Abstract
A family of inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) Ca2+ release channels plays a central role in Ca2+ signaling in most cells, but functional correlates of isoform diversity are unclear. Patch-clamp electrophysiology of endogenous type 1 (X-InsP3R-1) and recombinant rat type 3 InsP3R (r-InsP3R-3) channels in the outer membrane of isolated Xenopus oocyte nuclei indicated that enhanced affinity and reduced cooperativity of Ca2+ activation sites of the InsP3-liganded type 3 channel distinguished the two isoforms. Because Ca2+ activation of type 1 channel was the target of regulation by cytoplasmic ATP free acid concentration ([ATP]i), here we studied the effects of [ATP]i on the dependence of r-InsP3R-3 gating on cytoplasmic free Ca2+ concentration ([Ca2+]i). As [ATP]i was increased from 0 to 0.5 mM, maximum r-InsP3R-3 channel open probability (P o) remained unchanged, whereas the half-maximal activating [Ca2+]i and activation Hill coefficient both decreased continuously, from 800 to 77 nM and from 1.6 to 1, respectively, and the half-maximal inhibitory [Ca2+]i was reduced from 115 to 39 μM. These effects were largely due to effects of ATP on the mean closed channel duration. Whereas the r-InsP3R-3 had a substantially higher P o than X-InsP3R-1 in activating [Ca2+]i (<1 μM) and 0.5 mM ATP, the Ca2+ dependencies of channel gating of the two isoforms became remarkably similar in the absence of ATP. Our results suggest that ATP binding is responsible for conferring distinct gating properties on the two InsP3R channel isoforms. Possible molecular models to account for the distinct regulation by ATP of the Ca2+ activation properties of the two channel isoforms and the physiological implications of these results are discussed. Complex regulation by ATP of the types 1 and 3 InsP3R channel activities may enable cells to generate sophisticated patterns of Ca2+ signals with cytoplasmic ATP as one of the second messengers.
Keywords: allosteric regulation, calcium release channel, single-channel electrophysiology, patch clamp, Xenopus oocyte
INTRODUCTION
Modulation of free cytoplasmic Ca2+ concentration ([Ca2+]i) is a ubiquitous cellular signaling system. In many cell types, binding of ligands to plasma membrane receptors activates the hydrolysis of phosphatidylinositol 4,5-bisphosphate by membrane-bound phospholipase C, generating inositol 1,4,5-trisphosphate (InsP3). InsP3 causes the release of Ca2+ from the endoplasmic reticulum (ER) by binding to its receptor (InsP3R), which itself is a Ca2+ channel (Taylor and Richardson 1991; Berridge 1993; Putney and St. J. Bird 1993). A family of InsP3R isoforms (types 1, 2, and 3) with different primary sequences derived from different genes and alternatively spliced isoforms has been identified. The different isoforms have distinct and overlapping patterns of expression in different tissues. Most, if not all, mammalian cells express multiple isoforms whose absolute and relative expression levels can be modified by cell differentiation and physiological status, and which may associate as heterotetramers.
The functional correlates of this impressive diversity of InsP3R expression are largely unknown (see introduction of Mak et al. 2001, in this issue). Expression and single-channel recording of the recombinant rat type 3 InsP3R (r-InsP3R-3) channels in Xenopus oocyte nuclear membrane patches (Mak et al. 2000, Mak et al. 2001) demonstrated that they have remarkably similar ion permeation and channel gating properties as the Xenopus type 1 InsP3R (X-InsP3R-1). Of note, r-InsP3R-3 gating also exhibits a biphasic dependence on [Ca2+], with properties of the inhibitory Ca2+ site and allosteric tuning of that site by InsP3 highly similar to those properties of the endogenous X-InsP3R-1. In contrast, the r-InsP3R-3 channel is uniquely distinguished from the type 1 channel by enhanced Ca2+ sensitivity of, and lack of cooperativity between, the Ca2+ activation sites (see Mak et al. 2001, in this issue; Fig. 1). Interestingly, studies of the regulation by ATP of the X-InsP3R-1 channel revealed that the mechanism by which cytoplasmic free ATP stimulates its gating in low [Ca2+]i (<1 μM) is by specifically increasing the affinity of the Ca2+ activating site of the channel without affecting the degree of cooperativity for Ca2+ activation or the maximum open probability (P o; Mak et al. 1999). Although channel P o decreases when [ATP]i is decreased, this can be fully reversed by increasing [Ca2+]i, demonstrating that ATP is not a necessary agonist for activation of the InsP3R, but is rather an allosteric regulator, tuning the efficacy of Ca2+ to stimulate the activity of the InsP3-liganded InsP3R over a limited range of [Ca2+]i (10 nM–1 μM). Therefore, InsP3 and ATP are complementary allosteric regulators of InsP3R gating: InsP3 is a specific regulator of Ca2+ inhibition of Ca2+ release, tuning the functional affinity of the inhibitory Ca2+ binding sites of the channel (Mak et al. 1998, Mak et al. 2001), whereas ATP is a regulator of Ca2+-induced Ca2+ release, tuning the functional affinity of the activating Ca2+ binding sites.
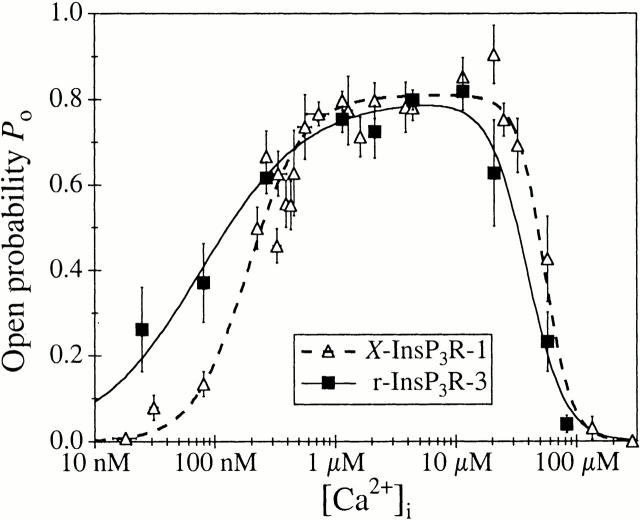
Figure 1.
Ca2+ dependencies of types 1 and 3 InsP3R channel P o in the presence of 0.5 mM ATP. 10 μM InsP3 was present in the pipet solutions used in experiments presented in all figures. Open triangles represent data for X-InsP3R-1 obtained from uninjected oocytes. Closed squares represent data for r-InsP3R-3 obtained from cRNA-injected oocytes. The curves (dashed for X-InsP3R-1 and solid for r-InsP3R-3) are the biphasic Hill equation fits from Mak et al. 2001.
Because the properties of the Ca2+ activation sites represented the only significant distinction between the types 1 and 3 InsP3R channels that could be discerned in our previous studies, and Ca2+ activation of X-InsP3R-1 channel gating is modulated by ATP, here we have investigated the effects of ATP on the gating properties of the recombinant r-InsP3R-3 channel. Surprisingly, our results reveal that the presence of ATP is absolutely necessary to confer distinct Ca2+ activation properties to the types 1 and 3 channels because, in its absence, Ca2+ activation of the two channels is very similar. ATP activates type 3 channel gating in a dramatically different manner compared with its effects on the type 1 channel: whereas maximal P o of both isoforms is similarly unaffected by [ATP]i, ATP abolishes the cooperativity of Ca2+ activation of r-InsP3R-3 besides increasing the functional affinity of the activating Ca2+ binding sites. Thus, complex allosteric dependencies on [ATP]i of the gating of types 1 and 3 InsP3R channels, coupled with differential levels of expression and subcellular localization, may enable cells to generate sophisticated Ca2+ signals, with cytoplasmic ATP as one of the second messengers.
