Abstract
The inositol 1,4,5-trisphosphate (InsP3) receptor (InsP3R) is an endoplasmic reticulum–localized Ca2+-release channel that controls complex cytoplasmic Ca2+ signaling in many cell types. At least three InsP3Rs encoded by different genes have been identified in mammalian cells, with different primary sequences, subcellular locations, variable ratios of expression, and heteromultimer formation. To examine regulation of channel gating of the type 3 isoform, recombinant rat type 3 InsP3R (r-InsP3R-3) was expressed in Xenopus oocytes, and single-channel recordings were obtained by patch-clamp electrophysiology of the outer nuclear membrane. Gating of the r-InsP3R-3 exhibited a biphasic dependence on cytoplasmic free Ca2+ concentration ([Ca2+]i). In the presence of 0.5 mM cytoplasmic free ATP, r-InsP3R-3 gating was inhibited by high [Ca2+]i with features similar to those of the endogenous Xenopus type 1 InsP3R (X-InsP3R-1). Ca2+ inhibition of channel gating had an inhibitory Hill coefficient of ∼3 and half-maximal inhibiting [Ca2+]i (K inh) = 39 μM under saturating (10 μM) cytoplasmic InsP3 concentrations ([InsP3]). At [InsP3] < 100 nM, the r-InsP3R-3 became more sensitive to Ca2+ inhibition, with the InsP3 concentration dependence of K inh described by a half-maximal [InsP3] of 55 nM and a Hill coefficient of ∼4. InsP3 activated the type 3 channel by tuning the efficacy of Ca2+ to inhibit it, by a mechanism similar to that observed for the type 1 isoform. In contrast, the r-InsP3R-3 channel was uniquely distinguished from the X-InsP3R-1 channel by its enhanced Ca2+ sensitivity of activation (half-maximal activating [Ca2+]i of 77 nM instead of 190 nM) and lack of cooperativity between Ca2+ activation sites (activating Hill coefficient of 1 instead of 2). These differences endow the InsP3R-3 with high gain InsP3–induced Ca2+ release and low gain Ca2+–induced Ca2+ release properties complementary to those of InsP3R-1. Thus, distinct Ca2+ signals may be conferred by complementary Ca2+ activation properties of different InsP3R isoforms.
Keywords: single-channel electrophysiology, patch-clamp, Xenopus oocyte, nucleus, Ca2+ release channel
INTRODUCTION
Modulation of cytoplasmic free Ca2+ concentration ([Ca2+]i) in response to the second messenger inositol 1,4,5-trisphosphate (InsP3) provides a ubiquitous signaling system. InsP3-mediated Ca2+ signals are often complex, being precisely controlled in both time and space as repetitive spikes or oscillations and as propagating waves that initiate at specific locations in the cell (Boitano et al. 1992; Lechleiter and Clapham 1992; Amundson and Clapham 1993; Atri et al. 1993; Berridge 1993; Rooney and Thomas 1993; Bootman and Berridge 1995; Clapham 1995; Toescu 1995). A family of InsP3 receptors (InsP3Rs) with different primary sequences derived from different genes has been identified (Furuichi et al. 1989; Mignery et al. 1989; Sudhof et al. 1991; Blondel et al. 1993; De Smedt et al. 1994; Maranto 1994) with alternatively spliced isoforms (Danoff et al. 1991; Nakagawa et al. 1991; Ferris and Snyder 1992). The InsP3Rs are ∼2,700 amino acid integral membrane proteins (Furuichi et al. 1994) that exist as tetramers (Supattapone et al. 1988; Maeda et al. 1991) in the endoplasmic reticulum (ER). Full-length sequences of cDNAs for three distinct isoforms (InsP3R-1, InsP3R-2, and InsP3R-3) are 60–80% homologous (Furuichi et al. 1989; Mignery et al. 1989; Sudhof et al. 1991; De Smedt et al. 1994; Maranto 1994; Joseph 1995). The different isoforms have distinct and overlapping patterns of expression in different tissues (Maranto 1994; Fujino et al. 1995; Furuichi and Mikoshiba 1995). Most cells express more than one isoform (Bush et al. 1994; De Smedt et al. 1994; Newton et al. 1994; Sugiyama et al. 1994; Fujino et al. 1995; Joseph et al. 1995; Nucifora et al. 1996), and expression levels, both absolute and relative to other isoforms, can be modified during cell differentiation (Nakagawa et al. 1991; Kume et al. 1993) and by use-dependent degradation (Magnusson et al. 1993; Wojcikiewicz et al. 1994; Honda et al. 1995; Wojcikiewicz 1995). In tissues that express more than one type of InsP3R, isoform-specific antibodies immunoprecipitate others, suggesting that receptors may associate in heteroligomeric complexes (Joseph et al. 1995; Monkawa et al. 1995; Wojcikiewicz and He 1995; Nucifora et al. 1996).
The diversity of InsP3R expression in mammalian cells is impressive, suggesting that cells require distinct InsP3Rs to provide unique Ca2+ signals and to regulate specific functions. Nevertheless, the functional correlates and physiological implications of this diversity are still unclear. Electrophysiological observations of all three isoforms have now been reported. The single-channel properties of the type 1 InsP3R have been examined by reconstitution of mammalian channels in lipid bilayer membranes (Bezprozvanny et al. 1991, Bezprozvanny et al. 1994; Watras et al. 1991; Bezprozvanny and Ehrlich 1994), as well as by patch-clamp of the outer nuclear membrane of Xenopus oocytes (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998; Stehno-Bittel et al. 1995). The type 2 receptor was recently examined by bilayer reconstitution (Perez et al. 1997; Ramos-Franco et al. 1998). To date, there have been two sets of reports of type 3 channel activity. In one, bilayer reconstitution of membranes from a cell type which expressed more type 3 relative to other isoforms was used (Hagar et al. 1998; Hagar and Ehrlich 2000); the other used patch-clamp electrophysiology of the outer nuclear membrane of Xenopus oocytes engineered to express the recombinant rat type 3 receptor (Mak et al. 2000). Together, these studies have demonstrated that the ion permeation properties of the different InsP3R isoforms are highly conserved, even across species (Mak et al. 2000). These results have therefore suggested that distinctions among channel isoforms may instead reside in their differential regulation or intracellular localization. To begin to address this issue, we describe here the regulation by [Ca2+]i and cytoplasmic InsP3 concentration ([InsP3]) of the gating of recombinant rat type 3 InsP3R (r-InsP3R-3) expressed in Xenopus oocytes. We used patch-clamp electrophysiology to study single recombinant channels in the outer membrane of the nuclear envelope of isolated Xenopus oocyte nuclei. In addition, the results obtained in this study have been compared with those obtained from the Xenopus type 1 InsP3R (X-InsP3R-1) in the same physiologically relevant membrane system (Mak et al. 1998). Our results reveal that, in addition to sharing similar permeation and gating properties, the two isoforms have highly similar responses to InsP3 and inhibition by high [Ca2+]i. However, important differences exist in the responses of the two isoforms to activation by [Ca2+]i. Distinct Ca2+ activation responses confer on these channels unique Ca2+-induced Ca2+ release (CICR) properties, which likely contribute to distinct spatial and temporal Ca2+ signals in cells expressing different and multiple InsP3R isoforms.
MATERIALS AND METHODS
Selection and Microinjection of Xenopus Oocytes
Maintenance of Xenopus laevis and surgical extraction of ovaries were carried out as described previously (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998). Because oocytes have endogenous InsP3R (X-InsP3R-1), it was necessary to distinguish them from expressed channels in our patch-clamp experiments (Mak et al. 2000). Endogenous X-InsP3R-1 channel activity detected in patch-clamp studies of oocyte nuclei is highly variable from batch to batch of oocytes, although the activity level among oocytes from the same batch is very consistent (Mak and Foskett 1994). Therefore, for each new batch of oocytes, a day of patch-clamping of isolated nuclei (at least 6 nuclei, 6–10 patches from each) was performed, to determine the endogenous channel expression level. Only batches with extremely low channel activity (<1 out of 15 patches exhibited InsP3R channel activity) were used for subsequent cRNA injections. Out of 496 nuclear patches from uninjected oocytes in selected batches, only 19 channels were detected in 11 patches. The mean number of InsP3R channels per nuclear patch was 0.038.
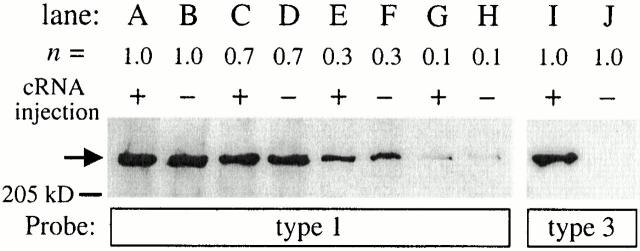
Oocytes selected for microinjection were defolliculated as described previously (Jiang et al. 1998). 23 nl of rat InsP3R-3 cRNA (1 μg/μl) was injected into the cytoplasm of defolliculated oocytes as described previously (Mak et al. 2000). cRNA-injected and uninjected but defolliculated control oocytes were placed in individual wells in 96-well plates containing 200 μl of ASOS (100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 5 mM HEPES, pH adjusted to 7.6 with NaOH; with 3 mM sodium pyruvate, 100 μg/ml gentamycin, and 100 μM N-acetyl-Leu-Leu-Norleucinal [Sigma-Aldrich]). 80 μl of ASOS in each well was changed daily. Nuclear patch-clamp experiments performed at various times after surgical extraction of the oocytes using control, uninjected oocytes maintained under identical conditions as cRNA-injected oocytes demonstrated that the probability of detection of endogenous InsP3R channel in a nuclear patch never increased over time after oocyte isolation (Mak et al. 2000). Furthermore, Western blot analysis (Fig. 1) showed that injection of the recombinant r-InsP3R-3 cRNA induced the expression of type 3 InsP3R in oocytes (Fig. 1, lanes I and J) without causing any detectable increase in the level of expression of the endogenous type 1 InsP3R (Fig. 1, lanes A, C, E, and G) compared with uninjected control oocytes (Fig. 1, lanes B, D, F, and H). The Western blots also showed that the InsP3R-1– and InsP3R-3–specific antibodies used in our experiments had very little cross-reactivity.
