Large conductance calcium-activated K+ channels, also referred to as “BK” or “maxi K” channels because of their big single-channel conductance (∼300 pS in symmetrical 150 mM KCl) are widely distributed in many different tissues (Latorre 1994; Kaczorowski et al. 1996). A signature feature of BK channels, in addition to their high K+ selectivity and conductance, is that they are activated by both intracellular calcium ion (Ca2+ i) and depolarization in a highly synergistic manner (Marty 1981; Barrett et al. 1982). It is this synergistic interaction that elevates BK channels to be guardians of calcium-driven processes and protectors of cellular integrity. Although Ca2+ i serves as a key messenger to trigger processes such as muscle contraction and secretion of transmitters, prolonged elevated Ca2+ i can be detrimental to the processes and deadly to cells. How then do BK channels serve as guardians? Consider the case in which the initial elevation of Ca2+ i arises from depolarization-induced opening of voltage-dependent calcium channels. The BK channels would sense both the level of Ca2+ i and the degree of depolarization. If both are high, then the synergistic action would readily open BK channels, which restores the resting membrane potential, turning off the voltage-dependent calcium channels to prevent Ca2+ i overload. If the Ca2+ i is less or the degree of depolarization is less, then the response of the BK channels would be less, allowing further Ca2+ i influx. In addition to protective and regulatory actions, such a positive feedback system based on BK channels has been used to facilitate oscillations in membrane potential, as occurs in cochlear hair cells for frequency tuning (Hudspeth and Lewis 1988; Wu et al. 1995). As expected, the underlying mechanisms are complex, and it has taken over 20 years of study to develop kinetic gating mechanisms that can account for the synergistic actions of voltage and Ca2+ i activation of BK channels over wide ranges of experimental conditions (see references in Cui and Aldrich 2000; Rothberg and Magleby 2000).
In addition to their activation by Ca2+ i, it has been known for some time that BK channels also are modulated by Mg2+ i (Golowasch et al. 1986; Oberhauser et al. 1988), but the mechanism of this modulation has remained obscure. This has now all changed, not in 20 years but essentially overnight, with the publication of two papers in this issue of the Journal of General Physiology. These landmark studies raise our knowledge about Mg2+ i action on BK channels from some very provocative observations to complete mechanistic descriptions that can account for the action of Mg2+ i over wide ranges of Ca2+ i and voltage. The work comes from the laboratories of Jianmin Cui (Shi and Cui 2001) and Chris Lingle (Zhang et al. 2001).
How is it possible that so much progress has been made in just a few years of study, rather than decades? Enter the ability of the kinetic model to explain complex phenomena and the power of molecular biology to dissect the physical basis of mechanism. However, before the new findings on Mg2+ can be discussed, it is first necessary to briefly review the structure and gating mechanism of BK channels. The four pore forming α subunits of BK channels (Slo) show homology with the pore-forming subunits of the six transmembrane superfamily of voltage-dependent K+ channels, including an S4 voltage sensor (Atkinson et al. 1991; Adelman et al. 1992; Butler et al. 1993; Diaz et al. 1998; Cui and Aldrich 2000). BK channels have an additional S0 transmembrane segment that places the NH2 terminus extracellular (Meera et al. 1997). The COOH terminus of BK channels exceeds the length of region S0–S6, being much longer than in typical K+ channels, and contains a high affinity Ca2+ binding site termed the calcium bowl (Wei et al. 1994; Schreiber and Salkoff 1997; Schreiber et al. 1999; Bian et al. 2001).