MATERIALS AND METHODS
Xenopus oocytes were obtained and selected for a low functional expression level of the endogenous X-InsP3R-1 as described previously (Mak et al. 2001). Of 89 nuclear patches obtained from isolated nuclei of selected uninjected oocytes, only 1 channel was detected. The mean number of InsP3R channels per nuclear patch was 0.011. Selected oocytes were microinjected with r-InsP3R-3 cRNA and used for nuclear patch-clamp experiments 4–5 d after injection. The mean number of InsP3R channels per nuclear patch for the cRNA-injected oocytes increased dramatically to 1.65. In 330 nuclear patches, 544 channels were detected in 167 patches, with 105 patches exhibiting multiple InsP3R channels. Assuming random, binomial association of X-InsP3R-1 and r-InsP3R-3 to form tetrameric channels (Mak et al. 2000), most (97.4%) of the channels detected in cRNA-injected oocytes are predicted to be homotetrameric r-InsP3R-3 channels.
Nuclear patch-clamp experiments were performed on isolated nuclei from cRNA-injected oocytes as described in the companion paper (see Mak et al. 2001, in this issue), except the pipet solutions used in this study contained a saturating concentration of 10 μM of InsP3 (used without further purification; Molecular Probes), various concentrations of Na2ATP, and either 0 or 3 mM MgCl2 as stated. Because of chelation of Mg2+ by ATP, the actual free Mg2+ in the solutions was 0 or 2.5 mM. The bath solutions used in all experiment had 140 mM KCl, 10 mM HEPES, 300 μM CaCl2, and 500 μM BAPTA ([Ca2+] = 500 nM), pH 7.3.
Data acquisition and analysis were performed as described in the companion paper (see Mak et al. 2001, in this issue).
RESULTS
ATP Activation of Recombinant Type 3 InsP3R
To study the effects of [ATP]i on gating of single recombinant r-InsP3R-3 channels in their native ER membrane environment, we performed patch-clamp experiments on nuclei isolated from oocytes injected with cRNA of r-InsP3R-3. Although the oocyte expresses endogenously a type 1 InsP3R, under the conditions of our experiments, >90% of the channels recorded were contributed by type 3 homotetramers (Mak et al. 2000). To facilitate comparisons with experimental results obtained for the type 1 InsP3R (Mak et al. 1999), the patch-clamp experiments were performed for the InsP3R-3 using similar experimental conditions. The pipet solutions contained various [Ca2+]i with 0.5 mM ATP alone, 3 mM Mg2+ alone, 0.5 mM ATP and 3 mM Mg2+ (calculated [ATP]i = 12 μM; calculated [Mg2+]i = 2.5 mM), or no ATP or Mg2+. To avoid possible effects of Ca2+ on InsP3 binding (Hagar and Ehrlich 2000; Meas et al. 2000), a functionally saturating InsP3 concentration of 10 μM was used.
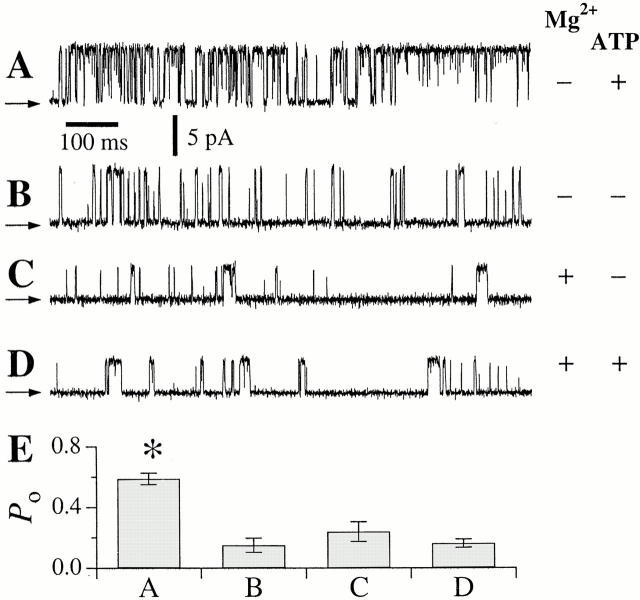
r-InsP3R-3 channel activities with a high P o of 0.6 (Fig. 2 A) and gating kinetics similar to those of the r-InsP3R-3 reported previously (see Mak et al. 2001, in this issue) were observed in pipet solutions containing 0.5 mM free ATP, and ∼250 nM free Ca2+. In the absence of ATP, in contrast, the r-InsP3R-3 channel had significantly lower P o of 0.2, either in the presence or absence of 3 mM Mg2+ (Fig. 2B and Fig. C). ATP did not affect the conductance of the r-InsP3R-3 channel (Fig. 2A and Fig. B). The time course of channel inactivation of the r-InsP3R-3 channel was not substantially different in the presence and absence of ATP.
Figure 2.
(A–D) Typical single-channel current traces of r-InsP3R-3 channels in the outer membrane of nuclei isolated from r-InsP3R-3 cRNA-injected oocytes under suboptimal [Ca2+]i, with various [ATP]i and [Mg2+]i. Arrows indicate closed channel current levels. (A) [Ca2+]i = 224 nM, [ATP]i = 0.5 mM; [Mg2+]i = 0 mM. (B) [Ca2+]i = 255 nM, [ATP]i = 0 mM; [Mg2+]i = 0 mM. (C) [Ca2+]i = 274 nM, [ATP]i = 0 mM; [Mg2+]i = 3.0 mM. (D) [Ca2+]i = 286 nM, [ATP]i = 12 μM; [Mg2+]i = 2.5 mM (0.5 mM total ATP; 3 mM total Mg2+). Reduction of InsP3R channel conductance in the presence of Mg2+ (C and D) is caused by permeant ion block of the channel by the divalent cation (Mak and Foskett 1998). (E) Single-channel P o of the r-InsP3R-3 channel in conditions specified in A–D. Asterisk represents P < 0.05.
To examine in more detail if the presence of the MgATP complex affects the P o of the r-InsP3R-3, similar experiments were performed using pipet solutions containing 3 mM total Mg2+ and 0.5 mM total ATP, so that the calculated [MgATP], free Mg2+ concentration ([Mg2+]i), and [ATP]i were 0.5 mM, 2.5 mM, and 12 μM, respectively. The P o of the r-InsP3R-3 channel remained low under these conditions (Fig. 2 D). Thus, the r-InsP3R-3 is activated by ATP free acid (ATP3− or ATP4−), but not by the MgATP complex (Fig. 2 E), suggesting that ATP hydrolysis or phosphorylation is not involved in ATP activation of the type 3 InsP3R. Therefore, this behavior is similar to that of the type 1 InsP3R channel (Mak et al. 1999).