Figure 1.
Expression of endogenous X-InsP3R-1 and recombinant r-InsP3R-3 in cRNA-injected (+) and control, uninjected (−) Xenopus oocytes. Western analysis was performed as described in Mak et al. 2000. (A–H) Immunoblotted with InsP3R-1-specific antibody (Joseph and Samanta 1993; Joseph et al. 1995); (I–J) immunoblotted with InsP3R-3-specific antibody (Transduction Labs.). Aliquots equivalent to n oocytes from the same lysate sample were used in the lanes.
Patch-clamp studies were performed 4–5 d after cRNA microinjection. The mean number of InsP3R per nuclear patch for the cRNA-injected oocytes increased dramatically by 47-fold, from 0.038 to 1.80. In 1,020 experiments, 1,831 channels were detected in 518 patches, with 354 of the patches exhibiting multiple InsP3R channels. If we assume a random, binomial association of X-InsP3R-1 and r-InsP3R-3 to form tetrameric channels, most (91.8%) of the channels detected in cRNA-injected oocytes were homotetrameric r-InsP3R-3 channels. This value probably underestimates the percentage of homotetrameric r-InsP3R-3 channels because of the higher probability of heterologously expressed channels to associate with other heterologously expressed channel monomers during protein biogenesis rather than with endogenous channels, due to the pronounced mismatch of the protein translation rates of expressed versus endogenous channels (Joseph et al. 2000).
Patch-clamping the Oocyte Nucleus
Patch-clamp experiments were performed as described (Mak and Foskett 1994, Mak and Foskett 1997, Mak and Foskett 1998). In brief, stage V or VI oocytes were opened mechanically just before use, and the nucleus was separated from the cytoplasm for patch-clamping. Like the X-IP3R-1 (Mak and Foskett 1994, Mak and Foskett 1997), the r-InsP3R-3 inactivated under constant [Ca2+]i, [InsP3], and applied potential (mean channel activity duration ∼2 min). Thus, experiments were done in the “on-nucleus” configuration, with the solution in the perinuclear lumen between the outer and inner nuclear membranes in apparent equilibrium with the bath solution (Mak and Foskett 1994). As inactivation was generally abrupt, with no detectable change in channel kinetics up to the disappearance of channel activity (Mak and Foskett 1997), kinetic measurements were made during the entire period the channels were active. All experiments were performed at room temperature with the pipet electrode at +20 mV relative to the reference bath electrode. Each experiment recorded the InsP3R channel activity at a specific [Ca2+]i and [InsP3], with no change of the pipet solution. Data acquisition was performed as described previously (Mak et al. 1998), with currents recorded with a filtering frequency of 1 kHz and a digitizing frequency of 5 kHz.
Analyses of Patch-clamp Current Traces
The patch-clamp current traces were analyzed using MacTac software (Bruxton) to identify channel opening and closing events using a 50% threshold (Mak et al. 1999). Current traces exhibiting one InsP3R channel, or two InsP3R channels determined to be identical and independently gated (Mak and Foskett 1997), were used for open probability (P o) evaluation, whereas only current traces with a single InsP3R channel were used for dwell time analysis. The number of channels in the membrane patch was assumed to be the maximum number of open channel current levels observed throughout the current record. Assuming that there are n identical and independent channels in the membrane patch, and each of the channels is Markovian with open probability of P o and open duration distribution characterized by a single exponential component of time constant τ, the mean dwell time of highest channel current level is τ/n (). If T is the minimum duration of an open event that is detectable in the experimental system, i.e., only events with duration longer than T will have amplitudes greater than the 50% threshold after filtering, then the rate of detection of the highest current level:
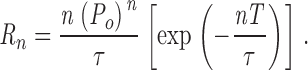 |
1 |
In our patch-clamp set up, T was empirically determined to be 0.2 ms using test pulses of variable duration. τ of InsP3R channels is ∼3–15 ms over the range of experimental conditions used. In experimental conditions with P o > 0.1, only current records with longer than 10 s of InsP3R channel activities were used. 10 s ≫ 1/R 3, so there is little uncertainty in the number of channels in the current traces used. In experimental conditions with P o < 0.1, only current records exhibiting one open channel current level with record duration >5/R 2 were used, to ensure that they were truly single-channel records.
Multiple conductance states were observed for recombinant r-InsP3R-3 (Mak et al. 2000). Only current traces exhibiting only the predominant main (M) conductance state were used for analyses. Each data point shown is the mean of results from at least four separate patch-clamp experiments performed under the same conditions. Error bars indicate the SEM.
Solutions for Patch-clamp Experiments
All patch-clamp experiments were performed with pipet solutions containing 140 mM KCl, 10 mM HEPES, and 0.5 mM Na2ATP, pH adjusted to 7.1 with KOH. By using K+ as the current carrier and appropriate quantities of the high affinity Ca2+ chelator, BAPTA (1,2-bis(O-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid [100–500 μM]; Molecular Probes), or the low affinity Ca2+ chelator, 5,5′-dibromo BAPTA (100–160 μM; Molecular Probes), or ATP (0.5 mM) alone to buffer Ca2+ in the experimental solutions, Ca2+ concentration was tightly controlled in our experiments. Total Ca2+ content (64–306 μM) in the solutions was determined by induction-coupled plasma mass spectrometry (Mayo Medical Laboratory). Free [Ca2+] were calculated using the Maxchelator software (C. Patton, Stanford University, Stanford, CA). The free [Ca2+] of all solutions was also directly measured, using Ca2+-selective minielectrodes (Baudet et al. 1994), and found to agree with the calculated [Ca2+] to within the accuracy of the electrode measurement (10%). Pipet solutions contained various concentrations of InsP3 (Molecular Probes) used with no further purification. The bath solutions used in all experiment had the same composition as the pipet solutions, except they lacked Na2ATP and had a free Ca2+ concentration of 220 nM.
RESULTS
Ca2+ Dependence of the Kinetic Properties of r-InsP3R-3 Gating
Gating of the InsP3R is sensitive to [Ca2+]i as well as [InsP3] (Bezprozvanny and Ehrlich 1995; Joseph 1995; Taylor and Traynor 1995). Low [Ca2+]i stimulate InsP3-activated channels, whereas higher [Ca2+]i are inhibitory (Taylor and Marshall 1992; Iino and Tsukioka 1994; Mak et al. 1998). The biphasic effects of [Ca2+]i on InsP3-mediated Ca2+ release are believed to underlie oscillations, waves, and transitions from localized to global cellular responses (Berridge 1993; Putney and St. J. Bird 1993; Toescu 1995). Whereas it is generally agreed that the type 1 isoform is inhibited by high [Ca2+]i, it has been suggested that the types 2 (Ramos-Franco et al. 1998) and 3 (Hagar et al. 1998) isoforms are not. In the X-InsP3R-1, Ca2+ is a true agonist (it gates the channel directly by binding to the channel), whereas InsP3 allosterically activates the Ca2+-liganded channel by reducing the Ca2+ affinity of the inhibitory binding sites on the channel (Mak et al. 1998). Because of the central role of [Ca2+]i in regulating the channel, we systematically investigated the effects of [Ca2+]i on the kinetic properties of the r-InsP3R-3 in native ER membrane.
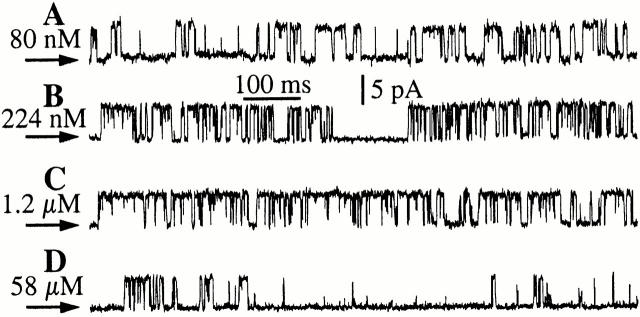
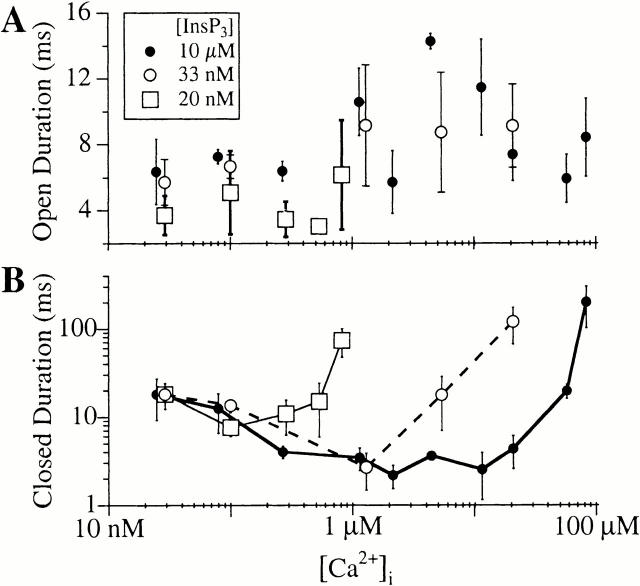
To examine specifically the effects of [Ca2+]i on r-InsP3R-3 channel gating, a functionally saturating concentration of InsP3 (10 μM) was applied to the cytoplasmic (pipet) side of the channel to stimulate it fully at all experimental [Ca2+]i (Fig. 2). At [Ca2+]i corresponding to resting levels in cells (10–100 nM), the P o of the r-InsP3R-3 channel was moderate (<0.5; Fig. 4), with the channel evidently active (Fig. 2 A). The P o increased to ∼0.8 when [Ca2+]i was raised from 100 nM to 1 μM, which was associated with decreasing mean closed duration (τc) (Fig. 3 and Fig. 4). Between [Ca2+]i of 1 and 25 μM, P o remained high (∼0.8; Fig. 3 and Fig. 4), with the channel exhibiting long sustained bursts of activities lasting up to several seconds, during which it only closed briefly (Fig. 2 C). As [Ca2+]i was increased beyond 25 μM, P o dropped precipitously, as a result of an increase in τc to >200 ms (Fig. 3 and Fig. 4). Within the more than three orders of magnitude range of [Ca2+]i examined (24.7 nM–82.8 μM), the mean open duration (τo) of the r-InsP3R-3 channel lay within a narrow range (4–16 ms) with no systematic dependence on [Ca2+]i (Fig. 3 A). In contrast, τc changed about two orders of magnitude (from 3 to 210 ms; Fig. 3 B) over the same range of [Ca2+]i, accounting for most of the strong dependence of channel P o on [Ca2+]i (Fig. 4).
