Abstract
Nitroreductase A catalyzes the divalent reduction of nitro compounds, quinones, and dyes by NADPH. In this paper, nitroreductase A is induced in Escherichia coli by exposure to paraquat in a manner that depends on the expression of soxR. Nitroreductase activity was only slightly induced by paraquat in a strain bearing a mutational defect in the gene encoding nitroreductase A, but it was ≈3-fold induced in the parental strain. Nitroreductase A thus appears to be a member of the soxRS regulon and probably contributes to the defenses against oxidative stress by minimizing the redox cycling attendant upon the univalent reduction of nitro compounds, quinones, and dyes.
The soxRS regulon of Escherichia coli positively regulates >10 genes in response to challenge with redox cycling agents, such as paraquat, that are capable of mediating the univalent reduction of dioxygen (1). The constitutive SoxR protein functions as the sensor of this form of oxidative stress and induces the production of SoxS, which, in turn, induces the members of this regulon (1–4). The SoxR protein is ordinarily kept largely reduced under the conditions prevailing in the E. coli cytosol (2). Constitutive mutants of soxR have been produced that have more negative redox potentials than does the wild-type protein; thus, favoring the oxidized, trancriptionally active form of soxR (4). The members of this regulon include MnSOD, to scavenge O2−; glucose-6-phosphate dehydrogenase, to supply NADPH; fumarase C, to replace the O2−-sensitive fumarases A and B; endonuclease IV, to participate in the repair of oxidized DNA; aconitase A, to compensate for the oxidative inactivation of aconitases; NADPH:ferredoxin reductase, to provide reduced ferredoxin; and micF, to diminish the porosity of the outer membrane (1, 5–7). Identification of the gene products induced by soxRS serves to increase our understanding of the manifold aspects of defense against oxidative stress.
It has recently been reported that there are several paraquat-inducible nitroblue tetrazolium reductases in E. coli and that NADPH:ferredoxin reductase is one of them (8). Earlier, we observed a paraquat-inducible nitroblue tetrazolium reductase and isolated this enzyme and determined the sequence of 21 N-terminal residues (unpublished results). However, a search of the databases available at that time (1994) did not yield a match. The report by Rodriguez et al. (8) stimulated a return to this unfinished project. Another search revealed that 19 out of the 21 amino acids in the N terminus of our nitroblue tetrazolium reductase are identical to that of nitroreductase A encoded by the nfsA gene (formerly designated mda A) (9, 10). It thus appeared that nitroreductase A might be a member of the soxRS regulon. This flavoenzyme catalyzes the divalent reduction of organic nitro compounds, quinones, and dyes (9). It could thereby prevent their univalent reductions and thus diminish the O2− production due to the redox cycling of these compounds. We now report that nitroreductase A is inducible by paraquat in a manner dependent upon SoxR and is thus a member of the SoxRS regulon.
MATERIALS AND METHODS
Nitrofurantoin, NADH, and NADPH were from Sigma. The strains of E. coli were DJ 901 (ΔsoxR), JTG936 (soxR constitutive), and GC4468 (parental). These were obtained from Bruce Demple (1, 11). Additional strains were JVQ1, which is defective in nfsA, and JVQ2, which is defective in both nfsA and nfsB, and the parental AB1157 (all were kindly provided by J. Whiteway and I. B. Lambert, ref. 12). Overnight cultures in LB medium were diluted 20-fold into fresh LB medium and, after 1 hr of incubation, 0.2 mM paraquat was added, where so indicated, and incubation was continued for another hour. Cells were then collected by centrifugation and were washed in chilled 50 mM Tris⋅Cl (pH 7.5) and were then resuspended in the same buffer and lysed with a French press. Clarified cell extracts were assayed for protein (13) and for nitroreductase activity at 25°C in the Tris buffer at pH 7.5. Nitrofurantoin was used at 25 μM, NADPH at 100 μM, and NADH at 50 μM. Nitrofurantoin has an extinction coefficient of 2.63 × 104 M−1 cm−1 at 373 nm (14). We followed its reduction at 378 nm, which minimized interference from the NAD(P)H absorption peak at 340 nm. The absorption of nitrofurantoin was identical at 373 nm and at 378 nm; hence we used the published Em.
RESULTS
Induction of Nitroreductase by Paraquat.
The oxygen-sensitive nitroreductases do not cause net reduction of nitro compounds under aerobic conditions because they catalyze univalent reduction and the resultant nitro radical anions are autoxidizable. The oxygen-insensitive nitroreductases, in contrast, catalyze divalent reduction of nitro compounds to stable products (12, 15, 16). Hence, when assaying the reduction of nitrofurantoin under aerobic conditions, we were following only the oxygen-insensitive activities. E. coli is able to produce three oxygen-insensitive nitroreductases, i.e., A, B, and another minor isoenzyme (9, 12, 15). The A isoenzyme is at least one order of magnitude more active with NADPH than with NADH, whereas the other two isoenzymes are active with either NADPH or NADH (9, 15).