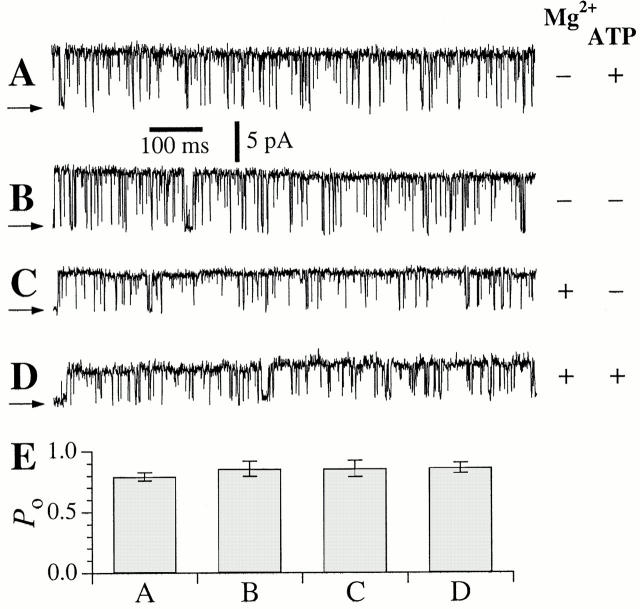
This activation of r-InsP3R-3 by ATP was prominently observed only at low [Ca2+]i (<1 μM). At optimal [Ca2+]i (>2 μM), the channel P o achieved the maximum value of ∼0.8 in both 0 or 0.5 mM free [ATP]i (Fig. 3). Thus, as in the case for X-InsP3R-1 (Mak et al. 1999), ATP is not essential for maximal activation of r-InsP3R-3.
Figure 3.
(A–D) Typical single-channel current traces of r-InsP3R-3 channels under optimal [Ca2+]i with various [ATP]i and [Mg2+]i. Arrows indicate closed channel current levels. (A) [Ca2+]i = 4.4 μM, [ATP]i = 0.5 mM; [Mg2+]i = 0 mM. (B) [Ca2+]i = 5.6 μM, [ATP]i = 0 mM; [Mg2+]i = 0 mM. (C) [Ca2+]i = 2.5 μM, [ATP]i = 0 mM; [Mg2+]i = 3.0 mM. (D) [Ca2+]i = 2.5 μM, [ATP]i = 12 μM; [Mg2+]i = 2.5 mM (0.5 mM total ATP; 3 mM total Mg2+). Reduction of InsP3R channel conductance in the presence of Mg2+ (C and D) is caused by permeant ion block of the channel by the divalent cation (Mak and Foskett 1998). (E) Single-channel open probability (P o) of the r-InsP3R-3 channel in conditions specified in A–D.
Effects of ATP on the Ca2+ Dependence of Types 1 and 3 InsP3R Gating
To determine the mechanisms by which ATP activates r-InsP3R-3 channel gating, we investigated systematically the effects of cytoplasmic ATP on the channel kinetics over a wide range of [Ca2+]i. In the absence of ATP, Ca2+ dependence of channel P o of the InsP3R-3 was biphasic (Fig. 4 A) and well fitted with the biphasic Hill equation (Mak et al. 1998):
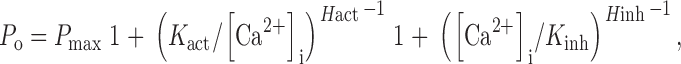 |
1 |
with a maximum open probability (P max) of 0.84 ± 0.02, a half-maximal activating [Ca2+]i (K act) of 800 ± 50 nM, an activation Hill coefficient (H act) of 1.6 ± 0.3, a half-maximal inhibitory [Ca2+]i (K inh) of 115 ± 15 μM, and an inhibition Hill coefficient (H inh) of 2 ± 0.5 (Table ).
Figure 4.
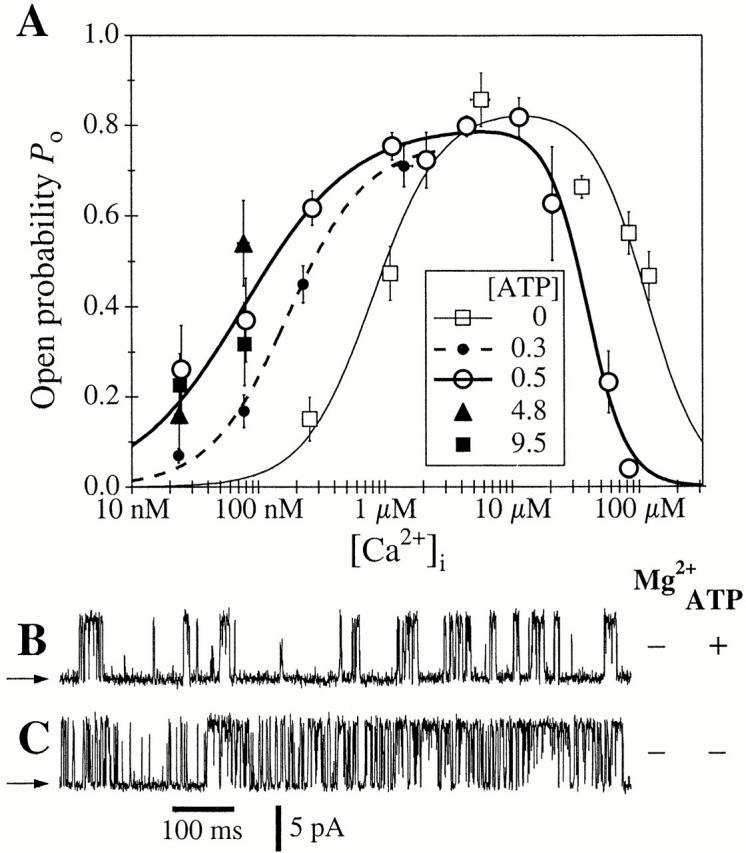
(A) Ca2+ dependencies of the r-InsP3R-3 channel P o in the presence of various [ATP]i. The symbols correspond to P o data in various [ATP]i (in millimolars) as tabulated in the graph. The curves for 0 and 0.5 mM ATP represent theoretical fits to the data using the biphasic Hill equation () whereas the curve for 0.3 mM ATP represents a fit using the activation Hill equation (). Hill equation parameters used are tabulated in Table . (B–C) Typical single-channel current traces of r-InsP3R-3 channels under inhibiting [Ca2+]i. Arrows indicate closed channel current levels. (B) [Ca2+]i = 58 μM, [ATP]i = 0.5 mM; [Mg2+]i = 0 mM. (C) [Ca2+]i = 83 μM, [ATP]i = 0 mM; [Mg2+]i = 0 mM.
Table 1.
Hill Equation Parameters for [Ca2+]i Dependence of InsP3R Isoforms
| InsP3Risoform | [ATP]i | P max | K act | H act | K inh | H inh | |
|---|---|---|---|---|---|---|---|
| mM | nM | μM | |||||
| A | Type 3 | 0.0 | 0.84 ± 0.02 | 800 ± 50 | 1.6 ± 0.3 | 115 ± 15 | 2.0 ± 0.5 |
| B | Type 3 | 0.3 | 0.76 ± 0.04 | 180 ± 30 | 1.4 ± 0.2 | N/A | N/A |
| C | Type 3 | 0.5 | 0.80 ± 0.03 | 77 ± 10 | 1.0 ± 0.1 | 39 ± 7 | 2.8 ± 0.4 |
| D | Type 1 | 0.0 | 0.80 ± 0.02 | 550 ± 50 | 1.9 ± 0.6 | 110 ± 15 | 4.0 ± 0.7 |
| E | Type 1 | 0.3 | N/A | 230 ± 30 | N/A | N/A | N/A |
| F | Type 1 | 0.5 | 0.81 ± 0.02 | 190 ± 20 | 1.9 ± 0.3 | 54 ± 3 | 3.9 ± 0.7 |
Parameters for the biphasic Hill equations () that fit the Ca2+ dependence of the P o of InsP3R channels under various experimental conditions. [ATP]i refers to the concentration of ATP free acid. Parameters for C are from Mak et al. 2001, parameters for E are from Mak et al. 1999. N/A, not available.
