Abstract
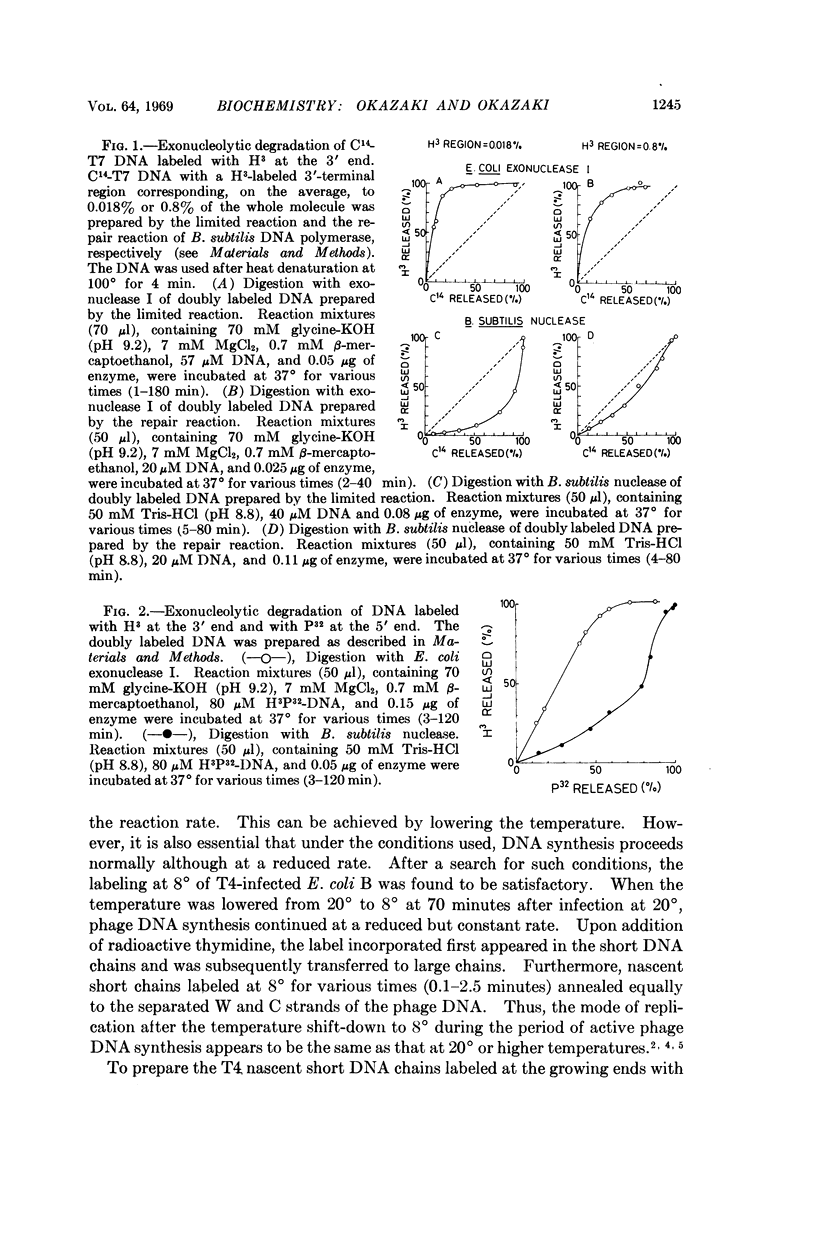
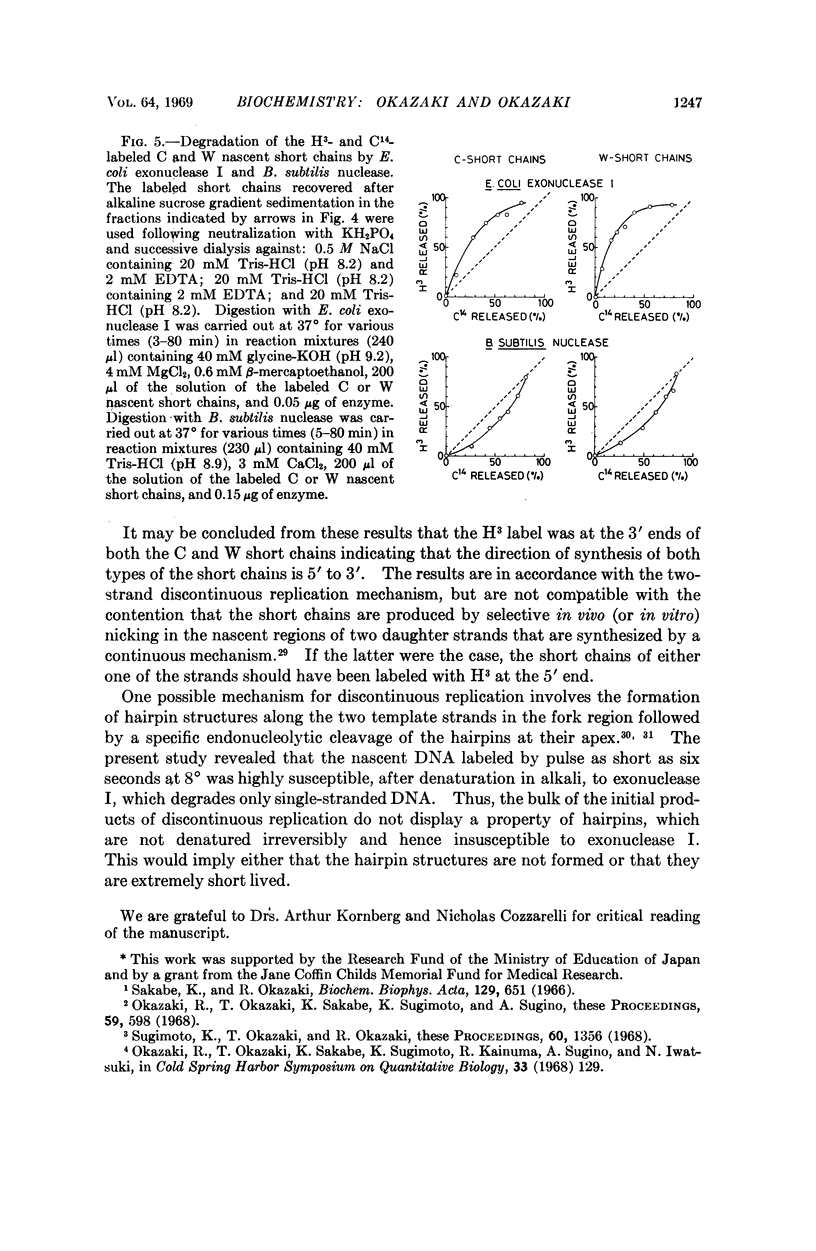
T4 nascent short chains labeled at their growing ends with H3-thymidine and uniformly with C14-thymidine were prepared, separated into complementary strands, and degraded by E. coli exonuclease I in the 3′ to 5′ direction or by B. subtilis nuclease in the 5′ to 3′ direction. The kinetics of release of H3 and C14 labels by both enzymes was consistent with the conclusion that the H3 label is at the 3′ end of the nascent short chains of both strands and that the short chains are products of discontinuous synthesis in the 5′ to 3′ direction along the two template strands.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J., Lehman I. R., Bessman M. J., Simms E. S., Kornberg A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. IV. LINKAGE OF SINGLE DEOXYNUCLEOTIDES TO THE DEOXYNUCLEOSIDE ENDS OF DEOXYRIBONUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 Jul 15;44(7):641–647. doi: 10.1073/pnas.44.7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoda J., Mathews E. DNA replication in vivo by a temperature-sensitive polynucleotide ligase mutant of T4. Proc Natl Acad Sci U S A. 1968 Nov;61(3):997–1004. doi: 10.1073/pnas.61.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., NUSSBAUM A. L. THE DEOXYRIBONUCLEASES OF ESCHERICHIA COLI. V. ON THE SPECIFICITY OF EXONUCLEASE I (PHOSPHODIESTERASE). J Biol Chem. 1964 Aug;239:2628–2636. [PubMed] [Google Scholar]
- Newman J., Hanawalt P. Intermediates in T4 DNA replication in a T4 ligase deficient strain. Cold Spring Harb Symp Quant Biol. 1968;33:145–150. doi: 10.1101/sqb.1968.033.01.018. [DOI] [PubMed] [Google Scholar]
