Abstract
In the presence of a low pH environment, the channel-forming T domain of diphtheria toxin undergoes a conformational change that allows for both its own insertion into planar lipid bilayers and the translocation of the toxin's catalytic domain across them. Given that the T domain contributes only three transmembrane segments, and the channel is permeable to ions as large as glucosamine+ and NAD−, it would appear that the channel must be a multimer. Yet, there is substantial circumstantial evidence that the channel may be formed from a single subunit. To test the hypothesis that the channel formed by the T domain of diphtheria toxin is monomeric, we made mixtures of two T domain constructs whose voltage-gating characteristics differ, and then observed the gating behavior of the mixture's single channels in planar lipid bilayers. One of these constructs contained an NH2-terminal hexahistidine (H6) tag that blocks the channel at negative voltages; the other contained a COOH-terminal H6 tag that blocks the channel at positive voltages. If the channel is constructed from multiple T domain subunits, one expects to see a population of single channels from this mixture that are blocked at both positive and negative voltages. The observed single channels were blocked at either negative or positive voltages, but never both. Therefore, we conclude that the T domain channel is monomeric.
Keywords: planar lipid bilayers, histidine tags, voltage gating, single channels, monomer
INTRODUCTION
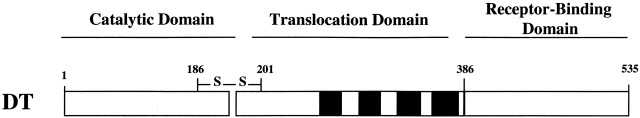
Diphtheria toxin (DT) is a single, 535–amino acid polypeptide secreted by Corynebacterium diphtheriae and is responsible for the disease diphtheria. The toxin has three functional domains (see Fig. 1): the NH2-terminal catalytic domain (residues 1–185), the COOH-terminal receptor-binding domain (residues 386–535), and the translocation, or T domain, lying between them (residues 202–378). The catalytic domain is connected to the T domain by a protease-susceptible loop and by an easily reducible disulfide bridge (for reviews see Madshus and Stenmark 1992; Falnes and Sandvig 2000.)
Figure 1.
Linear diagram of the protease-nicked form of diphtheria toxin (DT). All three functional domains are depicted. The catalytic domain remains connected to the translocation domain by a disulfide bridge between cysteines at 186 and 201. The four hydrophobic segments in the translocation domain are shown in black.
Cellular intoxication by DT is thought to proceed by the following mechanism. Pathogenic strains of Corynebacterium diphtheriae infect a host and secrete the bacteriophage-encoded toxin as a monomer. The receptor-binding domain targets it to the surface of cells harboring a heparin-binding epidermal growth factor–like precursor. While the toxin is on the surface of the cell, a host protease nicks the loop connecting the catalytic domain to the T domain, leaving the two domains still connected by their disulfide bridge. Internalization occurs via receptor-mediated endocytosis, and the toxin now finds itself in an acidifying endosome. The low pH of the endosome induces a conformational change in the toxin that inserts the T domain into the endosomal membrane and translocates the catalytic domain across the membrane into the reducing environment of the cytosol. Here, the disulfide bond linking the two domains is reduced, releasing the catalytic domain. Subsequent ADP-ribosylation of elongation factor 2 by this domain inhibits protein synthesis, thereby killing the cell. The endosome function in this process is to provide a low pH environment; experimentally it can be bypassed by exposing toxin-treated cells to a low pH, in which case the T domain translocates the catalytic domain directly across the plasma membrane (Draper and Simon 1980; Sandvig and Olsnes 1980).
The T domain alone, as well as whole toxin and a mutant lacking the R domain (CRM45), form channels in planar bilayers when the pH of the cis side (the solution to which T domain constructs are added) is below 6 (Donovan et al. 1981; Kagan et al. 1981). Associated with this channel formation, the entire catalytic domain along with ∼70 residues of the NH2 terminus of the T domain is translocated across the membrane (Oh et al. 1999). Thus the T domain contains all of the translocation machinery; no cellular components, or even the toxin's R domain, are required for translocation.
In the open channel state, the topology of the T domain consists of only three transmembrane segments (TH5, TH8, and TH9; Senzel et al. 2000), and yet ions as large as glucosamine+ and NAD− can traverse the channel (Hoch et al. 1985). This raises questions concerning the stoichiometry of the channel. How many subunits of DT are involved in making this channel and, thus, in translocating the catalytic domain? DT and CRM45 both aggregate at low pH (Carroll et al. 1986; Bell et al. 1997; Steere and Eisenberg 2000), but it is not known whether these aggregates are functional; in fact, purified dimers cannot infect host cells (Carroll et al. 1986). Remarkably, there is considerable circumstantial evidence that the channel may be a monomer. First, in scanning cysteine accessibility mutagenesis studies (Akabas et al. 1992), every cysteine mutant channel that reacted with thiol-specific methanethiosulfonate (MTS) derivatives yielded only one transition in single-channel conductance (Huynh et al. 1997), which is a result consistent with a monomeric channel. Second, the conductance of the D352C channel after reacting with MTS-ethylammonium is nearly identical to that of the D352K channel (Mindell et al. 1994; Huynh et al. 1997). This does not make sense for a multimeric channel, where the former has one positive charge (CH2-S-S-CH2-CH2-NH3 +) from its one MTS-ethylammonium reaction at D352C, and the latter would have n similar positively charged residues there (CH2-CH2-CH2-CH2-NH3 +), where n is the stoichiometry of the channel. Third, the conductance of the D352C channel is smaller than that of the wild-type channel, a consequence of replacing a negatively charged residue with a neutral one. The subsequent reaction with MTS-ethylsulfonate restores the channel conductance to that of wild type in a single step-change (Huynh et al. 1997). Again, it would be surprising, if this is a multimeric channel, that a mutant with only one negative charge at residue 352 had the same conductance as that of the wild-type channel with n negative charges there. The only observation suggesting that the T domain channel is composed of more than one subunit is that the rate of channel formation increases with about the second power of toxin concentration (Kagan et al. 1981). However, it is possible that a cooperative process facilitates toxin entry into the membrane, thereby giving a nonlinear dependence of the rate of channel formation on concentration, whereas the channel that actually forms is a monomer.