Figure 2.
Typical single-channel current traces of the r-InsP3R-3 at various [Ca2+]i in the presence of 10 μM InsP3. Arrows indicate closed channel current level in all current traces.
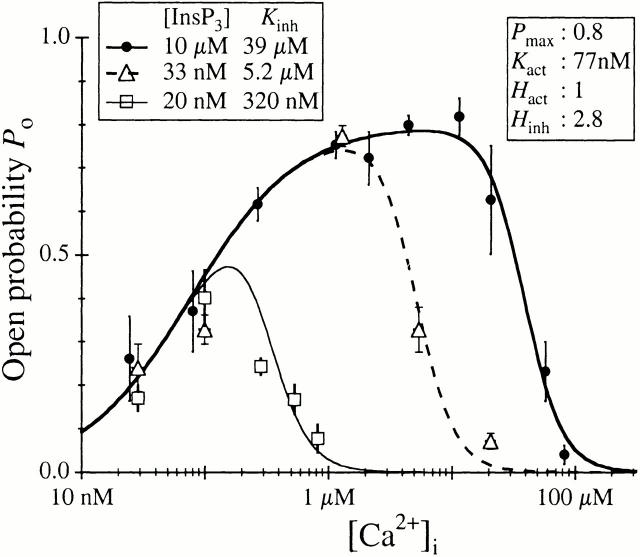
Figure 4.
Ca2+ dependence of r-InsP3R-3 channel open probability under various [InsP3]. Different symbols denote data for various [InsP3] as tabulated. The curves are theoretical fits using the Hill equation (), with K inh varying with [InsP3] as listed in the graph, whereas P max, K act, H act, and H inh remained independent of [InsP3] with values tabulated in the graph.
Figure 3.
Ca2+ dependencies of mean open channel duration (Α) and closed channel duration (B) of the r-InsP3R-3 activated by various concentrations of InsP3 as tabulated. In the closed channel duration graph, data points obtained with the same InsP3 concentration are connected with a line for clarity.
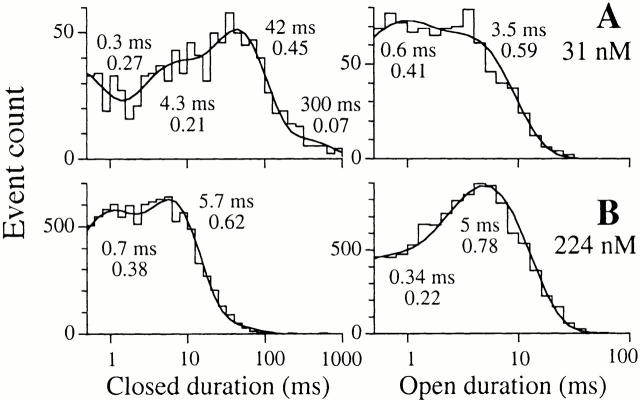
Detailed analyses of the r-InsP3R-3 channel dwell time histograms revealed that the channel has at least two distinguishable open kinetic states (Fig. 5): a long kinetic state with time constant (τ) of ∼8 ms and a short kinetic state with τ ∼1 ms. The relative weight of the short kinetic state decreased as the channel was activated by increasing [Ca2+]i, and then increased as high [Ca2+]i inhibited channel activity. The channel closed dwell time histograms revealed at least three distinguishable closed kinetic states (Fig. 5): (1) a long state with τ > 10 ms; (2) a medium state with 3.5 ms < τ < 10 ms; and (3) a short kinetic state with τ < 1 ms. The decrease in τc and, therefore, increase in channel P o, as the channel was activated by increases in [Ca2+]i, was achieved by both a decrease in the relative weights and the time constants of the long and medium closed kinetic states. Reversal of this trend increased τc and decreased P o when the channel was inhibited by [Ca2+]i > 25 μM (Fig. 5 D).
Figure 5.
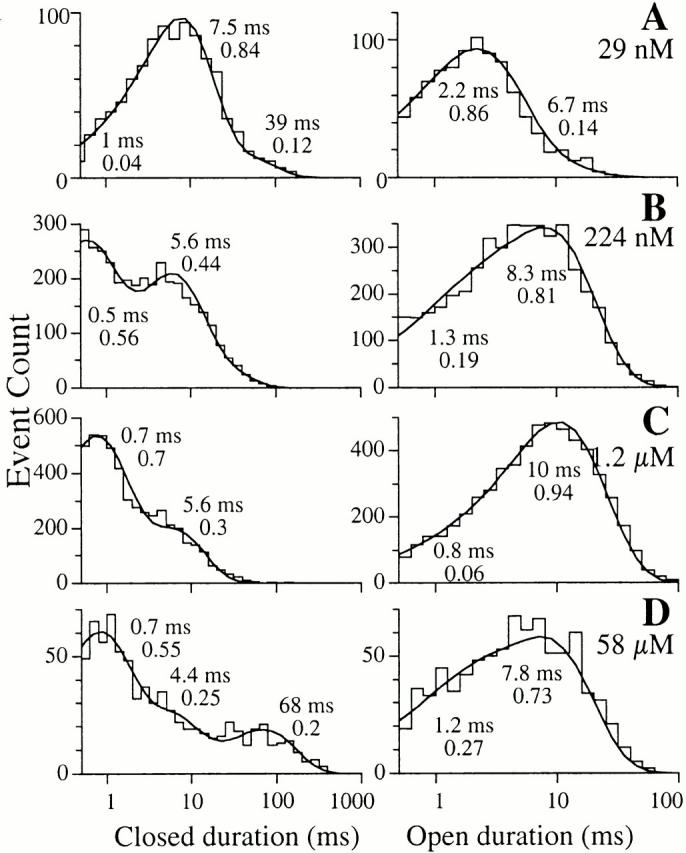
Single-channel open and closed channel duration histograms of r-InsP3R-3 in 10 μM InsP3, with 29 nM, 224 nM, 1.2 μM, and 57.5 μM Ca2+, respectively, as listed in the graphs. The open and closed duration histograms shown for each [Ca2+]i were derived from one patch-clamp experiment performed under the stated [Ca2+]i. Similar histograms were obtained from two additional experiments at each set of experimental conditions. The smooth curves are theoretical probability density functions consisting of two to four exponential components fitted to the histograms, as outlined in Sigworth and Sine 1987. The time constant and relative weight of each exponential component is labeled besides the corresponding peak in the curves. Duration histograms obtained with the same set of experimental conditions are fitted with the same number of exponential components and the time constants and relative weights of corresponding exponential components lie within ∼30% of the values shown.
The r-InsP3R-3 P o versus [Ca2+]i response in 10 μM InsP3 could be well fitted to a biphasic Hill equation (Fig. 4) so that:
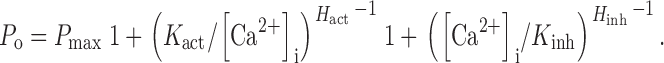 |
2 |
This suggests that the tetrameric InsP3R-3 channel can achieve a maximum open probability P max of 0.80 ± 0.03, with two distinct types of functional Ca2+ binding sites: activating sites with a half-maximal activating [Ca2+]i, K act, of 77 ± 10 nM and a Hill coefficient H act of 1.0 ± 0.1; and inhibitory sites with half-maximal inhibitory [Ca2+]i, K inh, of 39 ± 7 μM and Hill coefficient H inh of 2.8 ± 0.4. The Hill coefficient H act of ∼1 indicates that Ca2+ activation of the InsP3R-3 is not cooperative under our experimental conditions, whereas the large Hill coefficient H inh of 2.8 indicates that inhibition of the InsP3R by Ca2+ is a highly cooperative process.
InsP3 Sensitivity of the Ca2+ Dependence of r-InsP3R-3
The Ca2+ dependence of the gating of the X-InsP3R-1 is regulated by [InsP3] (Mak et al. 1998), and the different receptor isoforms are believed to differ in their affinities for InsP3 (Sudhof et al. 1991; Newton et al. 1994) and in the efficacy of InsP3 to activate them (Ramos-Franco et al. 1998). Therefore, we systematically investigated the InsP3 sensitivity of the Ca2+ dependence of r-InsP3R-3 gating. At [InsP3] < 100 nM, the r-InsP3R-3 became more sensitive to Ca2+ inhibition: at 33 nM, K inh decreased from 39 μM (determined at 10 μM InsP3) to 5.2 ± 0.8 μM (Fig. 4), whereas P max, H inh, K act, and H act were not affected. K inh further decreased to 320 ± 50 nM, in response to 20 nM InsP3. As in the case in saturating [InsP3], Ca2+ inhibition of P o in the presence of subsaturating (<100 nM) [InsP3] was caused mainly by an increase in the mean closed dwell time whereas the mean open dwell time remained relatively constant (Fig. 3). These results indicate that InsP3 activates the type 3 channel by tuning the efficacy of Ca2+ to inhibit it. No r-InsP3R-3 channel activities were observed in the absence of InsP3 in the pipet (in 25 nM Ca2+). Therefore, as in the case for X-InsP3R-1, InsP3 is a necessary agonist for r-InsP3R-3.
