Abstract
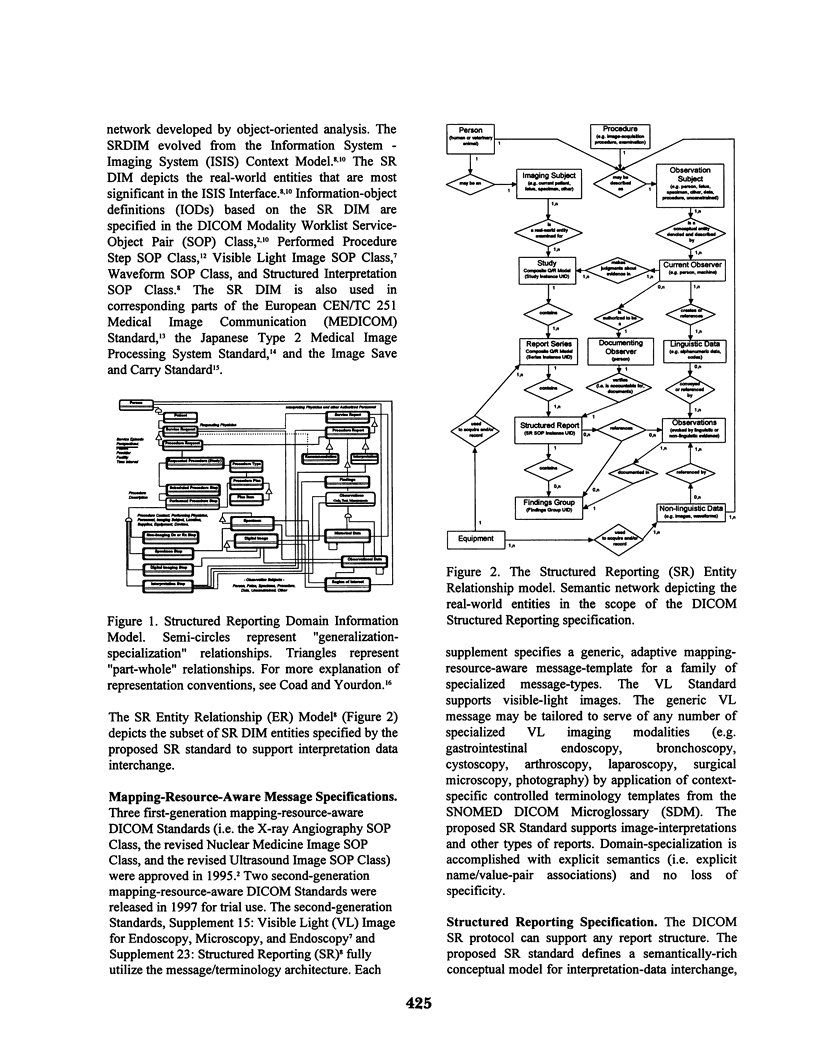
A standards-based message and terminology architecture has been specified to enable large-scale open and non-proprietary interchange of imaging-procedure descriptions and image-interpretation reports providing semantically-rich linkage of linguistic and non-linguistic information. The DICOM Structured Reporting Supplement, now available for trial use, embodies this interdependent message/terminology architecture. A DICOM structured report object is a self-describing information structure that can be tailored to support diverse clinical observation reporting applications by utilization of templates and context-dependent terminology from an external message/terminology mapping resource such as the SNOMED DICOM Microglossary (SDM), HL7 Vocabulary, or Terminology Resource for Message Standards (TeRMS).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidgood W. D., Jr, Horii S. C. Modular extension of the ACR-NEMA DICOM standard to support new diagnostic imaging modalities and services. J Digit Imaging. 1996 May;9(2):67–77. doi: 10.1007/BF03168859. [DOI] [PubMed] [Google Scholar]
- Bidgood W. D., Jr, Horii S. C., Prior F. W., Van Syckle D. E. Understanding and using DICOM, the data interchange standard for biomedical imaging. J Am Med Inform Assoc. 1997 May-Jun;4(3):199–212. doi: 10.1136/jamia.1997.0040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrey A. W., McDonald C. J., DeMoor G., Huff S. M., Leavelle D., Leland D., Fiers T., Charles L., Griffin B., Stalling F. Logical observation identifier names and codes (LOINC) database: a public use set of codes and names for electronic reporting of clinical laboratory test results. Clin Chem. 1996 Jan;42(1):81–90. [PubMed] [Google Scholar]
- Lindberg D. A., Humphreys B. L., McCray A. T. The Unified Medical Language System. Methods Inf Med. 1993 Aug;32(4):281–291. doi: 10.1055/s-0038-1634945. [DOI] [PMC free article] [PubMed] [Google Scholar]