All of this evidence, although not definitive, seems to argue against a channel composed of multiple subunits. In fact, for most protein systems, one would probably accept the above as ample evidence of monomericity. For DT though, the improbability of this proposal demands a closer look. (How can three transmembrane segments alone create a pore large enough to conduct K+ and Cl−, let alone glucosamine+ and NAD−?) Therefore, we have designed a set of experiments to test the hypothesis that the channel formed by the T domain of diphtheria toxin is monomeric.
MATERIALS AND METHODS
Constructs
Details concerning plasmid construction of the T domain with an NH2-terminal hexahistidine tag (H6 tag), as well as our methods of protein expression and purification for all the constructs used, were as previously reported (Zhan et al. 1995). To summarize, the NH2-terminal H6 construct was made by inserting wild-type T domain (DT residues 202–378) between the NdeI and XhoI sites of the Novagen pET-15b vector, which places an NH2-terminal H6 tag on the protein. The sequence of the H6 tag is: MGSSH6SSGLVPRGSHM-I202. This H6 tag has a thrombin cleavage site so that it can be removed after purification of the expressed protein on a nickel column. Using Stratagene's QuickChangeTM site-directed mutagenesis kit, we mutated the arginine in this thrombin cleavage site to a glutamine to minimize unintended proteolysis of this H6 tag by trace proteases.
The COOH-terminal H6 tag construct was engineered by inserting the same T domain into Novagen's pET-22b plasmid. To do this, an XhoI site was introduced at the COOH-terminal end of the T domain in the pET-15b vector, using the site-directed mutagenesis kit. The purified DNA from that mutant was digested with the restriction endonucleases NdeI and XhoI, gel-purified, and ligated into the pET-22b expression vector that had been cut with the same restriction enzymes and also gel-purified. This put the sequence (His)6 at the COOH terminus of the T domain. It was found that this protein was not an effective channel blocker. Again using the site-directed mutagenesis kit, we lengthened the H6 tag by inserting GGGMGSS between the COOH terminus of the T domain and the H6 tag (primers used were CGTATAATCGTCCCCTCGAGGGAGGTGGAATGGGATCGTCGCACCACCACCACCACCAC and its reverse compliment; nucleotides inserted are shown in bold type). This was a better blocker but not sufficiently distinctive to satisfy us. We therefore inserted additional residues SSGLVPR COOH-terminal of the H6 tag (primers used were CCACCACCACCACAGCAGCGGCCTCGTCCCCAGGTGAGATCCGGCTGC and its reverse compliment). This protein blocked the T domain channel effectively. Thus, our final COOH-terminal H6 construct is P378-LEGGGMGSSH6SSGLVPR. One can see that this is most of the NH2-terminal H6 tag oriented in the reverse direction.
The construct with both H6 tags was made as follows. Purified plasmid containing the T domain with COOH-terminal H6 tag was digested with the restriction endonucleases NdeI and Bpu1102, gel-purified, and cloned into the pET-15b expression vector. This put the NH2-terminal H6 tag sequence MGSSH6SSGLVPRGSHM upstream of residue 202 in our COOH-terminal H6 construct. Thus, the final sequence of the T domain with both NH2- and COOH-terminal H6 tags is: MGSSH6SSGLVPRGSHM-I202…P378-LEGGGMGSSH6SSGLVPR.
After expression and purification (Zhan et al. 1995) on a Novagen His-Bind column, the proteins were dialyzed into 20 mM Tris-Cl, pH 8.0, 1 mM EDTA and frozen at −80°C. The T domain with the NH2-terminal H6 tag was at a concentration of ∼3 mg/ml; that with the COOH-terminal H6 tag was at ∼1.5 mg/ml; and that with both the NH2-terminal and COOH-terminal H6 tags was ∼3 mg/ml.
Ratios for Mixtures
The appropriate ratio of NH2-terminal to COOH-terminal H6 tag T domain in the mixtures was determined as follows. The T domains were first individually incubated at 37°C for 2 h in 40% DMSO (Sigma-Aldrich) to break up most of the preformed aggregates (Carroll et al. 1986). This reliably disrupted >90% of the dimeric T domain, as assayed by 15% native PAGE (Fig. 2). They were then tested individually on bilayers to find dilutions that consistently gave from one to three single channels within a few minutes of being stirred into the cis solution. This was usually achieved with a 1:300 to 1:500 dilution (T domain construct:20 mM Tris-Cl, pH 8.0). The amount of each T domain in the individual dilutions was used to establish a suitable ratio in the mixtures of the original concentrated solutions.
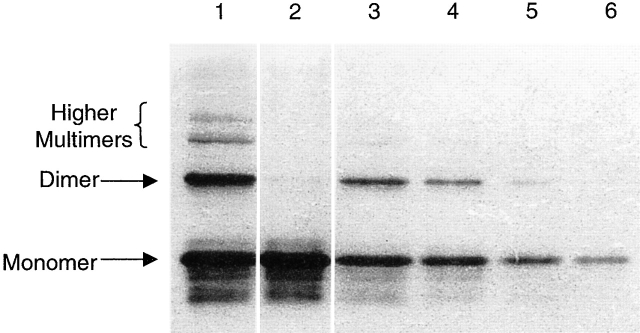
Figure 2.