These data can be described by a simple model similar to one derived for the X-InsP3R-1 (Mak et al. 1998), in which the InsP3-concentration dependence of K inh was fitted with a simple Hill equation ():
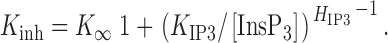 |
3 |
The results imply that the InsP3R has a single class of functional InsP3 binding sites with a half-maximal activating [InsP3], K IP3, of 55 ± 6 nM, a Hill coefficient H IP3 of 4.5 ± 1, and a maximum half-maximal inhibitory [Ca2+]i, K ∞, of 39 ± 7 μM at saturating [InsP3]. The large Hill coefficient H IP3 of ∼4 indicates that InsP3 activation of the InsP3R is highly cooperative, requiring InsP3 binding to perhaps all four monomers of the channel to relieve the Ca2+ inhibition and gate the channel open.
DISCUSSION
We recently described the first functional expression of the r-InsP3R-3, and examined its detailed permeation properties by patch-clamp electrophysiology of single channels in the Xenopus oocyte outer nuclear membrane (Mak et al. 2000). Because the endogenous Xenopus InsP3R, which is a type 1 isoform, had been studied in this same preparation (Mak et al. 1998), the channel properties of the two isoforms in the same environment could be compared. Despite the divergent isoform types and species differences, remarkably similar single-channel permeation properties of the two isoforms were observed. Both channels have nearly identical permeabilities to divalent and monovalent cations and gate to similar main and rarer subconductance states, both undergo use-dependent inactivation with similar kinetics, and they share a propensity to exist in channel clusters (Mak et al. 1998, Mak et al. 2000). Because three distinct InsP3R genes have been identified (Furuichi et al. 1989; Mignery et al. 1989; Sudhof et al. 1991; Blondel et al. 1993; Maranto 1994) and most cell types express more than one isoform (Bush et al. 1994; De Smedt et al. 1994; Newton et al. 1994; Sugiyama et al. 1994; Fujino et al. 1995; Joseph et al. 1995; Nucifora et al. 1996; De Smedt et al. 1997), it has been assumed that each isoform must possess unique properties. Thus, it was suggested that distinctions among isoforms might reside in channel regulation and/or localization (Mak et al. 2000). Therefore, in this study we examined the effects of [Ca2+]i and [InsP3], the major regulators of InsP3R channel activity, on the gating kinetics of the r-InsP3R-3 channel in native ER membrane.
Gating Properties and Ca2+ Inhibition of the r-InsP3R-3 and X-InsP3R-1 Channels Are Similar
Our results indicate that many gating properties of the main conductance state M of the X-InsP3R-1 and r-InsP3R-3 channels are similar in their responses to [Ca2+]i. Both channels have a P max of 0.8. Like the X-InsP3R-1 channel, the r-InsP3R-3 channel displays two distinct types of functional Ca2+ binding sites: (1) activating sites whose properties and their implications will be discussed in detail below; and (2) inhibitory sites, which have similar K inh (54 ± 3 μM for X-InsP3R-1 and 39 ± 7 μM for r-InsP3R-3) and H inh (3.9 ± 0.7 for X-InsP3R-1 and 2.8 ± 0.4 for r-InsP3R-3) in 10 μM InsP3. In both channels, the mean open channel durations remain within a narrow range (3–16 ms) over the whole range of [Ca2+]i examined. Thus, Ca2+-induced changes in P o of both channels were mainly due to large changes in the mean closed channel durations, which decreased as the channels became activated, and increased as P o decreased due to inhibition by high [Ca2+]i (Fig. 3; Mak et al. 1998). Analyses of the channel dwell time distributions revealed that the open channel dwell time distribution was not significantly affected by [Ca2+]i, as expected. The increase in mean closed channel durations as [Ca2+]i increased at inhibitory [Ca2+]i was mainly caused by an increase in the relative weight of a long closed kinetic state in both the r-InsP3R-3 (Fig. 5C and Fig. D) and the X-InsP3R-1 (data not shown).
Our results indicate that the recombinant r-InsP3R-3, when expressed and recorded in the oocyte outer nuclear membrane, exhibits inhibition of channel activity by high [Ca2+]i. This behavior agrees with that observed in recent studies demonstrating a biphasic dependence on [Ca2+]i of type 3 InsP3R activities measured by either 45Ca2+ efflux from loaded Ca2+ stores in permeabilized cells—16HBE14o- bronchial mucosal cells in Missiaen et al. 1998, Missiaen et al. 2000 and RINm5F cells in Swatton et al. 1999—that express the type 3 InsP3R as the major InsP3R isoform, or reduction of 45Ca2+ influx into microsomal vesicles isolated from COS-7 cells overexpressing exogenous type 3 InsP3R and sarco/ER ATPase by transient transfection (Boehning and Joseph 2000). However, these flux studies indicated that InsP3-induced Ca2+ flux was inhibited at a lower [Ca2+]i (∼1 μM) than we observed in our single-channel patch-clamp experiments (Fig. 4). The discrepancy between the results from cell-based assays and our single-channel measurements might possibly be explained by the difficulty in attaining sufficient control of the concentrations of InsP3 and Ca2+ in the microdomains around the InsP3R in the Ca2+ flux measurements. InsP3 concentrations used in the flux experiments—200 nM in Swatton et al. 1999, 1.5 μM in Missiaen et al. 2000, 3 μM in Missiaen et al. 1998, and 1 μM in Boehning and Joseph 2000—were lower than we used in our experiments (10 μM InsP3). Whereas such InsP3 concentrations were saturating in our patch-clamp experiments (K IP3 ∼ 55 nM), they might not be sufficient to ensure that the [InsP3] in the microenvironment around the InsP3R was saturating. Because InsP3 activates the channel by decreasing the sensitivity to Ca2+ inhibition (this study), Ca2+ release responses observed in the presence of a subsaturating concentration of InsP3 will be predicted to be associated with a lower half-maximal inhibitory [Ca2+]i, as observed in those studies. Alternately, there may be factors in the permeabilized cells and isolated microsomal vesicles associated with the InsP3R, for example phosphatidylinositol 4,5-bisphosphate (PIP2; Lupu et al. 1998), which were absent in our nuclear patch-clamp studies, that reduced its sensitivity to InsP3 activation or increased its sensitivity to Ca2+ inhibition in those studies.
These observations of biphasic Ca2+ dependence of the type 3 InsP3R are in contrast with observations in another recent study (Hagar et al. 1998), which suggested that Ca2+ activation was similar for the types 1 and 3 channels, and that high [Ca2+]i were not inhibitory to type 3 InsP3R gating. The reasons for these contradictory results are not clear. Different recording solutions and voltages were used in the two studies, and the numbers of channels and range of [InsP3] studied were significantly greater in this study. Nevertheless, it is difficult to understand how these variables could affect the channel gating response to Ca2+. Whereas recombinant channels of known subunit stoichiometry (nearly all predicted to be homotetramers) in native ER membrane were studied here, membrane proteins from cells (RIN-5F) that expressed multiple InsP3R isoforms (although type 3 was qualitatively the dominant isoform, from 77% [Swatton et al. 1999] to 96% [Wojcikiewicz and He, 1995]) reconstituted in artificial bilayers were examined in the other study (Hagar et al. 1998). It is important to note that the biphasic Ca2+ dependence of the recombinant type 3 channel observed in our study is very reminiscent of that of the endogenous type 1 channel. Thus, it is highly unlikely that the observed behavior of the type 3 channel is conferred upon it by heterologous expression. It seems possible that reconstitution procedures affect the channel, or that the reconstituted channels recorded were not homotetrameric type 3 channels. The maximum P o of the recombinant type 3 channel recorded in nuclear patch-clamp experiments was 0.8 in this study, but only 0.05 for the reconstituted channels recorded in the bilayer study. A similar disparity between the high P max observed for type 1 channels in nuclear membrane patches (0.8; Mak and Foskett 1997; Mak et al. 1998) compared with those reconstituted from cerebellar microsomes (0.05; Bezprozvanny et al. 1991; Kaftan et al. 1997) may be caused by PIP2 binding tightly to the InsP3 binding sites of reconstituted channels (Lupu et al. 1998), thus, preventing their effective activation by InsP3. It is possible that the channels observed in the RIN-5F membrane reconstitution study (Hagar et al. 1998) was similarly affected by their association with some cytosolic factor, absent in our nuclear patching studies, which reduced both their P max and sensitivity to Ca2+ inhibition.
It has been suggested (Michikawa et al. 1999) that an interaction between calmodulin and the type 1 InsP3R (Yamada et al. 1995; Cardy et al. 1997; Patel et al. 1997; Hirota et al. 1999) mediates Ca2+-dependent inhibition of type 1 InsP3R activity. Although there is no evidence at present to suggest that calmodulin directly interacts with the type 3 InsP3R (Yamada et al. 1995; Cardy et al. 1997), calmodulin has been also reported to influence Ca2+ inhibition of the type 3 InsP3R (Missiaen et al. 2000). However, in preliminary experiments, we have been unable to obtain any evidence linking calmodulin to Ca2+ inhibition of either the types 1 or 3 channels (our unpublished data). Nevertheless, it is possible that a cellular factor (e.g., calmodulin or a calmodulin-like molecule) is present in our nuclear patching system but not in the reconstituted bilayer system, which may influence the Ca2+ sensitivity of the type 3 channel and account for the distinct behaviors observed in our experiments and those in Hagar et al. 1998. Therefore, although the preponderance of the data at this time suggest that the Ca2+ inhibition properties of the types 1 and 3 isoforms are not significantly dissimilar, it remains to be determined whether this property of either or both channels is intrinsic to the channels themselves or conferred by regulatory interactions with other proteins.