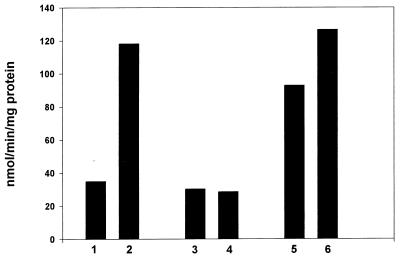
Fig. 1 presents the NADPH:nitrofurantoin reductase specific activities exhibited by the three strains of E. coli with and without paraquat induction. Bars 1 and 2 illustrate the 3.5-fold induction seen in the parental strain; bars 3 and 4 show that the ΔsoxR strain was incapable of such induction; bar 5 shows the high level of activity seen in the soxR constitutive strain; and bar 6 shows the modest further induction caused by paraquat in this strain. This induction with the soxR constitutive strain can be understood in terms of shifting the balance of reduced to oxidized SoxR further toward the oxidized state (2, 4).
Figure 1.
Inductions of the NADPH-dependent nitroreductase. The induction of NADPH:furantoin nitroreductase by paraquat was explored as described in Materials and Methods. Bars 1, 3, and 5, without exposure to paraquat; bars 2, 4, and 6, with exposure to paraquat. Bars 1 and 2, parental strain; bars 3 and 4, ΔsoxR strain; bars 5 and 6, soxR constitutive strain.
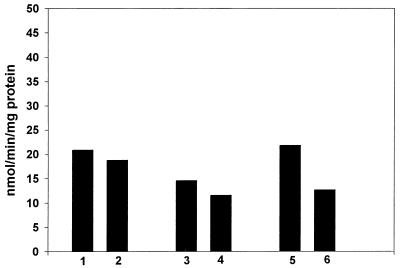
When the same experiment was repeated, but with NADH in place of NADPH, there was no induction by paraquat in any of the strains tested (Fig. 2). It is thus clear that the nitroreductase that was inducible by paraquat in the soxRS-dependent fashion must have been the A isoenzyme, which is specific for NADPH.
Figure 2.
Effect of paraquat on the NADH-dependent nitroreductase. Conditions as in Fig. 1 except that NADH was the electron donor.
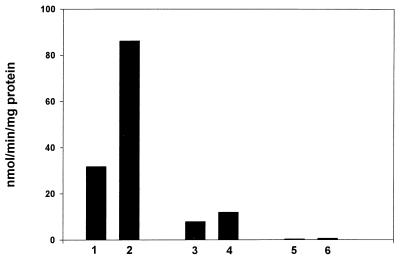
To more definitely establish that the induction of nitroreductase activity by paraquat was caused by the A isozyme, this induction was examined in nfsA, nfsA nsfB, and parental strains. As shown in Fig. 3, paraquat caused ≈3-fold induction of nitroreductase in the parental strain, but very little induction in the nfsA strain. The double mutant had only trivial nitroreductase activity and showed no response to paraquat. Hence, the nitroreductase A is the isozyme principally responsible for the paraquat effect. Nitroreductase B accounts for the activity seen in extracts of the nsfA mutant, and its slight induction by paraquat may be significant and worthy of further exploration.
Figure 3.
Effect of mutations in nfsA and nfsB on the inducibility of nitroreductase. Conditions as in Fig. 1: bar 1, parental strain; bar 2, parental + paraquat; bar 3, nfsA; bar 4, nfsA + paraquat; bar 5, nfsA nfsB; bar 6; nsfA nfsB + paraquat.
DISCUSSION
The oxygen-insensitive nitroreductase A is regulated as a member of the soxRS regulon. Thus it is inducible by paraquat in the parental, but not in the ΔsoxR, E. coli. This can be seen to make sense as part of the concerted defense against O2−. Thus the oxygen-insensitive nitroreductase converts organic nitro compounds to stable products by divalent reduction and thus makes them unavailable for redox cycling and O2− production under the catalytic influence of the oxygen-sensitive nitroreductases. It is also the case that nitroreductase A has a broad specificity and may exert a protective role by divalently reducing non-nitro compounds. Thus, it has been shown (9) that nitroreductase A can reduce a variety of quinones, ferricyanide, and 2,6-dichloroindophenol, in addition to a wide range of nitro compounds. In this regard it should be noted that overproduction of nitroreductase A protected E. coli against the quinone adriamycin (10) possibly by catalyzing its divalent reduction.
The ability of organic nitro compounds to mediate O2− production by cycles of univalent reduction and autoxidation is well supported (16). Indeed nitroquinoline-N-oxide has been seen to strongly induce the soxRS regulon, and this induction was protective (17). In addition, exposure of E. coli to 10 μM nitrofurantoin resulted in 50% inactivation of the O2−-sensitive dihydroxy acid dehydratase (18). This can be understood in terms of the oxidation by O2− of the [4Fe-4S] cluster at the active site of this dehydratase. Exposure of murine hepatocytes to nitrofurantoin increased the pool of labile iron (19), which can be explained by the release of iron from [4Fe-4S] clusters after their oxidation by O2−, and/or by diminishing the reductive reconstruction of such clusters, and possibly also by mobilization from ferritin by the nitrofurantoin anion radical.