Native 15% PAGE showing the disaggregation of preformed aggregates by DMSO. Lane 1 shows a heavily loaded (18 μg) sample of T domain with a COOH-terminal H6 tag. This sample was chosen for its unusually large dimer and multimer populations. (The band labeled “dimer” was so identified from its running at the same position as an unreduced T domain cysteine mutant.) Contrast this with lane 2, which shows the same quantity of protein after incubation in 40% DMSO for 2 h at 37°C. Notice the near absence of dimers and multimers. Lanes 3–6 are dilutions of lane 1 made to quantify the effects of the DMSO protocol. The dilutions are 6-, 12-, 30-, and 60-fold, respectively. Thus, it appears that >97% of the preformed dimers have been broken up. Samples of lanes 1 and 2 were also tested on bilayers and showed no noticeable difference in their ability to form channels, as assayed by the rate of channel entry. Thus, it can be concluded that preformed aggregates are not a major source of T domain channel-forming activity.
Purification of Monomeric T Domain
After treatment with DMSO, the mixtures were run on a Superdex G-75 sizing column (Pharmacia) in 50 mM NaCl, 10 mM Tris-Cl, pH 8.0, at a flow rate of 0.75 ml/min. Samples were collected in 0.5-ml fractions and concentrated 10-fold in a Savant UVS 400 Speed Vac® plus. Fig. 3 shows examples of how the peaks separated. All mixtures used for bilayer experiments were taken from fractions located to the right of the monomer peak. Purification was assayed on 15% native PAGE.
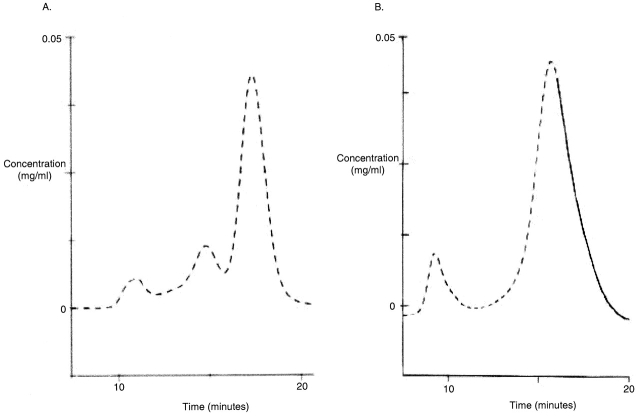
Figure 3.
Superdex G-75 elution profiles of T domain monomer, dimer, and higher order multimers. In both A and B, the column was equilibrated in 50 mM NaCl and 10 mM Tris-Cl, pH 8.0. The effluent was eluted at a flow rate of 0.75 ml/min, monitored at 280 nm, and 0.5-ml fractions were collected. The columns used in A and B were hand packed, and therefore contained different bead volumes; this is why the peak elution times are somewhat different. (A) To assess the separation of dimer and monomer, 200 μg of T domain with a cysteine in the NH2-terminal H6 tag (0.5 mg/ml) was partially reduced in 20 mM DTT and loaded on the column. One can see presumed multimers and other large molecular weight contaminants eluting in the void volume near 11 min, a dimeric peak eluting near 15 min, and a monomeric peak eluting after 17 min. (B) Approximately 120 μg of T domain with an NH2-terminal H6 tag was mixed with ∼180 μg of T domain with a COOH-terminal H6 tag, incubated in 40% DMSO for 2 h at 37°C (to disaggregate preformed dimers and multimers) and loaded on the column. In the void volume, just before 10 min, a presumed high molecular weight contaminant peak is seen. (We assume this to be a contaminant because Fig. 2 shows that higher T domain multimers are broken up by DMSO treatment.) A monomer peak is seen to elute after 15.5 min, and no dimer peak is observed. Only samples from the region of the spectrum drawn with a solid line were used in bilayer experiments.
Bilayer Experiments
Planar lipid (asolectin) bilayer membranes (∼70–100 μm in diameter) were made by a modification of the folded film method as described by Huynh et al. 1997. The solutions on both sides of the membrane contained 1 M KCl, 2 mM CaCl2, and 1 mM EDTA; in addition, the cis solution (the solution to which T domain constructs were added) contained 30 mM MES, pH 5.3, and the opposite trans solution contained 50 mM HEPES, pH 7.2. Voltages are those of the cis solution with respect to the trans solution, whose potential was held at virtual ground.
Current generated by single channels was measured by voltage clamping the cis side of the membrane to voltages between ±60 and 80 mV. Channels entered the membrane at positive voltages (Kagan et al. 1981); after a channel entered, the voltage was pulsed to negative values to determine the channel type: N-type H6 tag gating, C-type H6 tag gating, no gating at all, or both types of gating (see Fig. 4). If an entering channel left the membrane before application of this negative voltage pulse, it was not included in the dataset. Our method of counting channels was to take the maximum number of channels of each type seen coincidentally in the membrane. For example, if one C-type channelappeared, stayed a while and then left, and another C-type channel entered the membrane later, this was counted as only one C-type channel. This method of counting, by insuring our not counting the same channel multiple times, avoided biasing the data. If, however, two C-type channels were seen in the membrane at the same time, this and only this was counted as two C-type channels. To further clarify this point, if a membrane yielded a count of 3 N-type and 2 C-type channels, this meant that at some time A in the record there were 3 N-type channels coincidentally in the membrane, and at some time B (not necessarily the same as A) there were 2 C-type channels coincidentally in the membrane. Due to the rapid flickering nature of the COOH-terminal H6 tag block (see Fig. 4), we were unable to reliably count more than three of these channels in the membrane at one time, and thus usually stopped the experiment if three of these were seen coincidentally. It was found that pulsing to large negative voltages (−200 mV) would close (or drive out) all of the channels in the membrane, so that multiple experiments could be done on the same membrane. In the end, though, for each membrane, only the maximum number of channels of each type seen in the membrane coincidentally were counted.