- Newman J., Hanawalt P. Role of polynucleotide ligase in T4 DNA replication. J Mol Biol. 1968 Aug 14;35(3):639–642. doi: 10.1016/s0022-2836(68)80020-8. [DOI] [PubMed] [Google Scholar]
- OHSAKA A., MUKAI J. I., LASKOWSKI M., Sr THE USE OF PURIFIED MICROCOCCAL NUCLEASE IN IDENTIFYING THE NUCLEOTIDE TERMINUS BEARING A FREE 5'-MONOPHOSPHATE. J Biol Chem. 1964 Oct;239:3498–3504. [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- OKAZAKI T., KORNBERG A. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. XV. PURIFICATION AND PROPERTIES OF A POLYMERASE FROM BACILLUS SUBTILIS. J Biol Chem. 1964 Jan;239:259–268. [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo, II. Evidence for the second intermediate. Proc Natl Acad Sci U S A. 1968 Jun;60(2):691–698. doi: 10.1073/pnas.60.2.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K. An extracellular nuclease of Bacillus subtilis: some novel properties as a DNA exonuclease. Biochem Biophys Res Commun. 1966 Mar 22;22(6):611–619. doi: 10.1016/0006-291x(66)90190-2. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON C. C., INMAN R. B., KORNBERG A. ENZYMIC SYNTHESIS OF DEOXYRIBONUCLEIC ACID. 18. THE REPAIR OF PARTIALLY SINGLE-STRANDED DNA TEMPLATES BY DNA POLYMERASE. J Mol Biol. 1964 Jul;9:46–69. doi: 10.1016/s0022-2836(64)80090-5. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. I. PURIFICATION OF THE ENZYME AND CHARACTERIZATION OF THE PHOSPHATASE ACTIVITY. J Biol Chem. 1964 Jan;239:242–250. [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski P., Ginsberg B., Yudelevich A., Feiner L., Hurwitz J. Enzymatic mechanisms of the repair and breakage of DNA. Cold Spring Harb Symp Quant Biol. 1968;33:165–177. doi: 10.1101/sqb.1968.033.01.020. [DOI] [PubMed] [Google Scholar]