InsP3 Activates the r-InsP3R-3 Channel by Tuning Ca2+ Inhibition
All the observed gating properties of the r-InsP3R-3 channel over wide ranges of [InsP3] and [Ca2+]i could be well fitted with a biphasic Hill equation with the half-maximal inhibitory [Ca2+]i, K inh, being the only InsP3 concentration-sensitive parameter. Thus, the effect of InsP3 binding is not to enable activation of the r-InsP3R-3 by Ca2+, as expected for coagonist ligands and which has been generally assumed (Taylor and Richardson 1991; Mauger et al. 1994; Joseph 1995; Taylor and Traynor 1995), but rather it is to ameliorate inhibition of the channel by Ca2+. A similar analysis of the X-InsP3R-1 reached the same conclusion (Mak et al. 1998). The nearly identical behaviors of the types 1 and 3 channels from two different species strongly suggest that this is the mechanism by which InsP3 regulates all InsP3 receptors. The Hill coefficient H IP3 for both channels was ∼4, indicating that this process of InsP3 activation of InsP3R channel activity is highly cooperative (Meyer et al. 1988; Carter and Ogden 1997; Dufour et al. 1997), requiring InsP3 binding to probably all four monomers of the channel to relieve the Ca2+ inhibition and gate the channel open. Because InsP3 binding to the InsP3R is not cooperative (Taylor and Richardson 1991; Mauger et al. 1994; Joseph 1995; Taylor and Traynor 1995), the cooperative effects of [InsP3] on Ca2+ release that are observed in cells (Meyer et al. 1988; Schrenzel et al. 1995; Carter and Ogden 1997), therefore, are likely intrinsic to individual InsP3R channels.
Analysis of the effects of InsP3 on channel gating indicates that the functional half-maximal activating [InsP3], K IP3, of the r-InsP3R-3 is ∼55 nM. A similar analysis for the X-InsP3R-1 indicated that K IP3 ∼ 50 nM (Mak et al. 1998). For single reconstituted type 2 receptor channels, K IP3 ∼ 58 nM (Ramos-Franco et al. 1998). Therefore, these single-channel studies suggest that the functional InsP3 affinities of the three channel types are quite similar. Although these functional affinities are within the range of those determined in binding studies (∼2–200 nM; Taylor and Richardson 1991), the binding studies have suggested differential InsP3 sensitivities among the three isoforms (Cardy et al. 1997; Welch et al. 1997). Nevertheless, the published binding data are quite variable, possibly reflecting different experimental protocols involved in the binding and single-channel studies, regulation of InsP3 affinity (Benevolensky et al. 1994; Yoneshima et al. 1997), or selection for “activatable” receptors in single-channel studies. The latter may be relevant in light of the distinction in some binding studies between a high affinity (∼2 nM) inactive state and low affinity (20–60 nM) active state (Hingorani and Agnew 1992; Benevolensky et al. 1994; Marshall and Taylor 1994; Mauger et al. 1994). Importantly, the functional Hill coefficient for InsP3 binding to both X-InsP3R-1 and r-InsP3R-3 observed in nuclear patch-clamping is ∼4, suggesting a general requirement for all four monomers in a tetramer to bind InsP3.
These functional and binding affinities of the various InsP3R isoforms for InsP3 (∼50 nM) are very different from the functional InsP3 EC50 of 3.2 μM reported in a recent study of the regulation by InsP3 of the type 3 InsP3R reconstituted into lipid bilayers (in 160 nM Ca2+; Hagar and Ehrlich 2000). That study used the same experimental system as used in Hagar et al. 1998. Both reconstitution studies recorded a significantly lower maximum P o for both the type 3 (0.05 in Hagar et al. 1998 and 0.08 in Hagar and Ehrlich 2000) and type 1 (0.04 in Kaftan et al. 1997) channels than observed in our nuclear patching experimental system (0.8 for both types 1 and 3 InsP3R). Although we are again not certain about the causes of the discrepancies between the two experimental systems, it is possible that cellular factors like PIP2 may be associated with the InsP3R reconstituted into the planar lipid bilayer system and reduce the sensitivity of the InsP3R to InsP3 activation (Lupu et al. 1998).
The analyses of the kinetics of channel gating and the responses to InsP3 for both the types 1 and 3 InsP3R channels studied in native ER membrane now suggest a unifying model for channel activation by InsP3. Because [Ca2+]i affects the gating of the InsP3R primarily by regulating the closed state duration, and InsP3 regulates gating by tuning the channel sensitivity to Ca2+ inhibition, it follows that the kinetic basis for channel activation by InsP3 is the destabilization of the closed kinetic state(s). Consequently, InsP3 activates Ca2+ signaling by increasing the frequency of relatively stereotypic channel openings of (on average) ∼10 ms, each of which is the fundamental Ca2+ release event in Ca2+ signaling. InsP3 enhances the frequency of fundamental release events by reducing the Ca2+ affinity of the inhibitory binding site of the monomer to which it binds, in a process that is highly cooperative.
Ca2+ Activation of the r-InsP3R-3
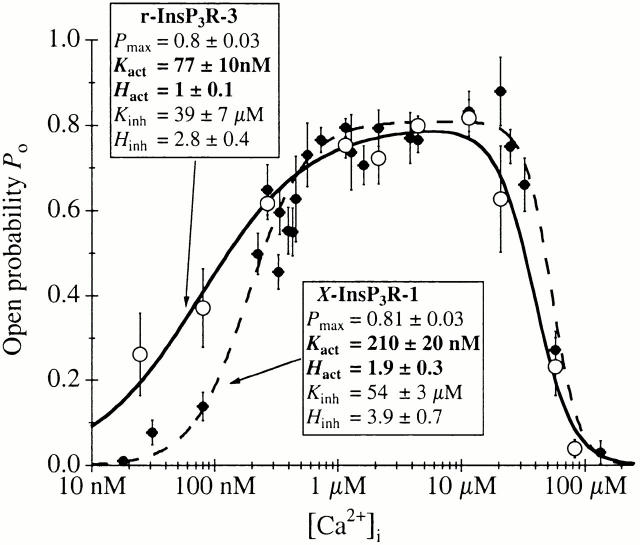
The activating Ca2+ binding sites of the r-InsP3R-3 had a half-maximal activating [Ca2+]i, K act, of 77 nM and H act of ∼1. These values contrast markedly with those obtained for the X-InsP3R-1 (Mak et al. 1998), where K act ∼ 210 nM and H act ∼ 2 (Fig. 6). Thus, in addition to a higher intrinsic sensitivity of the activating sites for Ca2+, the type 3 receptor lacks the apparent cooperativity among these sites that is observed in the type 1 receptor. This result agrees well with the flatter Ca2+ dependence of InsP3-induced Ca2+ release observed in B cells genetically engineered to express only InsP3R-3 compared with that observed in cells expressing InsP3R-1 only (Miyakawa et al. 1999). In light of the similarities between the two isoforms in their permeation and gating properties (Mak et al. 2000), and Ca2+ inhibition and regulation by InsP3 (this study), these differences in Ca2+ activation of the InsP3R channels represent the major distinguishing features between the two channels in our studies. Importantly, neither K act nor H act is affected by [InsP3] in either channel.
Figure 6.
Comparison of the Ca2+ dependencies of P o of r-InsP3R-3 and X-InsP3R-1 in 10 μM InsP3 and 0.5 mM ATP. Open circles represent data for r-InsP3R-3 from this study, fitted with the solid curve; closed circles represent data for X-InsP3R-1 taken from (Mak et al. 1998), fitted with the dashed curve. The curves are calculated using the Hill equation () with the tabulated parameters. Higher affinity and lack of cooperativity of the Ca2+ activation sites of the type 3 channel endow it with high gain IICR and low gain CICR. In contrast, lower affinity and presence of cooperativity of the Ca2+ activation sites of the type 1 channel confer low gain IICR and high gain CICR. Under resting [Ca2+]i, low levels of stimulation will trigger release of Ca2+ by IICR from the type 3 channel, which in turn will trigger further release by CICR from the type 1 channel.
The differences in the Ca2+ dependencies of the activation of the types 1 and 3 InsP3R are reflected in the dwell time distributions of the two isoforms at activating [Ca2+]i (<1 μM). Whereas the relative weight of the long open state (τ ∼ 8 ms) of both type 1 and type 3 InsP3R channels increased with [Ca2+]i during activation, most of the change in P o was the result of changes in the closed channel dwell time distribution. When [Ca2+]i was raised from 30 to 224 nM, there was a much more dramatic change in the closed channel dwell time distribution of the X-InsP3R-1 channel compared with that of the r-InsP3R-3 channel (Fig. 5 and Fig. 7). The rise in [Ca2+]i caused the predominant long closed state (τ > 10 ms), as well as the longest closed state (τ > 100 ms), of the X-InsP3R-1 channel to disappear (Fig. 7), resulting in the steep increase in channel P o with H act ∼ 2. In contrast, the long closed state (τ > 10 ms) of the r-InsP3R-3 had a low relative weight in 31 nM Ca2+, giving the channel a lower K act compared with the X-InsP3R-1. The disappearance of the long closed state with the rise in [Ca2+]i only caused a gentle increase in channel P o with H act ∼ 1.
Figure 7.
Single-channel open and closed channel duration histograms and the fitted theoretical probability density functions of X-InsP3R-1 in 10 μM InsP3 with 31 and 224 nM Ca2+, respectively, obtained as described in the legend to Fig. 5.