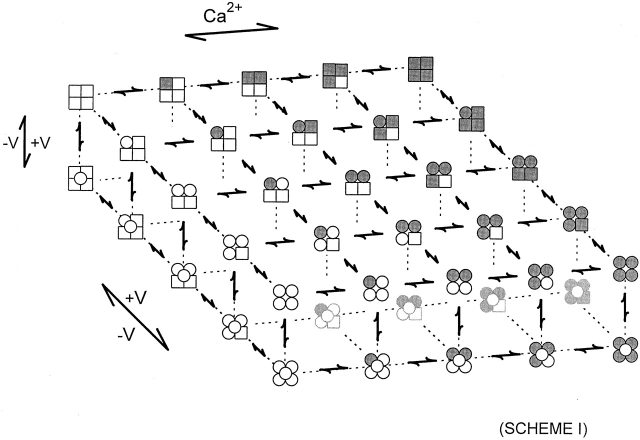
A gating mechanism that can account for the Ca2+ i and voltage activation of BK channels from 0 Ca2+ i to high Ca2+ i (1 mM) over a wide range of voltages is shown in Fig. 1 (Horrigan and Aldrich 1999; Rothberg and Magleby 1999, Rothberg and Magleby 2000; Cui and Aldrich 2000; Cox and Aldrich 2000). This two-tiered gating mechanism consists of 25 closed states on the upper tier and 25 open states on the lower tier. The tetrameric structure of BK channels (Shen et al. 1994) is reflected in the four subunits comprising each state of the channel. In Fig. 1, Ca2+ i binding to the calcium bowl is indicated by shading, and the depolarization-induced movement of each S4 voltage sensor is indicated by a transition from a square to a circle. It is the four potential configurations of each of the four subunits that leads to the large numbers of states on each tier. Since the theoretical basis for such large numbers of states was pointed out more than 30 years ago by Eigen 1968, why has it taken so many years to arrive at Fig. 1. One reason is that gating mechanisms are constructed in a stepwise manner, adding additional states only as necessary to account for the experimental data. Initial models for gating of BK channels were based on the relatively simple 10-state Monod-Wyman-Changeux (MWC) model (Monod et al. 1965), and provided reasonable descriptions of the gating over relatively wide ranges of experimental conditions (McManus and Magleby 1991; Wu et al. 1995; Cox et al. 1997; Cui et al. 1997).
Scheme S1.
It was only when improved analysis methods were used to investigate the extremes of gating at large, depolarized voltages and also at zero and high Ca2+ i that it became obvious that the gating was far more complex than the 10-state MWC model (Horrigan and Aldrich 1999; Horrigan et al. 1999; Rothberg and Magleby 1999; Nimigean and Magleby 2000; Talukder and Aldrich 2000). Not only would the theoretical states proposed by Eigen 1968 be needed, but the Eigen model would have to be expanded with an entire second tier to provide both the required numbers of states and also multiple transition pathways between the open and closed states at the extremes of gating at zero and high Ca2+ i, as shown in Fig. 1. The actual gating mechanism most likely is more complex than Fig. 1 (a third tier may be required to fully account for the brief closings), and there is the potential for up to 55 states per tier (Cox et al. 1997), but Fig. 1 will be sufficient to discuss the new findings on Mg2+.
Just as Eigen 1968 predicted the potential for large numbers of states many years ago from subunit structure, perhaps we can predict a gating mechanism to include the effects of Mg2+ i before looking in detail at the new findings. The previous observations by Golowasch et al. 1986 and Oberhauser et al. 1988 that 1–10 mM Mg2+ i increases the Hill coefficient for Ca2+ activation of BK channels from ∼2 to >4 in a dose-dependent manner, were an early indication that BK channels may contain low affinity Mg2+ binding sites that facilitate activation.
Given Fig. 1, and assuming that each one of the four subunits comprising the channel also has a separate Mg2+ binding site, what would the predicted model be? As each one of the 50 states in Fig. 1 now could bind either zero, one, two, three, or four Mg2+ ions, Fig. 1 would be expanded to 250 states. This is just the type of model that Shi and Cui 2001 and Zhang et al. 2001 have found consistent with the complex interactions between voltage, Ca2+, and Mg2+. Although a 250-state model may appear complex at first inspection, the basis for such a model is simplicity itself, following directly from four subunits, each with a voltage sensor, a high affinity Ca2+ binding site, a low affinity Mg2+ (divalent) binding site, and an additional (concerted) conformational change associated with channel opening.