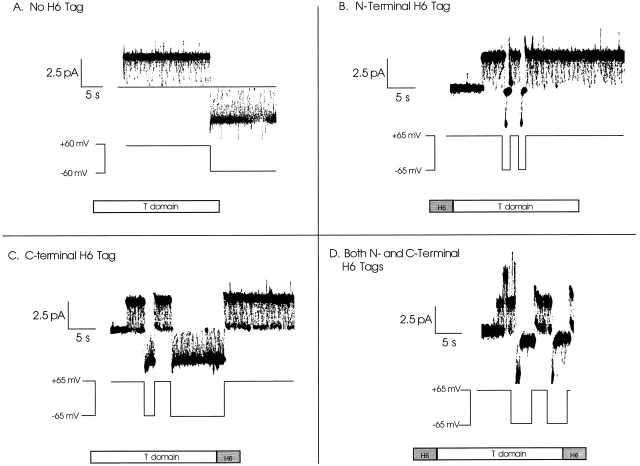
Figure 4.
Single-channel gating characteristics of the different T domain constructs. Before the start of each record, the construct was added to the cis compartment to a concentration of ∼1 ng/ml. Below each of the single channel records is a linear diagram of the illustrated construct showing the location of any H6 tags. (A) Single channel formed by wild-type T domain (lacking an H6 tag) is seen to remain open at both +60 and −60 mV, with unresolvably brief flickers to a zero-conductance closed state. (B) Single channel formed by T domain with an NH2-terminal H6 tag remains open at +65 mV like the wild-type channel and rapidly closes to zero conductance at −65 mV. (C) Single channel formed by T domain with a COOH-terminal H6 tag spends a good deal of time in the zero-conductance closed state at +65 mV and remains open at −65 mV like the wild-type channel. This blocking effect is even more exaggerated at higher positive voltages. (D) Single channel formed by T domain with both NH2- and COOH-terminal H6 tags is blocked at both +65 and −65 mV. (A second channel transiently appears during the first +65-mV pulse.) At positive voltages, the channel flickers rapidly between the open and closed states, spending about half of its time in each. At negative voltage pulses, the channel remains open briefly, before fully closing for the duration of the pulse. The solutions on both sides of the membrane were 1 M KCl, 2 mM CaCl2, and 1 mM EDTA; the cis solution contained 30 mM MES, pH 5.3, and the trans contained 50 mM HEPES, pH 7.2. The records were filtered at 100 Hz by the chart recorder.
RESULTS
The experiments to be described were performed in planar lipid bilayer membranes. Their rationale came from our earlier work, which showed that a histidine tag (H6 tag) attached to the NH2 terminus of the T domain rapidly and completely blocked the channel at cis negative voltages, whereas when the H6 tag was attached to the COOH terminus, the channel was blocked (a high frequency flickering block, occasionally entering a prolonged blocked state) at cis positive voltages (Senzel et al. 2000). These findings were used to demonstrate that the NH2 terminus of the protein (and its H6 tag) was on the trans side of the membrane and the COOH terminus (and its H6 tag) was on the cis side (Senzel et al. 2000). In the present study, we verified that, as expected, when the T domain has both NH2- and COOH-terminal H6 tags, the channel is blocked at both negative and positive voltages. This is summarized in Fig. 4.
We reasoned that if we mixed NH2-terminal and COOH-terminal H6-tagged T domains, and if the T domain channel is a multimer, then some fraction of channels should be blocked at both positive and negative voltages. For example, if the channel is a dimer, and equal amounts of NH2-terminal and COOH-terminal H6-tagged T domains were mixed, then on average (if the T domains have an equal preference to associate with one another) one quarter of the channels should be blocked only at negative voltages (both subunits have NH2-terminal H6 tags), one quarter blocked only at positive voltages (both subunits have COOH-terminal H6 tags) and one half blocked at both negative and positive voltages (one subunit has an NH2-terminal H6 tag and the other has a COOH-terminal H6 tag). If, however, the channel is a monomer, one expects to see only channels that are blocked at either negative or positive voltages, but not at both.
One could argue that before mixing these constructs, there already exist preformed homo-multimers and that these are responsible for the channel-forming activity. Thus, when mixtures are made, one sees channels that only gate at either negative or positive voltages and falsely asserts that these channels are monomeric. We attempted to circumvent this problem in two ways. First, we dissociated dimers and higher order aggregates by incubating the mixture in 40% DMSO (Carroll et al. 1986). This reduced the amount of dimer by as much as 95% (Fig. 2). Second, after this treatment, the mixture was applied to a size-exclusion column, and the monomeric band was purified away from any remaining dimer or higher aggregates. The monomer came off in several fractions, and only fractions from the trailing half of the monomer peak, furthest from the multimers, were used in our experiments (Fig. 3).