This behavior is dramatically different from the Ca2+ dependence of gating of the r-InsP3R-3 in the presence of 0.5 mM cytoplasmic free ATP (Fig. 4 A). At high, inhibitory [Ca2+]i, the presence of 0.5 mM ATP decreased the value of K inh from 115 to 39 μM such that, in [Ca2+]i > 50 μM, the r-InsP3R-3 channel activity was lower in the presence of 0.5 mM free ATP than in the absence of ATP (Fig. 4B and Fig. C). The value of H inh was somewhat higher in the presence of ATP. Of likely greater physiological significance, ATP induced a much lower K act of 77 ± 10 nM and reduced the value of H act to 1.0 ± 0.1 (Fig. 1 and Table ). Thus, cytoplasmic ATP decreased both the half-maximal activating [Ca2+]i as well as the activation Hill coefficient, with the result that the P o of the r-InsP3R-3 at [Ca2+]i < 500 nM is substantially higher in the presence of ATP than in its absence (Fig. 4 A). It is this dramatic increase of the Ca2+ affinity of the r-InsP3R-3 activating site(s) and loss of cooperativity between these sites in the tetrameric channel that give rise to the observed difference in channel activities of the r-InsP3R-3 and X-InsP3R-1 in activating [Ca2+]i (<1 μM) in the presence of ATP (Fig. 1).
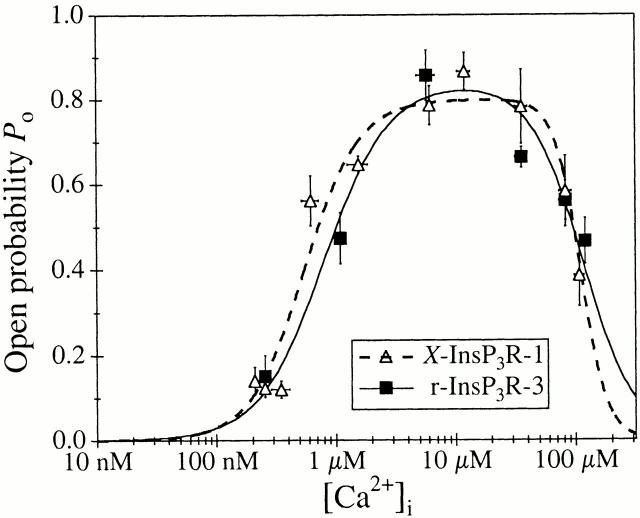
In sharp contrast, the biphasic Ca2+ dependence of the r-InsP3R-3 in the absence of cytoplasmic free ATP is remarkably similar to the biphasic Ca2+ dependence of the X-InsP3R-1 in the absence of ATP (Fig. 5), with P max = 0.80 ± 0.02, K act = 550 ± 50 nM, H act = 1.9 ± 0.6, K inh = 110 ± 15 μM, and H inh = 4.0 ± 0.7 (Table ). Thus, in the absence of cytoplasmic ATP, there are no significant differences between the responses to Ca2+ of the types 1 and 3 InsP3R channels.
Figure 5.
Ca2+ dependencies of types 1 and 3 InsP3R channel P o in the absence of ATP. Open triangles represent data for X-InsP3R-1 obtained from uninjected oocytes. Closed squares represent data for r-InsP3R-3 obtained from cRNA-injected oocytes. The curves (dashed for X-InsP3R-1 and solid for r-InsP3R-3) are the biphasic Hill equation fits using and parameters tabulated in Table .
Examination of the gating kinetics of the r-InsP3R-3 channel revealed that the mean open channel duration (τo) in the absence of cytoplasmic ATP lay within a narrow range between 3 and 12 ms over the range of [Ca2+]i studied (0.2–120 μM; Fig. 6). Within this range, τo varied with [Ca2+]i in a biphasic fashion, increasing as [Ca2+]i increased from 0.2 to 2 μM, and decreasing as [Ca2+]i further increase from 6 to 120 μM. This partly mirrored the variation of r-InsP3R-3 channel P o with [Ca2+]i (Fig. 4 A). In contrast, the mean closed channel duration (τc) decreased nearly an order of magnitude, from 18 to 2 ms, as [Ca2+]i was increased from 0.2 to 6 μM, and then remained low (∼2 ms) in high [Ca2+]i (6–120 μM). The changes in τc accounted for the major part of the Ca2+ activation of channel activity. These Ca2+ dependencies of τc and τo of the type 3 channel in the absence of ATP are reminiscent of those of X-InsP3R-1 in 0 mM ATP (Mak et al. 1999).
Figure 6.
Ca2+ dependencies of the mean open and closed channel durations of the r-InsP3R-3 channels in the presence of 0 (▪) or 0.5 mM (○) cytoplasmic free ATP. In the closed channel duration graph, data points obtained with the same InsP3 concentrations are connected with a line for clarity.
The presence of ATP enhanced r-InsP3R-3 channel activity at low [Ca2+]i (<2 μM) (Fig. 2 and Fig. 3) by both stabilizing the open channel kinetic state(s) and destabilizing the closed kinetic state(s) (Fig. 6). As [Ca2+]i increased from 10 to 83 μM, ATP reduced r-InsP3R-3 channel activity by stabilizing closed kinetic state(s) so that τc increased dramatically (Fig. 4 and Fig. 6).
Effects of ATP Concentration on Ca2+ Activation of r-InsP3R-3
The effects of cytoplasmic ATP concentration on the Ca2+ activation of the r-InsP3R-3 were studied in more detail in a series of patch-clamp experiments using various [ATP]i between 0 and 9.5 mM, over a wide range of [Ca2+]i between 20 nM and 6 μM. The pipet solutions again contained 10 μM InsP3, sufficient to saturate the InsP3R (Mak et al. 2001). For each ATP concentration used, the channel P o data over the range of [Ca2+]i employed could be well described by the activation Hill equation:
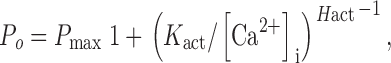 |
2 |
with P max ∼ 0.8 for all [ATP]i used (0–9.5 mM). Between [ATP]i of 0 and 500 μM, both K act and H act changed continuously (Fig. 4 A and Table ). At concentrations of ATP >500 μM, the activation of r-InsP3R-3 by Ca2+ exhibited no further systematic change (Fig. 4 A).
DISCUSSION
Inositol trisphosphate–mediated intracellular Ca2+ signaling is under complex regulation because the gating of the InsP3R Ca2+ release channel is sensitive to [Ca2+]i as well as to [InsP3]i (Bezprozvanny and Ehrlich 1995; Joseph 1995; Taylor and Traynor 1995). Gating of the InsP3-liganded channel requires Ca2+ binding to activation sites, whereas it is inhibited by Ca2+ binding to inhibition sites (Taylor and Marshall 1992; Iino and Tsukioka 1994; Mak et al. 1998), resulting in a biphasic dependence on [Ca2+]i. In patch-clamp studies of the endogenous X-InsP3R-1 channel (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998; Mak et al. 1998) or the recombinant r-InsP3R-3 channel (see Mak et al. 2001, in this issue) in the outer membrane of isolated Xenopus oocyte nuclei, gating of both the InsP3-liganded types 1 and 3 channels under optimal conditions exhibits robust activity, with a P max of ∼0.8 over a wide range of [Ca2+]i (Mak et al. 1998, Mak et al. 2001). In addition, InsP3 binding was found to activate both isoforms of the InsP3R by allosterically reducing the Ca2+ affinity of the inhibitory binding sites on the channel (Mak et al. 1998). When [InsP3]i is low, the channel is inhibited by Ca2+ because the Ca2+ affinity of the inhibitory site is higher than that of the activating site. At higher [InsP3]i, the channel becomes less sensitive to Ca2+ inhibition. When the affinity of the Ca2+ inhibitory site(s) falls below that of the activating site(s), the channel can become fully activated. Furthermore, the properties of the InsP3 binding site and the Ca2+ inhibition site are similar for the Xenopus type 1 and recombinant type 3 channels in the same nuclear membrane system: the functional affinity for InsP3 is ∼50 nM for both channels, and both channel isoforms exhibit K inh of ∼40–50 μM with Hill coefficient of 3–4 in the presence of 0.5 mM free ATP and saturating concentrations (∼10 μM) of InsP3.