Importantly, both the lower K act and lack of cooperativity confer upon the type 3 channel the ability to remain active even at low [Ca2+]i (<100 nM) when stimulated by InsP3, where the type 1 receptor would be nearly quiescent (Fig. 6). For example, at [Ca2+]i ∼ 50 nM, a resting level measured in many cell types, the P o of the type 3 channel in the presence of low concentrations of InsP3 (10 nM say) is ∼0.3, whereas the type 1 channel P o is nearly 10-fold lower (Fig. 6). At ∼25 nM Ca2+, gating of the type 3 receptor is relatively robust (P o ∼ 0.2), whereas type 1 channel activities can hardly be detected (Fig. 6).
Physiological Significance of Different Ca2+ Activation Properties of InsP3R-1 and InsP3R-3
The distinct properties of the Ca2+ activation sites of the InsP3R isoforms are likely to be of important physiological significance. Ca2+ released by the type 3 channel will serve to trigger release from other type 3 channels in a process of CICR. However, the results of our study now demonstrate that the type 3 receptor is designed to respond with only a limited dynamic range of CICR at resting [Ca2+]i (50 nM) and moderate stimulation ([InsP3] > 20 nM), as CICR can increase the frequency of fundamental release events by only ∼2.6-fold (Po increases from ∼0.3 at resting [Ca2+]i to a maximum of ∼0.8). The narrow dynamic range of CICR in the type 3 receptor is a consequence of its relatively robust channel activity at resting [Ca2+]i, which in turn derives from the high affinity of its Ca2+ activation sites, as well as their lack of cooperativity. In contrast, the type 3 receptor is poised to respond to low levels of stimulation that would be insufficient to activate the type 1 receptor. The same properties that confer the type 3 receptor with a low gain CICR, confer on it an exquisite sensitivity to weak stimuli at resting [Ca2+]i, as its Ca2+ release activity can increase from P o ∼ 0 to P o ∼ 0.3 when [InsP3] rises from 0 to <10 nM. Thus, in response to weak stimuli, i.e., low levels of InsP3, the InsP3R-3 behaves as a “switch”, imparting high gain to InsP3-induced Ca2+ release (IICR).
Although there is little difference in the functional InsP3 sensitivities of the types 1 and 3 InsP3Rs, their differential Ca2+ activation properties result in an apparent higher InsP3 sensitivity in vivo of the type 3 release channel under conditions of resting levels of Ca2+ in the cytoplasm. The high gain IICR property of the type 3 receptor will enable it to provide a “trigger” release of Ca2+ that could recruit other release channel types. In contrast, the type 1 receptor is relatively insensitive to low levels of InsP3 at resting [Ca2+]i (low gain IICR). However, it is well designed to provide a wide dynamic range of Ca2+ release activity at resting [Ca2+]i (50 nM) and moderate stimulation ([InsP3] > 20 nM) by CICR, which can increase the frequency of fundamental release events by ∼20-fold (Po increases from 0.05 to 0.8 as [Ca2+]i increases from 50 to 1,000 nM) (Fig. 6). Thus, the type 1 channel displays high gain CICR and low gain IICR. Because this behavior is complementary to the behavior of the type 3 channel, the presence of the two channels would be predicted to confer a distinct “[Ca2+]i repertoire” in response to stimulation, in contrast to the behaviors expected if either was the sole expressed isoform.
The efficacy of Ca2+ released through type 3 channels to trigger type 1 channel activity by CICR will depend on spatial proximity of the two channels and [InsP3], due to the limited range of Ca2+ diffusion in the cytoplasm. Functional X-InsP3R-1 and r-InsP3R-3 channels in the ER membrane have a high propensity to exist in clusters of up to 10 channels (Parker et al. 1996; Mak and Foskett 1997; Mak et al. 2000). Immunostaining has revealed overlapping as well as distinct (Lee et al. 1997; Yule et al. 1997; Monkawa et al. 1998) localization for the isoforms in different cell types. In several epithelial cell types, the type 3 InsP3R is exclusively and intimately associated with the apical plasma membrane. Electrophysiological and optical imaging experiments have demonstrated that low levels of stimulation are associated with Ca2+ release events in close proximity to the apical membrane (“trigger zone”; Kasai and Petersen 1994; Petersen 1996). With more intense stimulation, [Ca2+]i rises first in the trigger zone and then propagates as a wave to the basal pole of the cell (Kasai and Petersen 1994; Petersen 1996). We suggest, based on the results of this study, that the spatial restriction of the type 3 InsP3R to the apical region confers to this region an exquisite sensitivity to InsP3 and, therefore, provides a basis for these physiological observations. Our results can likely be generalized to predict that in cells which coexpress the types 1 and 3 receptors, the type 3 receptor will initiate the Ca2+ response, and the subsequent signal will be carried by CICR from the type 1 channel. Spatial restriction of the type 3 channel will enable these responses to be manifested as Ca2+ waves.
By regulating the affinity of the Ca2+ inhibition sites, the [InsP3] will also determine the efficacy of “cross-talk” from the type 3 to the type 1 receptor, as it defines the extent to which Ca2+ can be released. At low [InsP3] (<30 nM), CICR from the InsP3R-1 is limited by highly efficacious negative feedback by Ca2+. Because InsP3 binding to the InsP3R reduces Ca2+ inhibition of the channel, [Ca2+]i that can inhibit channel activity at low [InsP3] will be insufficient to inhibit it when [InsP3] is increased. In addition to enabling graded Ca2+ release from InsP3-sensitive stores, this mechanism enables more intense stimuli to promote greater diffusive spread of the local Ca2+ signal to other sites.
In summary, our results indicate that the types 1 and 3 InsP3R isoforms are functionally similar in terms of their permeation and gating properties, regulation by InsP3, and inhibition by cytoplasmic Ca2+. However, the isoforms are uniquely distinguished by their sensitivities to activation by Ca2+. Differential Ca2+ sensitivity of Ca2+ activation sites confers on each InsP3R isoform distinct and complementary release properties in response to cellular stimulation. The relative expression level and spatial localization of different InsP3R types will enable these properties to interact to generate complex Ca2+ signals, including graded release, oscillations, and waves.
Acknowledgments
We thank Dr. Graeme Bell (University of Chicago, Chicago, IL) for providing r-InsP3R-3 cDNA, and Dr. Suresh Joseph (Thomas Jefferson University) for InsP3R antibodies.
This work was supported by grants to J.K. Foskett from the National Institutes of Health (MH59937 and GM56328) and to D.-O.D. Mak from the American Heart Association (9906220U).
Footnotes
Abbreviations used in this paper: CICR, Ca2+-induced Ca2+ release; InsP3, inositol 1,4,5-trisphosphate; ER, endoplasmic reticulum; IICR, InsP3-induced Ca2+ release; PIP2, phosphatidylinositol 4,5-bisphosphate; P o, open probability; r-InsP3R-3, rat type 3 InsP3R; X-InsP3R-1, Xenopus type 1 InsP3R.
References
- Amundson J., Clapham D. Calcium waves. Curr. Opin. Neurobiol. 1993;3:375–382. doi: 10.1016/0959-4388(93)90131-h. [DOI] [PubMed] [Google Scholar]
- Atri A., Amundson J., Clapham D., Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys. J. 1993;65:1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudet S.B., Hove-Madsen L., Bers D.M. How to make and use calcium-specific mini- and microelectrodes. In: Nuccitelli R., editor. A Practical Guide to the Study of Calcium in Living Cells. Academic Press Inc; San Diego, CA: 1994. pp. 94–114. [PubMed] [Google Scholar]
- Benevolensky D., Moraru I.I., Watras J. Micromolar calcium decreases affinity of inositol trisphosphate receptor in vascular smooth muscle. Biochem. J. 1994;299:631–636. doi: 10.1042/bj2990631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellumconduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 1994;104:821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B.E. The inositol 1,4,5-trisphosphate (InsP3) receptor. J. Membr. Biol. 1995;145:205–216. doi: 10.1007/BF00232713. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B.E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I., Bezprozvannaya S., Ehrlich B.E. Caffeine-induced inhibition of inositol(1,4,5)-trisphosphate-gated calcium channels from cerebellum. Mol. Biol. Cell. 1994;5:97–103. doi: 10.1091/mbc.5.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G.I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 1993;268:11356–11363. [PubMed] [Google Scholar]
- Boehning D., Joseph S.K. Functional properties of recombinant type I and type III inositol 1,4,5-trisphosphate receptor isoforms expressed in COS-7 cells. J. Biol. Chem. 2000;275:21492–21499. doi: 10.1074/jbc.M001724200. [DOI] [PubMed] [Google Scholar]
- Boitano S., Dirksen E.R., Sanderson M.J. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science. 1992;258:292–295. doi: 10.1126/science.1411526. [DOI] [PubMed] [Google Scholar]
- Bootman M.D., Berridge M.J. The elemental principles of calcium signaling. Cell. 1995;83:675–678. doi: 10.1016/0092-8674(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Bush K.T., Stuart R.O., Li S.-H., Moura L.A., Sharp A.H., Ross C.A., Nigam S.K. Epithelial inositol 1,4,5-trisphosphate receptors. Multiplicity of localization, solubility, and isoforms. J. Biol. Chem. 1994;269:23694–23699. [PubMed] [Google Scholar]