After the above protocol, we performed experiments on two sets of purified mixtures of NH2- and COOH-terminal H6-tagged T domains. We observed 75 single channels, none of which gated at both negative and positive voltages. From the first mixture, a total of eight separate bilayer experiments were conducted yielding 35 single channels. (A typical record from one bilayer is shown in Fig. 5.) Of these, 17 showed N-type and 14 showed C-type H6-tagged gating characteristics, whereas 4 did not gate at either positive or negative voltages (see next paragraph). The failure to observe any channels that showed both N-type and C-type gating is, of course, which is consistent with a monomeric channel. If, for the sake of argument, it is assumed that the channel is dimeric, the probability of our not seeing a single channel that showed both N-type and C-type H6 tag gating in 35 events is 4.5 × 10−5 (see ). From the second mixture, 9 individual bilayer experiments were performed giving a total of 40 observed single channels. Of these, 26 showed N-type and 9 showed C-type H6-tagged gating characteristics, and 5 did not gate at either positive or negative voltages (see next paragraph). If the channel is dimeric, the probability of our not having seen one channel that showed both N-type and C-type H6 tag gating in 40 events is 1.3 × 10−4 (see ). In the combined two sets of mixtures, the probability of our not having seen even one channel that showed both N- and C-type gating is 5.9 × 10−9. If more than two T domains are required to construct the channel, then the odds of our not having seen even one channel that was blocked at both negative and positive voltages become even more minuscule.
Figure 5.
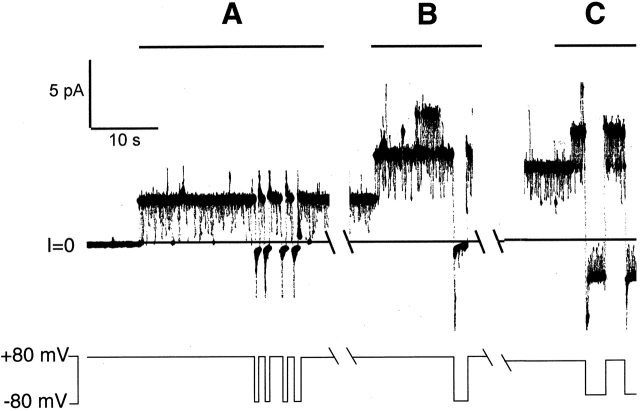
Channels formed in a bilayer treated with a mixture of NH2- and COOH-terminal H6-tagged T domains. Before its use in this experiment, the mixture was incubated for 2 h at 37°C in 40% DMSO, and then run on a sizing column to remove any preformed dimers or higher multimers. Approximately 1 ng of protein was stirred into the cis solution; the voltage was then held at +80 mV, awaiting the arrival of channels. In A, an N-type channel enters the membrane as evidenced by its gating characteristics: it remains open at +80 mV and rapidly closes at −80 mV. In B, with the N-type channel from A still in the membrane, a second N-type channel enters the membrane; both of the channels are open at +80 mV and rapidly close at −80 mV. Note that in B, a third channel transiently enters the membrane and, from its gating characteristic, appears to be a C-type channel. However, since we did not pulse to negative voltages, we could not preclude that this channel gated at both positive and negative voltages, and therefore it was not included in our dataset. In C, with the two N-type channels still present in the membrane, a third channel enters, and, from its gating characteristic, appears to be a C-type channel. Indeed, this is confirmed upon pulsing to a negative voltage, where two channels close (the original two N-types) and one channel remains open (the new C-type channel). (Two large current spikes that saturated the chart recorder are seen just before the negative voltage pulse.) Thus, this record was scored as two N-type channels and one C-type channel. (Each break in the record was ∼45 s. Throughout the first break, the voltage was held at +80 mV; during the second break, the voltage was switched from +80 to +70 mV.) The solutions were the same as in Fig. 4. The records were filtered at 100 Hz by the chart recorder.
There were nine single channels that did not gate at all. We attribute these “nulls” to a subpopulation of the NH2-terminal H6 tag proteins that had its NH2-terminal H6 tag cleaved off by trace amounts of protease in the solution. (The region of the T domain immediately downstream of the NH2-terminal H6 tag is exposed in the water-soluble crystal structure [Bennett and Eisenberg 1994] and could be a potential nicking site. In fact, on SDS-PAGE we saw faint bands with molecular weights slightly smaller than the T domain.) To test this hypothesis, we performed measurements on only the T domain with the NH2-terminal H6 tag. In eight separate bilayer experiments yielding 36 channels, 31 were blocked at negative voltages and 5 were nulls, a ratio similar to those seen in our mixing experiments. No nulls were seen in experiments with T domain having the COOH-terminal H6 tag.
We found that our extensive efforts to eliminate preformed dimers and aggregates were probably unnecessary. When we compared the activity of a sample that had a large preformed dimer population to its activity after receiving the 40% DMSO treatment (using the sample illustrated in Fig. 2), we saw no difference as assayed by the rate of channel entry into the membrane (results not shown). Thus, it appears that the preformed dimers and higher aggregates are not contributing significantly to channel formation.
With this in mind, we can also include in our dataset the results from a mixture that was DMSO treated, but was not run on the sizing column, and from a mixture that was neither DMSO treated nor run on the sizing column. In the former case, 21 single-channel events were recorded on six separate bilayers; of these events, 11 showed N-type gating, 9 showed C-type gating, and 1 did not gate at all. In the latter case, of 19 single-channel events observed on five bilayers, 9 showed N-type gating and 10 showed C-type gating. Combining these data with the results from the previous mixtures, we observed a total of 115 single channels in 28 bilayers and never saw a channel that showed both N-type and C-type gating.