In contrast, the properties of the Ca2+ activation sites differ between the two isoforms. In nuclear patch-clamp studies, the type 3 channel is uniquely distinguished from the type 1 channel by enhanced sensitivity of (K act of 77 nM instead of 190 nM) and lack of cooperativity between the Ca2+ activation sites (H act of 1 instead of ∼2) in the presence of 0.5 mM free ATP and saturating concentrations (10 μM) of InsP3 (Mak et al. 1998, Mak et al. 2001). As a result, the r-InsP3R-3 has a substantially higher P o than the endogenous X-InsP3R-1 in activating [Ca2+]i (<1 μM) in the presence of 0.5 mM ATP (Fig. 1). We have suggested that these properties endow the InsP3R-3 with high gain InsP3–induced Ca2+ release and low gain Ca2+–induced Ca2+ release properties, features which are complementary to the low gain InsP3–induced Ca2+ release and high gain Ca2+–induced Ca2+ release properties of InsP3R-1.
Our previous study of the regulation by ATP of the X-InsP3R-1 channel (Mak et al. 1999) revealed that the mechanism by which ATP stimulates gating of the InsP3R-1 involved increasing the affinity of the Ca2+ activating site of the channel specifically (i.e., decreasing the K act), without affecting H act or P max (Mak et al. 1999). Although channel P o decreased when [ATP]i was decreased, this could be fully reversed by increasing [Ca2+]i, demonstrating that ATP is not a necessary agonist for activation of the InsP3R, but is rather an allosteric regulator, tuning the efficacy of Ca2+ to stimulate the activity of the InsP3-liganded InsP3R-1 over a limited range of [Ca2+]i (10 nM to 1 μM).
Because the Ca2+ activation properties of channel gating was the major feature distinguishing the types 1 and 3 channels, and these properties of the type 1 channel were regulated by [ATP]i, we therefore investigated the effects of ATP on the gating of the type 3 channel.
ATP Tuning of the Affinities of the InsP3R Inhibitory Ca2+ Binding Sites
Our patch-clamp experimental data indicate that ATP decreases K inh of both types 1 and 3 InsP3R in very similar manner, whereas P max values remained unchanged (Table ). Thus, ATP reduces the channel activities of both InsP3R channels at [Ca2+]i > 10 μM by decreasing K inh, from ∼110 μM in the absence of ATP to ∼45 μM at 0.5 mM ATP (Fig. 1, Fig. 4, and Fig. 5). This reduction of K inh could not be reversed by the application of supersaturating [InsP3]i (10 μM) that was substantially higher than the half-maximal [InsP3]i ∼ 50 nM for X-InsP3R-1 (Mak et al. 1998) and r-InsP3R-3 (Mak et al. 2001). Thus, the more efficacious inhibition of InsP3R channel activities by high [Ca2+]i (>10 μM) in the presence of ATP is independent of any possible competitive inhibition of InsP3 binding to the InsP3R by ATP (Bezprozvanny and Ehrlich 1993; Hagar and Ehrlich 2000; Meas et al. 2000), and may be a mechanism through which [ATP]i can regulate feedback inhibition of InsP3R-mediated Ca2+ release.
Although binding assays have indicated that ATP can competitively inhibit binding of InsP3 to InsP3R-3 (Meas et al. 2000), no inhibition of the recombinant r-InsP3R-3 channel activities was observed in [Ca2+]i < 1 μM by even 9.5 mM ATP under our experimental conditions. This is likely due to our use of a supersaturating [InsP3]i (10 μM) that far exceeds the K IP3 of r-InsP3R-3 (∼55 nM; Mak et al. 2001). As InsP3 affects the InsP3R-3 channel activity solely by tuning the affinity of the inhibitory Ca2+ binding site(s) and has no effect on Ca2+ activation of the channel (Mak et al. 2001), effective InsP3 binding must be reduced to <0.3% (Mak et al. 2001) before effects of competitive inhibition of InsP3 binding by ATP would be observed in activating [Ca2+]i (<1 μM) in our experiments. This requires [ATP]i > 10 mM (Meas et al. 2000), which is higher than the range of [ATP]i used in our experiment. An inhibition by 7–10 mM ATP of InsP3R-3 single-channel activities in lipid bilayers in 160 nM Ca2+ and 2 μM InsP3 (Hagar and Ehrlich 2000) was probably caused by the reduced functional sensitivity to activation by InsP3 of the type 3 channel reconstituted into bilayers (EC50 of 3.2 μM in 160 nM Ca2+) compared with that observed in our experiments (see Mak et al. 2001, in this issue). In fact, enhancement, not inhibition, of InsP3R-3 channel activity by 10 mM ATP was also observed in the presence of 10 μM InsP3 in permeabilized cells using Ca2+ imaging (Miyakawa et al. 1999). Similarly, no evidence of ATP inhibition of InsP3 binding to InsP3R-1 was observed in our characterization of regulation by ATP (0–9.5 mM) of Ca2+ activation of X-InsP3R-1 (Mak et al. 1999), again because of application of supersaturating [InsP3] (Mak et al. 1998). Therefore, differential inhibition of InsP3 binding to types 1 and 3 InsP3R reported in Meas et al. 2000 has no impact on our characterization of ATP regulation of InsP3R channel activities in Mak et al. 1999 and this study.
ATP Enhancement of Ca2+ Activation of r-InsP3R-3 Channel
Our systematic single-channel patch-clamp experimental data demonstrated that, in the nuclear membrane system, cytoplasmic free ATP, but not MgATP, enhanced the activation by Ca2+ (<1 μM) of recombinant type 3 InsP3R channel through an increase of the Ca2+ affinity and decrease of the cooperativity of the activating sites of the channel.
The effects of cytoplasmic ATP on the activity of the InsP3R-3 have been studied previously primarily by Ca2+ release assays using cells that expressed endogenous type 3 InsP3R as the only (Miyakawa et al. 1999) or major (Missiaen et al. 1998; Meas et al. 2000) InsP3R isoform. Enhancement of Ca2+ release by ATP was observed in all the studies. The half-maximal ATP concentrations of 341 and 177 μΜ reported for ATP activation of Ca2+ release (Missiaen et al. 1998) and ATP inhibition of photoaffinity labeling (Meas et al. 2000), respectively, of InsP3R-3 are comparable to our experimental results with the activation of r-InsP3R-3 channel saturated by 0.5 mM ATP. Our patch-clamp data indicate that ATP decreases the apparent Hill coefficient of Ca2+ activation of the InsP3R-3 channel, whereas ATP did not apparently have such an effect on Ca2+ release in permeabilized 16HBE14o- cells (Missiaen et al. 1998). This difference is probably due to the very different experimental systems used. Whereas our patch-clamp experiments measure directly the single-channel activities of the InsP3R under rigorously controlled experimental conditions, measurements of Ca2+ flux characterize the activities of heterogeneous populations of unknown numbers of InsP3R containing various isoforms (Sienaert et al. 1998) and possibly heterooligomers, from which the single-channel activities of the InsP3R can only be inferred indirectly. The effects of cytoplasmic free ATP on InsP3-induced Ca2+ release in permeabilized B cells genetically engineered to express individual InsP3R isoforms was reported in Miyakawa et al. 1999. Under the experimental conditions used in that study (in 300 nM Ca2+), the reduction in InsP3-induced Ca2+ release when [ATP]i was decreased from 10 to 0 mM was smaller in cells expressing InsP3R-3 only than in cells expressing InsP3R-1 only. This result agrees qualitatively with our single-channel results. A similar change of [ATP]i decreased P o from ∼0.6 to ∼0.2 in r-InsP3R-3 (Fig. 4 A), whereas P o changed from 0.8 to 0.2 in X–InsP3R-1 (Mak et al. 1999).