What then are the effects of Mg2+ i on BK channels reported in these new studies? One effect, the reduction of single-channel current amplitude by millimolar concentrations of intracellular divalent cations due to voltage-dependent fast block has been known for some time (Ferguson 1991), and will not be considered here. To discuss the other effects of Mg2+ i, first, it is necessary to explain the assay method typically used to characterization the Ca2+ activation of BK channels. For a fixed Ca2+ i, the holding potential is changed and the membrane conductance or open probability (Po) of the BK channel or channels is measured. This produces a sigmoid curve, with normalized conductance (or Po) changing from zero to one as the voltage moves in the depolarizing direction. Because of the synergistic interaction between voltage and Ca2+ i, the voltage for half activation (V0.5) shifts to the left for higher Ca2+ i (i.e., with higher Ca2+ i, less depolarization is needed for the same level of activation). Alternatively, if some factor such as Mg2+ i facilitates the activation of the channel, then this would be indicated by a leftward shift in V0.5 for a fixed Ca2+ i, as less depolarization would be required for the same level of activation.
Using this assay, Zhang et al. 2001 found a continuous monotonic leftward shift in V0.5 as Ca was increased more than four orders of magnitude, from <10−6 to >10−2 M. A series of exhaustive experiments over wide ranges of Ca2+ i, Mg2+ i, and voltage then suggested two binding sites with different properties. For divalent cation concentrations, <300 μM, the leftward shift and altered kinetics of activation were specific for Ca2+ i, suggesting a high affinity Ca2+ binding site. Once the high affinity site was saturated with Ca2+, the addition of 1–100 mM Mg2+ i was just as effective as the addition of 1–100 mM Ca2+ i in continuing the leftward shift and altering the kinetics, suggesting a low affinity nonspecific divalent site. Ca2+ i was not required for the facilitating effect of Mg2+ i, as 10 mM Mg2+ i gave similar leftward shifts in the presence or absence of 4, 10, 100, and 300 μM Ca2+ i. Increases in Ca2+ at the high affinity site greatly increased the rate of activation, whereas increases in Ca2+ and/or Mg2+ at the low affinity site gave only small increases in the rate of activation, while greatly slowing deactivation. Zhang et al. 2001 suggested that the differential effects on the kinetics of low and high concentrations of cations indicated separate sites of action and separate regulatory mechanisms. Zhang et al. 2001 found that the interactions between Ca2+ i, Mg2+ i, and voltage were consistent with Fig. 1 expanded to 250 states to include four independent low affinity Ca2+/Mg2+ binding sites in addition to the four independent high affinity Ca2+ binding sites and the four voltage sensors per channel. In their model Mg2+ i increases Po by binding more tightly to the open states once they have been activated, slowing the rate at which channels leave the open states. For both the experimental data and the theoretical predictions of the 250 state model, the Hill coefficient for activation by Ca2+ i increased with Mg2+ i and also with depolarization. These effects of Mg2+ i (Golowasch et al. 1986; Oberhauser et al. 1988) and depolarization (Cox et al. 1997) are consistent with previous findings.
Whereas Zhang et al. 2001 coaxed the secrets of Mg2+ i modulation from the channel with a heavy dose of kinetics applied over a wide range of Ca2+ i, Mg2+ i, and voltage, Shi and Cui 2001 examined fewer conditions, but selected them to maximize the effects and also applied the power of molecular biology to dissect the binding sites. Consistent with the findings of Zhang et al. 2001, Shi and Cui 2001 found that the Mg2+ i induced leftward shift in V0.5 first became significant as Mg2+ i approach 1 mM, and that the leftward shift then continued up to the examined concentration of 100 mM Mg2+ i. A compelling superimposed plot showed that the leftward shift was independent of whether Ca2+ i was either 0 or 110 μM, suggesting independent mechanisms of action for the high affinity Ca2+ site and the low affinity Mg2+ i site.
