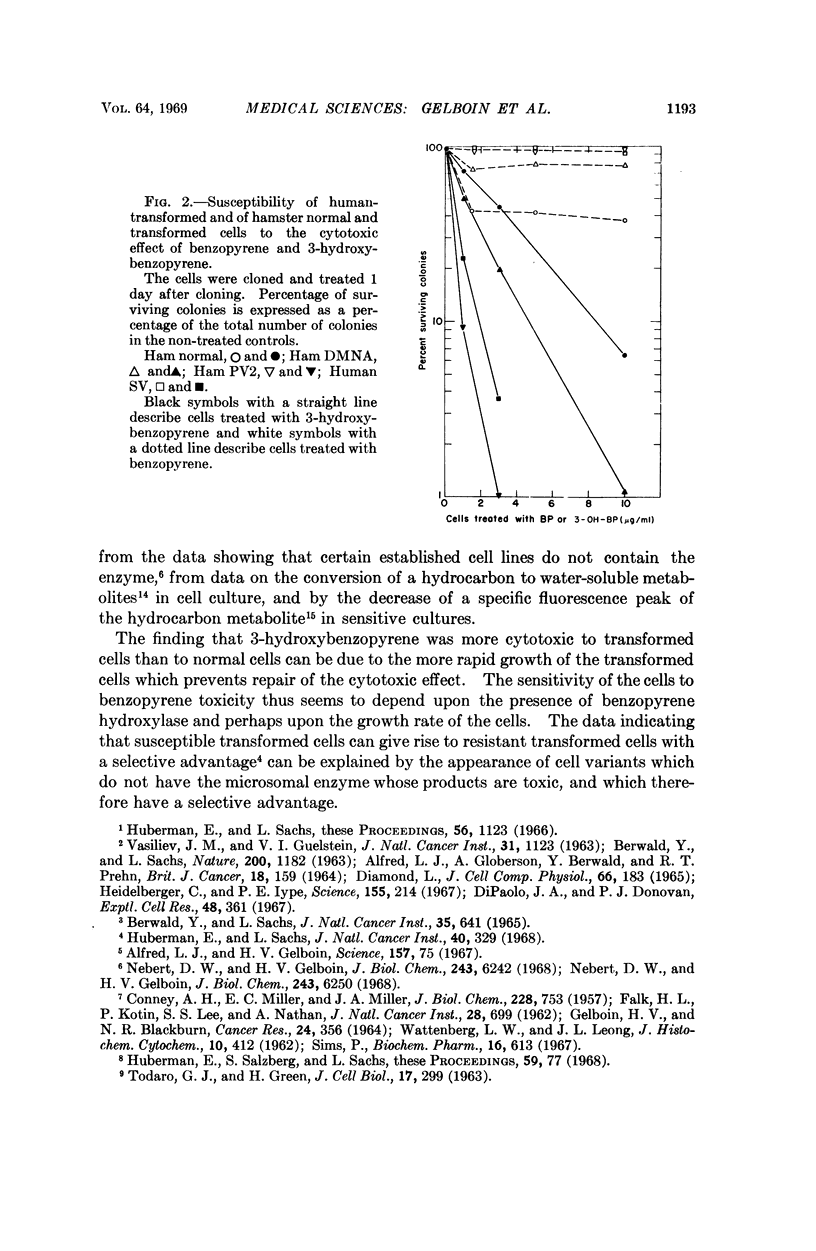
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALFRED L. J., GLOBERSON A., BERWALD Y., PREHN R. T. DIFFERENTIAL TOXICITY RESPONSE OF NORMAL AND NEOPLASTIC CELLS IN VITRO TO 3,4-BENZOPYRENE AND 3-METHYLCHOLANTHRENE. Br J Cancer. 1964 Mar;18:159–164. doi: 10.1038/bjc.1964.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfred L. J., Gelboin H. V. Benzpyrene hydroxylase induction by polycyclic hydrocarbons in hamster embryonic cells grown in vitro. Science. 1967 Jul 7;157(3784):75–76. doi: 10.1126/science.157.3784.75. [DOI] [PubMed] [Google Scholar]
- Andrianov L. N., Belitsky G. A., Ivanova O. J., Khesina A. Y., Khitrovo S. S., Shabad L. M., Vasiliev J. M. Metabolic degradation of 3,4-benzopyrene in the cultures of normal and neoplastic fibroblasts. Br J Cancer. 1967 Sep;21(3):566–575. doi: 10.1038/bjc.1967.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwald Y., Sachs L. In vitro transformation of normal cells to tumor cells by carcinogenic hydrocarbons. J Natl Cancer Inst. 1965 Oct;35(4):641–661. [PubMed] [Google Scholar]
- CONNEY A. H., MILLER E. C., MILLER J. A. Substrate-induced synthesis and other properties of benzpyrene hydroxylase in rat liver. J Biol Chem. 1957 Oct;228(2):753–766. [PubMed] [Google Scholar]
- Diamond L., Sardet C., Rothblat G. H. The metabolism of 7,12-dimethylbenz(a)anthracene in cell cultures. Int J Cancer. 1968 Nov 15;3(6):838–849. doi: 10.1002/ijc.2910030617. [DOI] [PubMed] [Google Scholar]
- Dipaolo J. A., Donovan P. J. Properties of Syrian hamster cells transformed in the presence of carcinogenic hydrocarbons. Exp Cell Res. 1967 Nov;48(2):361–377. doi: 10.1016/0014-4827(67)90361-8. [DOI] [PubMed] [Google Scholar]
- Huberman E., Sachs L. Cell susceptibility to transformation and cytotoxicity by the carcinogenic hydrocarbon benzo[a]pyrene. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1123–1129. doi: 10.1073/pnas.56.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Sachs L. Susceptibility of cells transformed by polyoma virus and simian virus 40 to the cytotoxic effect of the carcinogenic hydrocarbon benzo[a]pyrene. J Natl Cancer Inst. 1968 Feb;40(2):329–336. [PubMed] [Google Scholar]
- Huberman E., Salzberg S., Sachs L. The in vitro induction of an increase in cell multiplication and cellular life span by the water-soluble carcinogen dimethylnitrosamine. Proc Natl Acad Sci U S A. 1968 Jan;59(1):77–82. doi: 10.1073/pnas.59.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. II. Cellular responses during enzyme induction. J Biol Chem. 1968 Dec 10;243(23):6250–6261. [PubMed] [Google Scholar]
- TODARO G. J., GREEN H., GOLDBERG B. D. TRANSFORMATION OF PROPERTIES OF AN ESTABLISHED CELL LINE BY SV40 AND POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jan;51:66–73. doi: 10.1073/pnas.51.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Green H., Swift M. R. Susceptibility of human diploid fibroblast strains to transformation by SV40 virus. Science. 1966 Sep 9;153(3741):1252–1254. doi: 10.1126/science.153.3741.1252. [DOI] [PubMed] [Google Scholar]
- VASILIEV J. M., GUELSTEIN V. I. SENSITIVITY OF NORMAL AND NEOPLASTIC CELLS TO THE DAMAGING ACTION OF CARCINOGENIC SUBSTANCES: A REVIEW. J Natl Cancer Inst. 1963 Nov;31:1123–1151. [PubMed] [Google Scholar]