Under our experimental conditions, only free ATP, not the MgATP complex, enhanced r-InsP3R-3 channel activity, as in the case for the X-InsP3R-1 (Mak et al. 1999). However, Ca2+ flux measurements in permeabilized cells suggested that MgATP also enhanced Ca2+ release mediated by the InsP3R-3 (Meas et al. 2000). This discrepancy may be due to the fact that single-channel P o is directly measured in our nuclear patch-clamp experiments, whereas Ca2+ flux measurements are affected by the Ca2+ conductance of the InsP3R channels as well as their P o. Because Mg2+ is a permeant blocking ion of the InsP3R (Mak and Foskett 1998; Mak et al. 2000), the presence of Mg2+ would be expected to reduce InsP3-induced Ca2+ flux through the InsP3R, as observed in Meas et al. 2000. Thus, addition of ATP could generate an apparent increase in the observed Ca2+ flux because the added ATP lowered the concentration of free Mg2+ by forming the MgATP complex, thus alleviating the Mg2+ blockage of the InsP3R.
Recently, the effects of ATP on InsP3R-3 channel properties were studied (Hagar and Ehrlich 2000) by reconstituting into planar lipid bilayers InsP3R in microsomes isolated from RIN-m5F cells that express mainly type 3 InsP3R (77–96%; Wojcikiewicz and He 1995; Swatton et al. 1999). Under the lipid bilayer experimental conditions, ATP (<6 mM) activated the channels by decreasing the mean closed channel durations and increasing the mean open channel durations, but did not affect the channel conductance. This agrees qualitatively with our patch-clamp results (Fig. 2, Fig. 4, and Fig. 6). However, the reconstituted InsP3R-3 in planar lipid bilayers exhibited a half-maximal activating [ATP]i of 2.8 mM, whereas the r-InsP3R-3 in our nuclear membrane patches was fully activated by 0.5 mM ATP (Fig. 4 A). The cause of the discrepancy between the two experimental systems is uncertain; the microenvironment (lipid membranes, buffer solutions, and transmembrane voltages) experienced by the InsP3R-3 channel in the two studies were very different. However, as the planar lipid bilayer system consistently recorded a significantly lower maximum P o (∼0.05) for both the type 3 (Hagar et al. 1998; Hagar and Ehrlich 2000) and type 1 (Kaftan et al. 1997) InsP3R than was observed in our experimental system (0.8 for both types 1 and 3 InsP3R), it is possible that cellular factors, like phosphatidylinositol 4,5-bisphosphate (Lupu et al. 1998), may be associated with the InsP3R reconstituted into the planar lipid bilayer system, and reduce the channel activities of the InsP3R and the efficacy of ATP to activate it.
Molecular Models for ATP Regulation of Ca2+ Activation of the InsP3R
In a previous study of ATP regulation of the single-channel activity of the X-InsP3R-1 (Mak et al. 1999), it was demonstrated that ATP activates the X-InsP3R-1 channel not by increasing the P max, but by increasing the apparent affinity of the activating Ca2+ binding site(s), i.e., decreasing K act. Within the range of [ATP]i used in that study (0–9.5 mM), [ATP]i had no observable effect on the value of H act. The data could be interpreted by an empirical model described by a modified Michaelis-Menten equation, in which [ATP]i only affects the functional affinity of the activating Ca2+ binding site(s) of the X-InsP3R-1 with no effects on the cooperativity of those sites (Mak et al. 1999).
In contrast, the regulation by ATP of the r-InsP3R-3 observed in this study is dramatically different. Whereas P max of the r-InsP3R-3 was similarly unaffected by [ATP]i, both H act and K act of the type 3 channel were reduced by ATP. Furthermore, these effects of ATP on the Ca2+ activation of the r-InsP3R-3 were saturated by 0.5 mM ATP (Fig. 4 A), whereas increasing [ATP]i up to several mM continued to further decrease K act of the type 1 channel (Mak et al. 1999). Of particular interest is that the Ca2+ activation responses of the two isoforms become essentially the same in the absence of ATP (Fig. 5). Remarkably, therefore, the major feature distinguishing the types 1 and 3 channel isoforms (Mak et al. 2001) is dependent on the presence of ATP. In the absence of ATP, the permeation and gating behaviors of the two isoforms are indistinguishable in our nuclear patch-clamp studies.
How can we account for the distinct regulation by ATP of the Ca2+ activation properties of the two channel isoforms? Analysis of the primary sequence of the type 1 InsP3R (Mignery et al. 1990) revealed two putative ATP binding sites (Yamada et al. 1994), only one of which is conserved in the sequence of the type 3 InsP3R (Maranto 1994; Yamada et al. 1994; Yamamoto-Hino et al. 1994). Glutathione-S-transferase (GST)–fusion proteins containing the putative type 1–specific ATP-binding sequence or the ATP-binding sequence present in both types 1 and 3 InsP3R have both been shown to bind ATP in vitro (Maes et al. 1999). Therefore, it is possible that the functional ATP binding sites responsible for the regulation by ATP are distinct between the types 1 and 3 channels. Accordingly, whereas ATP binds with a functional affinity of ∼0.27 mM to the functional ATP binding site in type 1 InsP3R and increases the sensitivity of the channel to Ca2+ activation without affecting the cooperativity of Ca2+ activation (Mak et al. 1999), it might bind with a higher affinity to a different functional ATP binding site in the type 3 InsP3R. In this model, binding to this distinct site in the type 3 channel would change, through a different molecular mechanism, the number and cooperativity of Ca2+ binding site(s) involved in the Ca2+ activation of the r-InsP3R-3, as well as the sensitivity of the channel to Ca2+ activation.
Alternately, the regulation of InsP3R by ATP and Ca2+ can be accounted for by the molecular Monad-Wyman-Changeux (MWC) model (Monod et al. 1965) for allosteric systems. In this allosteric model, the InsP3R channel can exist in two conformations, one active and one inactive. In the absence of ligands, the channel mostly exists in the inactive conformation. Both ATP and Ca2+ regulate the InsP3R channel as activating heterotropic ligands (Monod et al. 1965) by preferentially binding to and stabilizing the active conformation of the channel. Although not a general feature of the MWC model, our experiment results showed that ATP and Ca2+ are not equivalent heterotropic ligands of the InsP3R channel. The InsP3R channel had low P o at low [Ca2+]i despite the presence of saturating [ATP]i, whereas the channel exhibited high P o at optimal [Ca2+]i even in the absence of ATP (Fig. 4 A and 5; Mak et al. 1999). To account for this non-equivalence in a modified MWC model, we assume that Ca2+ must bind to one or more of the activating Ca2+ binding sites in the channel before the channel can be active, whereas ATP binding is not necessary.