- Sakabe K., Okazaki R. A unique property of the replicating region of chromosomal DNA. Biochim Biophys Acta. 1966 Dec 21;129(3):651–654. doi: 10.1016/0005-2787(66)90088-8. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Okazaki T., Imae Y., Okazaki R. Mechanism of DNA chain growth. 3. Equal annealing of T4 nascent short DNA chains with the separated complementary strands of the phage DNA. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1343–1350. doi: 10.1073/pnas.63.4.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Okazaki T., Okazaki R. Mechanism of DNA chain growth, II. Accumulation of newly synthesized short chains in E. coli infected with ligase-defective T4 phages. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1356–1362. doi: 10.1073/pnas.60.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Ogawa T. Replication of phage lambda DNA. Cold Spring Harb Symp Quant Biol. 1968;33:533–551. doi: 10.1101/sqb.1968.033.01.061. [DOI] [PubMed] [Google Scholar]
- Tsukada K., Moriyama T., Lynch W. E., Lieberman I. Polydeoxynucleotide intermediates in DNA replication in regenerating liver. Nature. 1968 Oct 12;220(5163):162–164. doi: 10.1038/220162a0. [DOI] [PubMed] [Google Scholar]
- Yudelevich A., Ginsberg B., Hurwitz J. Discontinuous synthesis of DNA during replication. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1129–1136. doi: 10.1073/pnas.61.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]