- Cardy T.J.A., Traynor D., Taylor C.W. Differential regulation of types-1 and -3 inositol trisphosphate receptors by cytosolic Ca2+ . Biochem. J. 1997;328:785–793. doi: 10.1042/bj3280785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T.D., Ogden D. Kinetics of Ca2+ release by InsP 3 in pig single aortic endothelial cellsevidence for an inhibitory role of cytosolic Ca2+ in regulating hormonally evoked Ca2+ spikes. J. Physiol. 1997;504:17–33. doi: 10.1111/j.1469-7793.1997.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham D.E. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- Danoff S.K., Ferris C.D., Donath C., Fischer G., Munemitsu S., Ullrich S., Snyder S.H., Ross C.A. Inositol 1,4,5-trisphosphate receptorsdistinct neuronal and non-neuronal forms generated by alternative splicing differ in phosphorylation. Proc. Natl. Acad. Sci. USA. 1991;88:2951–2955. doi: 10.1073/pnas.88.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt H., Missiaen L., Parys J.B., Bootman M.D., Mertens L., Van den Bosch L., Casteels R. Determination of relative amounts of inositol trisphosphate receptor mRNA isoforms by ratio polymerase chain reaction. J. Biol. Chem. 1994;269:21691–21698. [PubMed] [Google Scholar]
- De Smedt H., Missiaen L., Parys J.B., Henning R.H., Sienaert I., Vanlingen S., Gijsens A., Himpens B., Casteels R. Isoform diversity of the inositol trisphosphate receptor in cell types of mouse origin. Biochem. J. 1997;322:575–583. doi: 10.1042/bj3220575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour J.F., Arias I.M., Turner T.J. Inositol 1,4,5-trisphosphate and calcium regulate the calcium channel function of the hepatic inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1997;272:2675–2681. doi: 10.1074/jbc.272.5.2675. [DOI] [PubMed] [Google Scholar]
- Ferris C.D., Snyder S.H. Inositol phosphate receptors and calcium disposition in the brain. J. Neurosci. 1992;12:1567–1574. doi: 10.1523/JNEUROSCI.12-05-01567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino I., Yamada N., Miyawaki A., Hasegawa M., Furuichi T., Mikoshiba K. Differential expression of type 2 and type 3 inositol 1,4,5-trisphosphate receptor mRNAs in various mouse tissuesin situ hybridization study. Cell Tissue Res. 1995;280:201–210. doi: 10.1007/BF00307790. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Mikoshiba K. Inositol 1,4,5-trisphosphate receptor-mediated Ca2+ signaling in the brain. J. Neurochem. 1995;64:953–960. doi: 10.1046/j.1471-4159.1995.64030953.x. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400 . Nature. 1989;342:32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Kohda K., Miyawaki A., Mikoshiba K. Intracellular channels. Curr. Opin. Neurobiol. 1994;4:294–303. doi: 10.1016/0959-4388(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Hagar R.E., Ehrlich B.E. Regulation of the type III InsP3 receptor by InsP3 and ATP. Biophys. J. 2000;79:271–278. doi: 10.1016/S0006-3495(00)76289-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagar R.E., Burgstahler A.D., Nathanson M.H., Ehrlich B.E. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature. 1998;396:81–84. doi: 10.1038/23954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani S.R., Agnew W.S. Assay and purification of neuronal receptors for inositol 1,4,5-trisphosphate. Methods Enzymol. 1992;207:573–591. doi: 10.1016/0076-6879(92)07041-l. [DOI] [PubMed] [Google Scholar]
- Hirota J., Michikawa T., Natsume T., Furuichi T., Mikoshiba K. Calmodulin inhibits inositol 1,4,5-trisphosphate-induced calcium release through the purified and reconstituted inositol 1,4,5-trisphosphate receptor type 1. FEBS Lett. 1999;456:322–326. doi: 10.1016/s0014-5793(99)00973-4. [DOI] [PubMed] [Google Scholar]
- Honda Z., Takano T., Hirose N., Suzuki T., Muto A., Kume S., Mikoshiba K., Itoh K., Shimizu T. Gq pathway desensitizes chemotactic receptor-induced calcium signaling via inositol trisphosphate receptor down-regulation. J. Biol. Chem. 1995;270:4840–4844. doi: 10.1074/jbc.270.9.4840. [DOI] [PubMed] [Google Scholar]
- Iino M., Tsukioka M. Feedback control of inositol trisphosphate signalling by calcium. Mol. Cell. Endocrinol. 1994;98:141–146. doi: 10.1016/0303-7207(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Jiang Q.S., Mak D., Devidas S., Schwiebert E.M., Bragin A., Zhang Y., Skach W.R., Guggino W.B., Foskett J.K., Engelhardt F. Cystic fibrosis transmembrane conductance regulator–associated ATP release is controlled by a chloride sensor. J. Cell Biol. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.K., Samanta S. Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. Evidence for association of the receptor with ankyrin. J. Biol. Chem. 1993;268:6477–6486. [PubMed] [Google Scholar]
- Joseph S.K. The inositol trisphosphate receptor family. Cell. Signalling. 1995;8:1–7. doi: 10.1016/0898-6568(95)02012-8. [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Lin C., Pierson S., Thomas A.P., Maranto A.R. Heteroligomers of type-I and type-III inositol trisphosphate receptors in WB rat liver epithelial cells. J. Biol. Chem. 1995;270:23310–23316. doi: 10.1074/jbc.270.40.23310. [DOI] [PubMed] [Google Scholar]
- Joseph S.K., Bokkala S., Boehning D., Zeigler S. Factors determining the composition of inositol trisphosphate receptor hetero-oligomers expressed in COS cells. J. Biol. Chem. 2000;275:16084–16090. doi: 10.1074/jbc.M000506200. [DOI] [PubMed] [Google Scholar]
- Kaftan E.J., Ehrlich B.E., Watras J. Inositol 1,4,5-trisphosphate (InsP3) and calcium interact to increase the dynamic range of InsP3 receptor-dependent calcium signaling. J. Gen. Physiol. 1997;110:529–538. doi: 10.1085/jgp.110.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Petersen O.H. Spatial dynamics of second messengersIP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994;17:95–101. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Kume S., Muto A., Aruga J., Nakagawa T., Michikawa T., Furuichi T., Nakade S., Okano H., Mikoshiba K. The Xenopus IP3 receptorstructure, function, and localization in oocytes and eggs. Cell. 1993;73:555–570. doi: 10.1016/0092-8674(93)90142-d. [DOI] [PubMed] [Google Scholar]
- Lechleiter J.D., Clapham D.E. Molecular mechanisms of intracellular calcium excitability in X. laevis oocytes. Cell. 1992;69:283–294. doi: 10.1016/0092-8674(92)90409-6. [DOI] [PubMed] [Google Scholar]
- Lee M.G., Xu X., Zeng W.Z., Diaz J., Wojcikiewicz R.J.H., Kuo T.H., Wuytack F., Racymaekers L., Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J. Biol. Chem. 1997;272:15765–15770. doi: 10.1074/jbc.272.25.15765. [DOI] [PubMed] [Google Scholar]
- Lupu V.D., Kaznacheyeva E., Krishna U.M., Falck J.R., Bezprozvanny I. Functional coupling of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1998;271:14067–14070. doi: 10.1074/jbc.273.23.14067. [DOI] [PubMed] [Google Scholar]
- Maeda N., Kawasaki T., Nakade S., Yokota N., Taguchi T., Kasai M., Mikoshiba K. Structural and functional characterization of inositol 1,4,5-trisphosphate receptor channel from mouse cerebellum. J. Biol. Chem. 1991;266:1109–1116. [PubMed] [Google Scholar]
- Magnusson A., Haug L.S., Walaas S.I., Ostvold A.C. Calcium-induced degradation of the inositol (1,4,5)-trisphosphate receptor/Ca2+-channel. FEBS Lett. 1993;323:229–232. doi: 10.1016/0014-5793(93)81345-z. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel inositol 1,4,5-trisphosphate receptor currents revealed by patch clamp of isolated Xenopus oocyte nuclei. J. Biol. Chem. 1994;269:29375–29378. [PubMed] [Google Scholar]
- Mak D.-O.D., Foskett J.K. Single-channel kinetics, inactivation, and spatial distribution of inositol trisphosphate (IP3) receptors in Xenopus oocyte nucleus. J. Gen. Physiol. 1997;109:571–587. doi: 10.1085/jgp.109.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D, Foskett J.K. Effects of divalent cations on single–channel conduction properties of Xenopus IP3 receptor. Am. J. Physiol. 1998;275:C179–C188. doi: 10.1152/ajpcell.1998.275.1.C179. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. Inositol 1,4,5-trisphosphate activation of inositol trisphosphate receptor Ca2+ channel by ligand tuning of Ca2+ inhibition. Proc. Natl. Acad. Sci. USA. 1998;95:15821–15825. doi: 10.1073/pnas.95.26.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Foskett J.K. ATP regulation of type 1 inositol 1,4,5-trisphosphate receptor channel gating by allosteric tuning of Ca2+ activation. J. Biol. Chem. 1999;274:22231–22237. doi: 10.1074/jbc.274.32.22231. [DOI] [PubMed] [Google Scholar]
- Mak D.-O.D., McBride S., Raghuram V., Yue Y., Joseph S.K., Foskett J.K. Single-channel properties in endoplasmic reticulum membrane of recombinant type 3 inositol trisphosphate receptor. J. Gen. Physiol. 2000;115:241–255. doi: 10.1085/jgp.115.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto A.R. Primary structure, ligand binding, and localization of the human type 3 inositol 1,4,5-trisphosphate receptor expressed in intestinal epithelium. J. Biol. Chem. 1994;269:1222–1230. [PubMed] [Google Scholar]