In the MWC model, InsP3R channel activity can exhibit a dependence on the concentration of one of its ligands (Ca2+) with a Hill coefficient >1 regardless of the number of Ca2+ required to bind to the channel to open it (our unpublished data). The MWC model also predicts that the apparent half-maximal activating concentration (K act) of one ligand (Ca2+) can vary in the presence of different concentrations of the other ligand (ATP), even though the dissociation constants for the ligands of both conformations of the channel remains unchanged. Furthermore, heterotropic effects of Ca2+ and ATP on the InsP3R channel can change the Hill coefficient for Ca2+ activation (H act) of the channel without changing the number of Ca2+ required to bind to the channel before it can adopt the active conformation. Thus, according to the MWC model, binding of ATP, a heterotropic ligand, to the r-InsP3R-3 channel can abolish the cooperativity of Ca2+ and simultaneously decrease its half-maximal activating concentration (Monod et al. 1965). The magnitudes of changes in the observed K act and H act for Ca2+ activation of an InsP3R isoform due to heterotropic effects of ATP, and the range of [ATP]i over which the changes occur, will depend on relevant parameters of that isoform, including the relative stability of the active and inactive conformations, and the affinities of those conformations for the ligands. With a different set of parameters for the type 1 InsP3R, the binding of ATP can continuously change the observed K act for Ca2+ activation over a wide range of [ATP]i without affecting the value of H act observably. Therefore, despite the observed differences in the regulation by ATP of the Ca2+ activation of the types 1 and 3 InsP3R, it is possible that ATP regulates Ca2+ activation of the two InsP3R isoforms through the same MWC allosteric mechanism, with the different channel isoforms possessing different sets of relevant parameters. Because there are a large number of parameters involved in a MWC model for a tetrameric channel interacting with two ligands (Changeux and Edelstein 1998; Jones 1999), detailed numerical fittings by the MWC model of the r-InsP3R-3 channel open probability and dwell time distribution data, similar to those performed in Rothberg and Magleby 1999, will be necessary to determine if the regulation by ATP and Ca2+ of channel gating of InsP3R (both types 1 and 3) can be well described by such a model.
Differential Regulation by ATP of Ca2+ Activation of the Types 1 and 3 InsP3R Isoforms
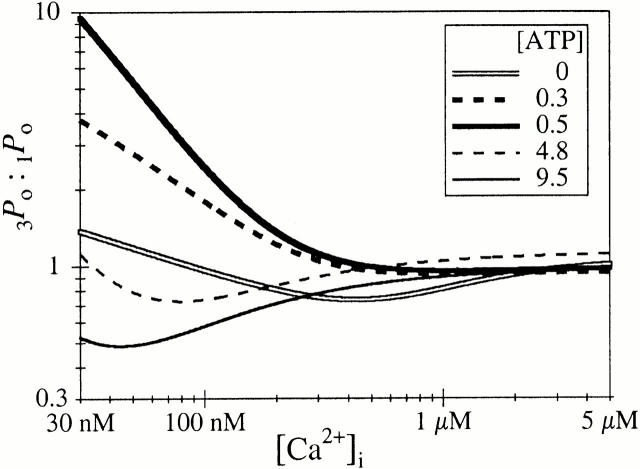
We characterized previously the permeation properties, propensity to cluster, and regulation by Ca2+ and InsP3 of the type 3 InsP3R channel (Mak et al. 2000), and concluded that the only parameter that distinguishes the types 1 and 3 isoforms in the same membrane under identical experimental conditions is their Ca2+ activation properties. However, the results of this study reveal that cytoplasmic ATP is critical to establishing this difference between the Ca2+ responses of the two isoforms. In the absence of ATP, the biphasic Ca2+ responses of the X-InsP3R-1 and r-InsP3R-3 are very similar (Fig. 5). This may have important consequences in cells that express both isoforms. As a result of the difference in the regulation of the two InsP3R isoforms by ATP, the relative level of activation of the two InsP3-liganded isoforms by [Ca2+]i (between 10 and 1,000 nM) will vary in a complex pattern with changes in [ATP]i, as depicted in Fig. 7. When [ATP]i is <0.5 mM, the InsP3R-1 channel is mostly less sensitive to activation by Ca2+ than is InsP3R-3. This is because ATP decreases the K act of InsP3R-3 to a greater extent than that of InsP3R-1, and decreases H act of InsP3R-3 but does not affect that of InsP3R-1. Whereas increases of [ATP]i (from 0.5 to 9.5 mM) continue to decrease K act of the type 1 channel (Mak et al. 1999), the effects of ATP on Ca2+ activation of the type 3 channel are saturated at 500 μM. Thus, at 4.8 mM ATP, P o of InsP3R-1 in [Ca2+]i > 35 nM is higher than that of InsP3R-3, although the type 3 channel is still more active than the type 1 isoform in [Ca2+]i < 35 nM. At 9.5 mM ATP, InsP3R-1 is more active than InsP3R-3 in most [Ca2+]i.
Figure 7.
Variation of the ratio between the P o of r-InsP3R-3 (3 P o) and that of X–InsP3R-1 (1 P o), with [Ca2+]i in the presence of different [ATP]i as tabulated. P o are calculated with , using the parameters (P max, K act, and H act) tabulated in Table . 3 P o for 4.8 and 9.5 mM ATP were calculated using the same parameters as in 0.5 mM ATP. P max of X-InsP3R-1 was assumed to be 0.8 in 0.3 mM ATP.
Whereas the MgATP concentration in the cytoplasm is in the range of 3–8 mM (Corkey et al. 1986; Dunne et al. 1988; Flatman 1991; Kargacin and Kargacin 1997; Maechler et al. 1998), the concentration of ATP4−, the ligand of the InsP3R (Mak et al. 1999; and this study), is in the range of 400–600 μM. Thus, under normal physiological conditions in the cytoplasm, the type 3 channel will be more sensitive than the type 1 channel to activation by Ca2+. Nevertheless, with the close physical proximity of the ER and mitochondria (Otsu et al. 1990; Satoh et al. 1990; Simpson et al. 1997; Rizzuto et al. 1998), it is possible that the concentration of free ATP in the microdomains in which the InsP3R are located may vary over a wide range without substantial change in the overall cytoplasmic ATP concentration, so that [ATP]i detected by the InsP3R may be elevated to levels at which the type 1 InsP3R becomes more active than the type 3 InsP3R. Thus, the complex dependencies of the types 1 and 3 InsP3R channel activities on [ATP]i, coupled with differential levels of expression and subcellular localization, may enable the cell to generate sophisticated patterns of Ca2+ signals with cytoplasmic ATP as one of the second messengers.
Acknowledgments
We thank Dr. Graeme Bell (University of Chicago, Chicago, IL) for providing r-InsP3R-3 cDNA.
This work was supported by grants to J.K. Foskett from the National Institutes of Health (MH59937 and GM56328) and to D.-O.D. Mak from the American Heart Association (9906220U).
Footnotes
Abbreviations used in this paper: ER, endoplasmic reticulum; InsP3, inositol 1,4,5-trisphosphate; MWC, Monad-Wyman-Changeux; P o, open probability; r-InsP3R-3, rat type 3 InsP3R; X-InsP3R-1, Xenopus type 1 InsP3R.