The seemingly paradoxical findings that mutants lacking nitroreductase A were more resistant to the toxicity of certain nitro compounds may be due to the greater toxicity of the products of reduction of those nitro compounds (12). The nitro compounds used in these studies are not the compounds that elicited the evolution of nitroreductases. It might be supposed that nitro compounds produced by nitration of endogenous targets, such as tyrosyl residues, by peroxynitrite in the presence of CO2 (20), impose a toxicity that can be muted by the action of the nitroreductases. Another possibility is that the biologically important substrates of nitroreductase A are quinones, whose divalent reduction by this enzyme preempts univalent reduction and subsequent autoxidation. Clearly more work will be needed to fully explain the protective functions of the oxygen-insensitive nitroreductases.
Most of the target genes of the soxRS regulon are also regulated by the marRAB regulon, which provides protection against various drugs (21). It is therefore likely that nitroreductase A is under the control of marRAB and probably can be induced by other transcriptional regulators such as the Rob-protein (21).
Acknowledgments
We are grateful to Dr. B. Demple and to Drs. J. Whiteway and I. B. Lambert who generously provided the strains of E. coli used in this work; to Dr. L. Benov for help with some of these experiments; and to Dr. W. F. Beyer for the N-terminal sequence of the paraquat-inducible NADPH:nitroblue tetrazolium reductase. This work was supported by grants from the Amyotrophic Lateral Sclerosis Association, the National Institutes of Health, the Council for Tobacco Research–U.S.A., Inc., and the North Carolina Biotechnology Center Collaborative Funding Assistant Program, and received support from Aeolus Pharmaceuticals, Inc.
References
- 1.Demple B. Gene. 1996;179:53–57. doi: 10.1016/s0378-1119(96)00329-0. [DOI] [PubMed] [Google Scholar]
- 2.Gaudu P, Moon N, Weiss B. J Biol Chem. 1997;272:5082–5086. doi: 10.1074/jbc.272.8.5082. [DOI] [PubMed] [Google Scholar]
- 3.Ding H, Demple B. Proc Natl Acad Sci USA. 1997;97:8445–8449. doi: 10.1073/pnas.94.16.8445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hidalgo E, Ding H, Demple B. Cell. 1997;88:121–129. doi: 10.1016/s0092-8674(00)81864-4. [DOI] [PubMed] [Google Scholar]
- 5.Fridovich I. J Exp Biol. 1998;201:1203–1209. doi: 10.1242/jeb.201.8.1203. [DOI] [PubMed] [Google Scholar]
- 6.Liochev S I. Free Radical Res. 1996;25:269–284. [Google Scholar]
- 7.Liochev S I, Hausladen A, Beyer W F, Fridovich I. Proc Natl Acad Sci USA. 1994;91:1328–1331. doi: 10.1073/pnas.91.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez R E, Krapp A R, Carrilo N. Microbiology. 1998;144:2375–2376. doi: 10.1099/00221287-144-9-2375. [DOI] [PubMed] [Google Scholar]
- 9.Zenno S, Koike H, Kumar A N, Jayamaran R, Tanokura M, Saigo K. J Bacteriol. 1996;178:4508–4514. doi: 10.1128/jb.178.15.4508-4514.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee P K, Sternberg N L. Proc Natl Acad Sci USA. 1995;92:8950–8954. doi: 10.1073/pnas.92.19.8950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg J T, Monach P, Chou J H, Josephy P O, Demple B. Proc Natl Acad Sci USA. 1990;87:6181–6185. doi: 10.1073/pnas.87.16.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whiteway J, Koziarz P, Veall J, Sandhu N, Kumar P, Hoecher B, Lambert I B. J Bacteriol. 1998;180:5529–5539. doi: 10.1128/jb.180.21.5529-5539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.McOsker, C. C. & Fitzpatrick, P. M. (1994) J. Antimicrob. Chemother.33, Suppl. A., 23–30. [DOI] [PubMed]
- 15.McGalla D R, Olive P, Tu Y, Fan M L. Can J Microbiol. 1975;21:1485–1491. doi: 10.1139/m75-220. [DOI] [PubMed] [Google Scholar]
- 16.Peterson F J, Mason R P, Hovsepian J, Holtzman J I. J Biol Chem. 1979;254:4009–4014. [PubMed] [Google Scholar]
- 17.Nunoshiba T, Demple B. Cancer Res. 1993;53:3250–3252. [PubMed] [Google Scholar]
- 18.Brown O R, Smyk-Randall E, Draczynska-Lusiak B, Fee J. Arch Biochem Biophys. 1995;319:10–22. doi: 10.1006/abbi.1995.1262. [DOI] [PubMed] [Google Scholar]
- 19.Stäubli A, Boelsterli U A. Am J Physiol. 1998;274:G1031–G1037. doi: 10.1152/ajpgi.1998.274.6.G1031. [DOI] [PubMed] [Google Scholar]
- 20.Squadrito G L, Prior W A. Free Radical Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 21.Ariza R R, Li Z, Ringstad N, Demple B. J Bacteriol. 1995;177:1651–1661. doi: 10.1128/jb.177.7.1655-1661.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]