DISCUSSION
Associated with the translocation of diphtheria toxin's catalytic domain across a planar lipid bilayer, the toxin's T domain forms a channel. The T domain in this channel, which is permeable to ions as large as glucosamine+ and NAD− (Hoch et al. 1985), contributes only three transmembrane segments (Senzel et al. 2000). Therefore, it seems obvious that the channel must be a multimer, yet several independent pieces of evidence, reviewed in the introduction, suggest the contrary. The experiments described in this paper were directed at resolving this question. We mixed in a test tube T domain molecules having an NH2-terminal histidine tag (H6 tag) with those having a COOH-terminal H6 tag and observed the resulting channels formed by this mixture in planar lipid bilayers. Channels formed by T domain molecules with an NH2-terminal H6 tag show characteristic blocking at negative voltages, whereas those formed by T domain molecules with a COOH-terminal H6 tag block at positive voltages (Fig. 4). We reasoned that if the channel is a multimer, then some of the channels formed from the mixture should have both of these gating characteristics, just as do channels formed by T domain molecules that have both an NH2-terminal and a COOH-terminal H6 tag (Fig. 4). We recorded 115 channels in 28 bilayers and never saw one channel manifesting both N-type and C-type H6 tag gating! Therefore, we conclude, contrary to common sense, that the T domain channel contains only one T domain molecule.
What are some possible arguments against this conclusion? It might be contended that the channels were generated from dimers or multimers that preexisted in the NH2-terminal and COOH-terminal H6-tagged T domain solutions, and so naturally we would not see any channels with both N-type and C-type H6 tag gating. This is a very unlikely possibility on two grounds. First, after incubating a T domain solution that had a relatively large dimer content for 2 h at 37°C in 40% DMSO, which converted almost all of the dimers and higher aggregates to monomers (Fig. 2), we found the channel-forming ability of the solution unchanged, indicating that preformed dimers and higher multimers are not a significant source of channels. Second, in two sets of experiments, we went to the extreme of removing residual preformed dimers and multimers from mixtures of NH2-terminal and COOH-terminal H6-tagged T domains that had undergone the DMSO treatment, by running the mixtures on a molecular sizing column and using, for the experiments, only fractions from the trailing half of the monomer peak (Fig. 3).
Even though preexisting dimers and multimers are not the source of channel-forming activity, this does not preclude that T domain monomers can come together on or within the membrane to form a multimeric channel. For this to account for our failure to see even one channel out of 115 that manifested both N-type and C-type H6-tagged gating, however, one would have to assume that NH2-terminal H6-tagged T domains and COOH-terminal H6-tagged T domains associate exclusively with themselves; i.e., heteromultimer formation is precluded. Although this is logically possible, we can think of no justification for this assumption. In fact, if one argues that somehow a T domain with a COOH-terminal H6 tag cannot associate with one that has an NH2-terminal H6 tag, then why would T-domains containing both an NH2-terminal and COOH-terminal H6 tag associate in the postulated multimeric channel? One would have to invoke a special attraction of NH2-terminal H6 tags and/or COOH-terminal H6 tags with themselves; we can see no physical justification for this. One might also argue that even though NH2-terminal H6-tagged T domains can form heteromultimers with COOH-terminal H6-tagged T domains, for some reason these do not form functional channels. Again, it is difficult to reconcile this position with the ability of T domains that have both an NH2-terminal and a COOH-terminal H6 tag to form channels. Thus, it seems to us that to believe that the T domain channel is a multimer, one must argue that heteromeric channels are formed by NH2-terminal and COOH-terminal H6-tagged T domains, but some of these exhibit only N-type gating, whereas others exhibit only C-type gating. Asymmetric structures can be envisioned that fulfill this condition, but this leads us into a fantasyland that is best avoided.
Throughout the analyses and discussions in this paper, we have tacitly assumed that only a single H6 tag is required for channel blocking. If this is not the case, one can imagine new scenarios in which the channel is multimeric, and yet no channels formed from our mixture exhibit both N-type and C-type gating. For example, if the channels were a trimer and required at least two NH2- or COOH-terminal H6 tags to get NH2- or COOH-terminal gating, respectively, we would observe only N-type and C-type gating channels, never channels showing both types of gating. In fact, in general, if the channel is formed by an odd number (n) of subunits and requires (n + 1)/2 NH2- or COOH-terminal H6 tags to get N- or C-type gating, respectively, the above statement would hold. (If n is even, the equivalent assumption that (n/2) + 1 H6 tags are required for blocking predicts a significant number of channels showing neither N- nor C-type gating.) We think that the requirement of multiple H6 tags to affect channel blocking is a priori unlikely; we know of no precedent for this in the channel literature. Moreover, if more than one H6 tag were required to completely block the channel, we would anticipate substates (partial block) when less than one H6 tag entered the channel. We have never observed this.
This paper presents what we feel are compelling arguments for the monomeric nature of the T domain channel formed in planar lipid bilayers. The channels formed by whole toxin in planar bilayers are indistinguishable from these (Silverman et al. 1994), and therefore it is not much of a stretch to assert that they too are monomeric. The mechanism by which the catalytic domain of the toxin is translocated across planar bilayers in association with channel formation by its T domain (Oh et al. 1999) remains to be resolved, but whatever it is, we feel that it is now established that only one T domain molecule is involved in the process. The structure of the T domain channel, having only three transmembrane segments (Senzel et al. 2000), remains a mystery and probably involves the lipids in its architecture.