References
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. ATP modulates the function of inositol 1,4,5-trisphosphate-gated channels at two sites. Neuron. 1993;10:1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 1995;145:205–216. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- Changeux J.-P., Edelstein S.J. Allosteric receptors after 30 years. Neuron. 1998;21:959–980. doi: 10.1016/s0896-6273(00)80616-9. [DOI] [PubMed] [Google Scholar]
- Corkey B.E., Duszynski J., Rich T.L., Matschinsky B., Williamson J.R. Regulation of free and bound magnesium in rat hepatocytes and isolated mitochondria. J. Biol. Chem. 1986;261:2567–2574. [PubMed] [Google Scholar]
- Dunne M.J., West-Jordan J.A., Abraham R.J., Edwards R.T.H., Petersen O.H. The gating of nucleotide-sensitive K+ channels in insulin-secreting cells can be modulated by changes in the ratio of ATP4−/ADP3− and by non-hydrolyzable derivatives of both ATP and ADP. J. Membr. Biol. 1988;104:165–172. doi: 10.1007/BF01870928. [DOI] [PubMed] [Google Scholar]
- Flatman P.W. Mechanisms of magnesium transport. Annu. Rev. Physiol. 1991;53:259–271. doi: 10.1146/annurev.ph.53.030191.001355. [DOI] [PubMed] [Google Scholar]
- Hagar R.E., Burgstahler A.D., Nathanson M.H., Ehrlich B.E. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar R.E., Ehrlich B.E. Regulation of the type III InsP3 receptor by InsP3 and ATP. Biophys. J. 2000;79:271–278. doi: 10.1016/S0006-3495(00)76289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Tsukioka M. Feedback control of inositol trisphosphate signalling by calcium. Mol. Cell. Endocrinol. 1994;98:141–146. doi: 10.1016/0303-7207(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Jones S.W. Commentarya plausible model. J. Gen. Physiol. 1999;114:271–275. doi: 10.1085/jgp.114.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.K. The inositol trisphosphate receptor family. Cell Signalling. 1995;8:1–7. doi: 10.1016/0898-6568(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Kaftan E.J., Ehrlich B.E., Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J. Gen. Physiol. 1997;110:529–538. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargacin M.E., Kargacin G.J. Predicted changes in concentrations of free and bound ATP and ADP during intracellular Ca2+ signaling. Am. J. Physiol. 1997;273:C1416–C1426. doi: 10.1152/ajpcell.1997.273.4.C1416. [DOI] [PubMed] [Google Scholar]
- Lupu V.D., Kaznacheyeva E., Krishna U.M., Falck J.R., Bezprozvanny I. Functional coupling of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1998;271:14067–14070. doi: 10.1074/jbc.273.23.14067. [DOI] [PubMed] [Google Scholar]
- Maechler P., Wang H.Y., Wollheim C.B. Continuous monitoring of ATP levels in living insulin secreting cells expressing cytosolic firefly luciferase. FEBS Lett. 1998;422:328–332. doi: 10.1016/s0014-5793(97)01618-9. [DOI] [PubMed] [Google Scholar]
- Maes K., Missiaen L., Parys J.B., Sienaert I., Bultynck G., Zizi M., De Smet P., Casteels R., De Smedt H. Adenine-nucleotide binding sites on the inositol 1,4,5-trisphosphate receptor bind caffeine, but not adenophostin A or cyclic ADP-ribose. Cell Calcium. 1999;25:143–152. doi: 10.1054/ceca.1998.0011. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D, Foskett J.K. Effects of divalent cations on single-channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 1998;275:C179–C188. doi: 10.1152/ajpcell.1998.275.1.C179. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca2+ activation. J. Biol. Chem. 1999;274:22231–22237. doi: 10.1074/jbc.274.32.22231. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Raghuram V., Yue Y., Joseph S.K., Foskett J.K. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 2000;115:241–255. doi: 10.1085/jgp.115.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. Regulation by Ca2+ and inositol 1,4,5-trisphosphate (InsP3) of single recombinant type 3 InsP3 receptor channelsCa2+ activation uniquely distinguishes types 1 and 3 InsP3 receptors. J. Gen. Physiol. 2001;117:435–446. doi: 10.1085/jgp.117.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto A.R. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J. Biol. Chem. 1994;269:1222–1230. [PubMed] [Google Scholar]
- Meas K., Missiaen L., De Smet P., Vanlingen G., Callewaert G., Parys J.B., De Smedt H. Differential modulation of inositol 1,4,5-trisphosphate receptor type 1 and type 3 by ATP. Cell Calcium. 2000;27:257–267. doi: 10.1054/ceca.2000.0121. [DOI] [PubMed] [Google Scholar]
- Mignery G.A., Newton C.L., Archer B.T.I, Sudhof T.C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1990;265:12679–12685. [PubMed] [Google Scholar]
- Missiaen L., Parys J.B., Sienaert I., Maes K., Kunzelmann K., Takahashi M., Tanzawa K., De Smedt H. Functional properties of the type-3 InsP3 receptor in 16HBE14o- bronchial mucosal cells. J. Biol. Chem. 1998;273:8983–8986. doi: 10.1074/jbc.273.15.8983. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosake T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.-P. On the nature of allosteric transitionsa plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Otsu H., Yamamoto A., Maeda N., Mikoshiba K., Tashiro Y. Immunogold localization of inositol 1,4,5-triphosphate (InsP3) receptor in mouse cerebellar Purkinje cells using three monoclonal antibodies. Cell Struct. Funct. 1990;15:163–173. doi: 10.1247/csf.15.163. [DOI] [PubMed] [Google Scholar]
- Putney J.W., Jr., St. J. Bird G. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr. Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- Rothberg B.S., Magleby K.L. Gating kinetics of single large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism. J. Gen. Physiol. 1999;114:93–124. doi: 10.1085/jgp.114.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Ross C.A., Villa A., Supattapone S., Pozzan T., Snyder S.H., Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cellsquantitative immunogold labeling reveals concentration in an ER subcompartment. J. Cell Biol. 1990;111:615–624. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienaert I., Huyghe S., Parys J.B., Malfait M., Kunzelmann K., De Smedt H., Verleden G.M., Missiaen L. ATP-induced Ca2+ signals in bronchial epithelial cells. Pflügers Arch. 1998;436:40–48. doi: 10.1007/s004240050602. [DOI] [PubMed] [Google Scholar]
- Simpson P.B., Mehotra S., Lange G.D., Russell J.T. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J. Biol. Chem. 1997;272:22654–22661. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- Swatton J.E., Morris S.A., Cardy T.J.A., Taylor C.W. Type 3 inositol trisphosphate receptors in RINm5F cells are biphasically regulated by cytosolic Ca2+ and mediate quantal Ca2+ mobilization. Biochem. J. 1999;344:55–60. [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Marshall I.C.B. Calcium and inositol 1,4,5-trisphosphate receptorsa complex relationship. Trends Biochem. Sci. 1992;17:403–407. doi: 10.1016/0968-0004(92)90009-x. [DOI] [PubMed] [Google Scholar]
- Taylor C.W., Traynor D. Calcium and inositol trisphosphate receptors. J. Membr. Biol. 1995;145:109–118. doi: 10.1007/BF00237369. [DOI] [PubMed] [Google Scholar]
- Taylor C.W., Richardson A. Structure and function of inositol trisphosphate receptors. Pharmacol. Ther. 1991;51:97–137. doi: 10.1016/0163-7258(91)90043-l. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H., He Y. Type I, II and III inositol 1,4,5-trisphosphate receptor coimmunoprecipitation as evidence for the existence of heterotetrameric receptor complexes. Biochem. Biophys. Res. Commun. 1995;213:334–341. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- Yamada N., Makino Y., Clark R.A., Pearson D.W., Mattei M.-G., Guénet J.-L., Ohama E., Fujino I., Miyawaki A., Furuichi T., Mikoshiba K. Human inositol 1,4,5-trisphosphate type-1 receptor, InsP 3R1structure, function, regulation of expression and chromosomal localization. Biochem. J. 1994;302:781–790. doi: 10.1042/bj3020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Hino M., Sugiyama T., Hikichi K., Mattei M.G., Hasegawa K., Sekine S., Sakurada K., Miyawaki A., Furuichi T., Hasegawa M., Mikoshiba K. Cloning and characterization of human type 2 and type 3 inositol 1,4,5-trisphosphate receptors. Recept. Chan. 1994;2:9–22. [PubMed] [Google Scholar]