- Marshall I.C.B., Taylor C.W. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem. J. 1994;301:591–598. doi: 10.1042/bj3010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger J.-P., Lièvremont J.-P., Piétri-Rouxel F., Hilly M., Coquil J.-F. The inositol 1,4,5-trisphosphate receptorkinetic properties and regulation. Mol. Cell. Endocrinol. 1994;98:133–139. doi: 10.1016/0303-7207(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly Cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988;240:653–655. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Michikawa T., Hirota J., Kawano S., Hiraoka M., Yamada M., Furuichi T., Mikoshiba K. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,14,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- Mignery G.A., Sudhof T.C., Takei K., De Camilli P. Putative receptor for inositol 1,4,5-trisphosphate similar to ryanodine receptor. Nature. 1989;342:192–195. doi: 10.1038/342192a0. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Parys J.B., Sienaert I., Maes K., Kunzelmann K., Takahashi M., Tanzawa K., De Smedt H. Functional properties of the type-3 InsP3 receptor in 16HBE14o- bronchial mucosal cells. J. Biol. Chem. 1998;273:8983–8986. doi: 10.1074/jbc.273.15.8983. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Bultynck G., Vanlingen S., De Smet P., Callewaert G., Parys J.B. Calmodulin increases the sensitivity of type 3 inositol-1,4,5-trisphosphate receptors to Ca2+ inhibition in human bronchial mucosal cells. Mol. Pharmacol. 2000;57:564–567. doi: 10.1124/mol.57.3.564. [DOI] [PubMed] [Google Scholar]
- Miyakawa T., Maeda A., Yamazawa T., Hirose K., Kurosake T., Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO (Eur. Mol. Biol. Organ.) J. 1999;18:1303–1308. doi: 10.1093/emboj/18.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monkawa T., Miyawaki A., Sugiyama T., Yoneshima H., Yamamoto-Hino M., Furuichi T., Saruta T., Hasegawa M., Mikoshiba K. Heterotetrameric complex formation of inositol 1,4,5-trisphosphate receptor subunits. J. Biol. Chem. 1995;270:14700–14704. doi: 10.1074/jbc.270.24.14700. [DOI] [PubMed] [Google Scholar]
- Monkawa T., Hayashi M., Miyawaki A., Sugiyama T., Yamamoto-Hino M., Hasegawa M., Furuichi T., Mikoshiba K., Saruta T. Localization of inositol 1,4,5-trisphosphate receptors in the rat kidney. Kidney Int. 1998;53:296–301. doi: 10.1046/j.1523-1755.1998.00763.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Okano H., Furuichi T., Aruga J., Mikoshiba K. The subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in a tissue-specific and developmentally-specific manner. Proc. Natl. Acad. Sci. USA. 1991;88:6244–6248. doi: 10.1073/pnas.88.14.6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton C.L., Mignery G.A., Südhof T.C. Co-expression in vertebrate tissues and cell lines of multiple inositol 1,4,5-trisphosphate (InsP3) receptors with distinct affinities for InsP3 . J. Biol. Chem. 1994;269:28613–28619. [PubMed] [Google Scholar]
- Nucifora F.C., Jr., Sharp A.H., Milgram S.L., Ross C.A. Inositol 1,4,5-trisphosphate receptors in endocrine cellslocalization and association in hetero- and homotetramers. Mol. Biol. Cell. 1996;7:949–960. doi: 10.1091/mbc.7.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Choi J., Yao Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocyteshot spots, puffs and blips. Cell Calcium. 1996;20:105–121. doi: 10.1016/s0143-4160(96)90100-1. [DOI] [PubMed] [Google Scholar]
- Patel S., Morris S.A., Adkins C.E., O'Beirne G., Taylor C.W. Ca2+-independent inhibition of inositol trisphosphate receptors by calmodulinredistribution of calmodulin as a possible means of regulating Ca2+ mobilization. Proc. Natl. Acad. Sci. USA. 1997;94:11627–11632. doi: 10.1073/pnas.94.21.11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P.J., Ramos-Franco J., Fill M., Mignery G.A. Identification and functional reconstitution of the type 2 inositol 1,4,5-trisphosphate receptor from ventricular cardiac myocytes. J. Biol. Chem. 1997;272:23961–23969. doi: 10.1074/jbc.272.38.23961. [DOI] [PubMed] [Google Scholar]
- Petersen O.H. New aspects of cytosolic calcium signaling. News Physiol. Sci. 1996;11:13–17. [Google Scholar]
- Putney J.W., Jr., St. J. Bird G. The inositol phosphate-calcium signaling system in nonexcitable cells. Endocr. Rev. 1993;14:610–631. doi: 10.1210/edrv-14-5-610. [DOI] [PubMed] [Google Scholar]
- Ramos-Franco J., Fill M., Mignery G.A. Isoform-specific function of single inositol 1,4,5-trisphosphate receptor channels. Biophys. J. 1998;75:834–839. doi: 10.1016/S0006-3495(98)77572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T.A., Thomas A.P. Intracellular calcium waves generated by Ins(1,4,5)P3-dependent mechanisms. Cell Calcium. 1993;14:674–690. doi: 10.1016/0143-4160(93)90094-m. [DOI] [PubMed] [Google Scholar]
- Schrenzel J., Demaurex N., Foti M., Van Delden C., Jacquet J., Mayr G., Lew D.P., Krause K.H. Highly cooperative Ca2+ elevations in response to ins(1,4,5)P3 microperfusion through a patch-clamp pipette. Biophys. J. 1995;69:2378–2391. doi: 10.1016/S0006-3495(95)80107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth F.J., Sine S.M. Data transformations for improved display and fitting of single channel dwell time histograms. Biophys. J. 1987;52:1047–1054. doi: 10.1016/S0006-3495(87)83298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehno-Bittel L., Lückhoff A., Clapham D.E. Calcium release from the nucleus by InsP3 receptor channels. Neuron. 1995;14:163–167. doi: 10.1016/0896-6273(95)90250-3. [DOI] [PubMed] [Google Scholar]
- Sudhof T.C., Newton C.L., Archer B.T., Ushkaryov Y.A., Mignery G.A. Structure of a novel InsP3-receptor. EMBO (Eur. Mol. Biol. Organ.) J. 1991;10:3199–3206. doi: 10.1002/j.1460-2075.1991.tb04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Furuya A., Monkawa T., Yamamoto-Hino M., Satoh S., Ohmori K., Miyawaki A., Hanai N., Mikoshiba K., Hasegawa M. Monoclonal antibodies distinctively recognizing the subtypes of inositol 1,4,5-trisphosphate receptorapplication to the studies on inflammatory cells. FEBS Lett. 1994;354:149–154. doi: 10.1016/0014-5793(94)01099-4. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Worley P.F., Baraban J.M., Snyder S.H. Solubilization, purification and isolation of an inositol trisphosphate receptor. J. Biol. Chem. 1988;263:1530–1534. [PubMed] [Google Scholar]
- Swatton J.E., Morris S.A., Cardy T.J.A., Taylor C.W. Type 3 inositol trisphosphate receptors in RINm5F cells are biphasically regulated by cytosolic Ca2+ and mediate quantal Ca2+ mobilization. Biochem. J. 1999;344:55–60. [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W., Richardson A. Structure and function of inositol trisphosphate receptors. Pharmacol. Ther. 1991;51:97–137. doi: 10.1016/0163-7258(91)90043-l. [DOI] [PubMed] [Google Scholar]
- Taylor C.W., Marshall I.C.B. Calcium and inositol 1,4,5-trisphosphate receptorsa complex relationship. Trends Biochem. Sci. 1992;17:403–407. doi: 10.1016/0968-0004(92)90009-x. [DOI] [PubMed] [Google Scholar]
- Taylor C.W., Traynor D. Calcium and inositol trisphosphate receptors. J. Membr. Biol. 1995;145:109–118. doi: 10.1007/BF00237369. [DOI] [PubMed] [Google Scholar]
- Toescu E.C. Temporal and spatial heterogeneities of Ca2+ signalingmechanisms and physiological roles. Am. J. Physiol. 1995;269:G173–G185. doi: 10.1152/ajpgi.1995.269.2.G173. [DOI] [PubMed] [Google Scholar]
- Watras J., Bezprozvanny I., Ehrlich B.E. Inositol 1,4,5-trisphosphate-gated channels in cerebellumpresence of multiple conductance states. J. Neurosci. 1991;11:3239–3245. doi: 10.1523/JNEUROSCI.11-10-03239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W., Williams A.J., Tinker A., Mitchell K.E., Deslongchamps P., Lamothe J., Gerzon K., Bidasee K.R., Besch H.R., Jr., Airey J.A. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry. 1997;36:2939–2950. doi: 10.1021/bi9623901. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H. Type I, II, and III inositol 1,4,5-trisphosphate receptors are unequally susceptible to down-regulation and are expressed in markedly different proportions in different cell types. J. Biol. Chem. 1995;270:11678–11683. doi: 10.1074/jbc.270.19.11678. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H., He Y. Type I, II and III inositol 1,4,5-trisphosphate receptor coimmunoprecipitation as evidence for the existence of heterotetrameric receptor complexes. Biochem. Biophys. Res. Commun. 1995;213:334–341. doi: 10.1006/bbrc.1995.2134. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz R.J.H., Furuichi T., Nakade S., Mikoshiba K., Nahorski S.R. Muscarinic receptor activation down-regulates the type I inositol 1,4,5-trisphosphate receptor by accelerating its degradation. J. Biol. Chem. 1994;269:7963–7969. [PubMed] [Google Scholar]
- Yamada M., Miyawaki A., Saito K., Nakajima T., Yamamoto-Hino M., Ryo Y., Furuichi T., Mikoshiba K. The calmodulin-binding domain in the mouse type 1 inositol 1,4,5-trisphosphate receptor. Biochem. J. 1995;308:83–88. doi: 10.1042/bj3080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneshima H., Miyawaki A., Michikawa T., Furuichi T., Mikoshiba K. Ca2+ differentially regulates the ligand-affinity states of type 1 and type 3 inositol 1,4,5-trisphosphate receptors. Biochem. J. 1997;322:591–596. doi: 10.1042/bj3220591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule D.I., Ernst S.A., Ohnishi H., Wojcikiewicz R.J.H. Evidence that zymogen granules are not a physiologically relevant calcium pool. Defining the distribution of inositol 1,4,5-trisphosphate receptors in pancreatic acinar cells. J. Biol. Chem. 1997;272:9093–9098. doi: 10.1074/jbc.272.14.9093. [DOI] [PubMed] [Google Scholar]