There is a feeling in the literature that in real life (i.e., the cell) the translocation of the catalytic domain across the endosomal membrane into the cytosol is accomplished through an oligomer of DT (Steere and Eisenberg 2000). The results in this paper do not, of course, directly address this issue. All we can say in light of the present results and previous work (Oh et al. 1999) is that there is no necessity to invoke DT oligomers in cell intoxication, with the following caveat. The rate of channel formation increases with about the second power of toxin concentration (Kagan et al. 1981). Thus, a cooperative interaction of two or more DT molecules may promote toxin entry into the membrane, even though the ultimate intoxicating unit is a monomer. Interestingly, proteins in a molten globule-like state promote the transmembrane insertion of the T domain (Ren et al. 1999). Since at low pH, DT itself partly unfolds into a molten globule-like state (London 1992), it may catalyze within the acidic endosome its own insertion into the endosomal membrane. In this sense, oligomerization may promote cellular toxicity.
There are also data using liposomes that suggest that oligomerization is involved in DT pore formation. For example, Sharpe and London 1999 report that the size of liposome-entrapped molecules that are released is an increasing function of DT concentration (at pH 4.5). They interpret this to mean that the pores formed by DT increase in size as the concentration of DT within the membrane increases. Furthermore, they observed that DT oligomerizes within the liposomal membrane under their experimental conditions. Thus, they conclude that the pore formed by DT is an oligomer and that the pore size is a function of the number of subunits in the oligomer. We do not know how to relate our results to these, given the very different experimental conditions and techniques involved. In a previous paper (Senzel et al. 2000), we discussed the difficulties involved in trying to reconcile planar lipid bilayer experiments with those on liposomes.
Acknowledgments
We thank Jean-Claude Schwartz for his help with the size exclusion columns, Drs. Karen Jakes, Paul Kienker, Stephen Slatin, and Myles Akabas for their helpful discussions and readings of the manuscript, and Dr. Paul Kienker for his penetrating comments and critique of the .
This work was supported by National Institutes of Health grants T-32-GM07288 (to M. Gordon) and GM-29210 (to A. Finkelstein).
We wish to determine how unlikely it would be for us to observe in our mixing experiments no channels that show both N- and C-type gating, if the T domain channel was a multimer. We assume that all T domain monomers have an equal preference to associate with one another, independent of the H6 tag's location or absence. Suppose, for concreteness, that the channel is a dimer.
Let fN, fC, and fO be the fraction of monomers in the mixture with NH2-terminal H6 tag, COOH-terminal H6 tag, and no H6 tag, respectively. More precisely, fN, fC, and fO can be considered as the probabilities for each type of monomer to contribute to a dimer. It follows that,
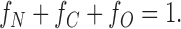 |
1 |
Then the probability that a dimer channel has at least one NH2-terminal H6 tag but no COOH-terminal H6 tag (i.e., the probability that it shows only N-type gating) is:
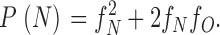 |
2a |
Likewise, the probabilities of a dimer channel showing only C-type gating, no gating, and both N- and C-type gating, respectively are:
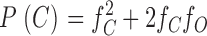 |
2b |
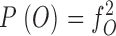 |
2c |
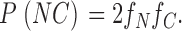 |
2d |
(We assume that a channel shows N-type gating if at least one of its subunits has an NH2-terminal H6 tag, and shows C-type gating if at least one of its subunits has a COOH-terminal H6 tag.)
Let [N], [C], and [O] be the number of channels observed in a set of experiments that show N-type, C-type, or no gating, respectively. (No channels were observed that showed both N- and C-type gating; i.e., [NC] = 0.) To use the observed numbers of channels (with [NC] = 0) to estimate the fractions of each type of monomer, and from this P(NC), which does not equal zero, we use the ratio of the probabilities:
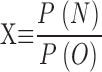 |
3a |
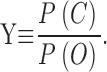 |
3b |
Combining , c, and and , we obtain
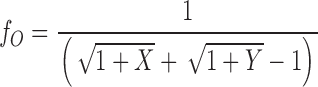 |
4a |
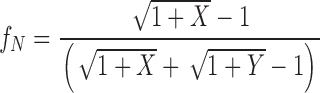 |
4b |
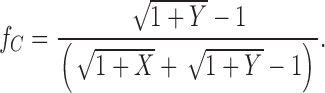 |
4c |
We can now substitute fN and fC from the above formulae into to calculate P(NC) from our data.
In our first set of experiments (results), we had: [N] = 17, [C] = 14, and [O] = 4. (Total number of events = 35.) From and we then have:
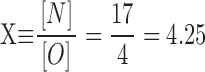 |
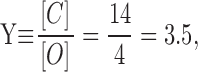 |
and substituting these into c we get:
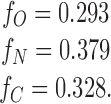 |
Therefore,
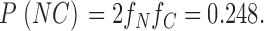 |
This is the probability of a dimeric channel showing both N-type and C-type H6 tag gating. The probability of our not seeing one such channel in our 35 events is:
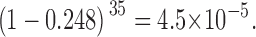 |
In our second set of experiments: [N] = 26, [C]=9, and [B]=5 (total number of events = 40), and by the same calculations as above:
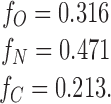 |
and therefore
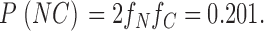 |
The probability of our not seeing one channel in our 40 events that shows both N-type and C-type H6 tag gating is:
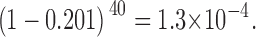 |
Thus, if the channel were a dimer, the probability of our not having seen even one channel in our two sets of experiments that showed both N-type and C-type H6 gating is
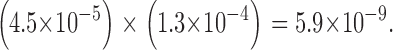 |
If the channel were a trimer, tetramer, or larger multimer, similar calculations would give probabilities much smaller than even this.
Footnotes
Abbreviations used in this paper: DT, diphtheria toxin; DTT, dithiothreitol; MTS, methanethiosulfonate.
To avoid continually having to use the expressions “C-type H6-tagged gating channel” or “N-type H6-tagged gating channel” we mercifully shorten these to “C-type channel” or “N-type channel.”
It is not definitely known whether the H6 tag sterically blocks the channel or binds externally and induces a conformational change that closes the channel. We think the former is much more likely, as the latter would require two separate external allosteric binding sites, one on the cis side and one on the trans side.
We cannot preclude that there is a contaminant, common to the different preparations and purification procedures of T domain, CRM45, and whole toxin, which contributes to the channel structure.
References
- Akabas M.H., Stauffer D.A., Xu M., Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Bell C.E., Poon P.H., Schumaker V.N., Eisenberg D. Oligomerization of a 45 kilodalton fragment of diphtheria toxin at pH 5.0 to a molecule of 20-24 subunits. Biochemistry. 1997;36:15201–15207. doi: 10.1021/bi971301x. [DOI] [PubMed] [Google Scholar]
- Bennett M.J., Eisenberg D. Refined structure of monomeric diphtheria toxin at 2.3 Å resolution. Protein Sci. 1994;3:1464–1475. doi: 10.1002/pro.5560030912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S.F., Barbieri J.T., Collier R.J. Dimeric form of diphtheria toxinpurification and characterization. Biochemistry. 1986;25:2425–2430. doi: 10.1021/bi00357a019. [DOI] [PubMed] [Google Scholar]
- Donovan J.J., Simon M.I., Draper R.K., Montal M. Diphtheria toxin forms transmembrane channels in planar lipid bilayers. Proc. Natl. Acad. Sci. USA. 1981;78:172–176. doi: 10.1073/pnas.78.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R.K., Simon M.I. The entry of diphtheria toxin into the mammalian cell cytoplasmevidence for lysosomal involvement. J. Cell Biol. 1980;87:849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falnes P.O., Sandvig K. Penetration of protein toxins into cells. Curr. Opin. Cell Biol. 2000;12:407–413. doi: 10.1016/s0955-0674(00)00109-5. [DOI] [PubMed] [Google Scholar]
- Hoch D.H., Romero-Mira M., Ehrlich B.E., Finkelstein A., DasGupta B.R., Simpson L.L. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayersrelevance to translocation of proteins across membranes. Proc. Natl. Acad. Sci. USA. 1985;82:1692–1696. doi: 10.1073/pnas.82.6.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh P.D., Cui C., Zhan H., Oh K.J., Collier R.J., Finkelstein A. Probing the structure of the diphtheria toxin channel. Reactivity in planar lipid bilayer membranes of cysteine-substituted mutant channels with methanethiosulfonate derivatives. J. Gen. Physiol. 1997;110:229–242. doi: 10.1085/jgp.110.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B.L., Finkelstein A., Colombini M. Diphtheria toxin fragment forms large pores in phospholipid bilayer membranes. Proc. Natl. Acad. Sci. USA. 1981;78:4950–4954. doi: 10.1073/pnas.78.8.4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London E. Diphtheria toxinmembrane interaction and membrane translocation. Biochim. Biophys. Acta. 1992;1113:25–51. doi: 10.1016/0304-4157(92)90033-7. [DOI] [PubMed] [Google Scholar]
- Madshus I., Stenmark H. Entry of ADP-ribosylating toxins into cells. Curr. Top. Microbiol. Immunol. 1992;175:1–26. doi: 10.1007/978-3-642-76966-5_1. [DOI] [PubMed] [Google Scholar]
- Mindell J., Silverman J.A., Collier R.J., Finkelstein A. Structure function relationships in diphtheria toxin channelsII. A residue responsible for the channel's dependence on trans pH. J. Membr. Biol. 1994;137:29–44. doi: 10.1007/BF00234996. [DOI] [PubMed] [Google Scholar]
- Oh K.J., Senzel L., Collier R.J., Finkelstein A. Translocation of the catalytic domain of diphtheria toxin across planar phospholipid bilayers by its own T domain. Proc. Natl. Acad. Sci. USA. 1999;96:8467–8470. doi: 10.1073/pnas.96.15.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Kachel K., Kim H., Malenbaum S., Collier R.J., London E. Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science. 1999;284:955–957. doi: 10.1126/science.284.5416.955. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J. Cell Biol. 1980;87:828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senzel L., Gordon M., Blaustein R.O., Oh K.J., Collier R.J., Finkelstein A. Topography of diphtheria toxin's T domain in the open channel state. J. Gen. Physiol. 2000;115:421–434. doi: 10.1085/jgp.115.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe J.C., London E. Diphtheria toxin forms pores of different sizes depending on its concentration in membranesprobable relationship to oligomerization. J. Membr. Biol. 1999;171:209–221. doi: 10.1007/s002329900572. [DOI] [PubMed] [Google Scholar]
- Silverman J.A., Mindell J.A., Zhan H., Finkelstein A., Collier R.J. Structure-function relationships in diphtheria toxin channelsdetermining a minimal channel-forming domain. J. Membr. Biol. 1994;137:17–28. doi: 10.1007/BF00234995. [DOI] [PubMed] [Google Scholar]
- Steere B., Eisenberg D. Characterization of high-order diphtheria toxin oligomers. Biochemistry. 2000;39:15901–15909. doi: 10.1021/bi0011678. [DOI] [PubMed] [Google Scholar]
- Zhan H., Oh K.J., Shin Y.K., Hubbell W.L., Collier R.J. Interaction of the isolated transmembrane domain of diphtheria toxin with membranes. Biochemistry. 1995;34:4856–4863. doi: 10.1021/bi00014a043. [DOI] [PubMed] [Google Scholar]