To locate the low affinity site, Shi and Cui 2001 examined the effect of Mg2+ i on Slo3, a pH- sensitive K+ channel that is insensitive to Ca2+ i and does not have a functional calcium bowl (Schreiber et al. 1998, Schreiber et al. 1999). For the Slo3 channel, V0.5 was unaffected by 10 mM Mg2+ i, indicating the absence of a functional low affinity site. Replacing the core (S0–S8) of the Slo3 channel with the core (S0–S8) of the Slo1 (BK) channel to form a Slo1 core/Slo3 tail channel then restored the leftward shift in V0.5 induced by 10 mM Mg2+ i. Thus, the low affinity Mg2+ site is located on the core (S0–S8) of the BK channel. Since the Slo1 core/Slo3 tail channel does not contain a high affinity site (calcium bowl), these results clearly establish that the low and high affinity sites are different. Shi and Cui were able to account for their observations using the equivalent of a 250-state model in which each subunit of the channel had a separate high affinity Ca2+ binding site and a low affinity Mg2+ binding site. Shi and Cui also suggested that high concentrations of Mg2+ i could compete for Ca2+ at the high affinity site, reducing its expected effect. Sharing of information between the two groups when the papers were in review alerted Zhang et al. 2001 to the possibility of Mg2+ i competition at the high affinity Ca2+ site, allowing them to further refine their analysis before publication. (One cannot help but wonder how much faster science might progress if there were more sharing of information before publication.)
The findings of Zhang et al. 2001 and Shi and Cui 2001 were in close agreement, with estimates of the various equilibrium constants within a factor of two of each other. One minor difference is that Zhang et al. argue that the unusual behavior of the Hill coefficients for Ca2+ i action arises from the differential contribution of the high and low affinity sites to the Hill plots at different voltages and Mg2+ i. On the other hand, Shi and Cui 2001 argue that the inhibitory effect of Mg2+ i on the high affinity site together with the activation effects on the low affinity sites contribute to the increased Hill coefficients in the presence of Mg2+ i. Often when there are differences in model-dependent conclusions of this type, the differences may reflect that the underlying models are still too simple, so that the conclusions become sensitive to the data being analyzed.
What then is the mechanism of action of Mg2+ i? These studies suggest that Mg2+ i activates BK channels independently of voltage and Ca2+ i by binding to the open configuration of the channel. The low affinity Mg2+ i sites do not participate in the Ca2+-dependent steps that influence the rate of activation. Thus, there are three separate factors that act to shift the equilibrium from the closed to the open states for BK channels: depolarization, Ca2+ i acting at high affinity sites, and Mg2+ i acting at separate low affinity sites. Under physiological conditions, Ca2+ i tends to be low compared with the millimolar concentrations of Mg2+ i, so that Mg2+ i would be the main ion bound to the low affinity sites. However, for BK channels close to active Ca2+ channels, it is possible that Ca2+ i could increase activity by binding to both the high and the low affinity sites.
These papers answer the fundamental question of how Ca2+ i over a range spanning more than four orders of magnitude can shift V0.5 in a monotonic fashion with pCa. The conclusions in this and previous work that multiple allosteric regulators (voltage, Ca2+ i, and Mg2+ i) can separately and independently regulate gating is of interest to how channels can be modulated. The fact that the low and high affinity sites map to different parts of the channel suggests that perhaps such allosteric regulatory domains are modular in nature. Finally, these results provide an explanation for the observation in these and previous studies (Golowasch et al. 1986; Oberhauser et al. 1988) that Mg2+ i increases the Hill coefficients for activation of the channel by Ca2+ i. The interactions between the various allosteric modulators are sufficient to account for the observations, so that it is not necessary to propose a Ca2+ i-induced change in the numbers of apparent Ca2+ binding sites.
What are the future directions of research in this area? Since kinetic models are seldom complete, they just get closer and closer to the underlying processes, it would be worthwhile to examine the effects of Mg2+ i using single-channel recording to determine if the proposed models for Mg2+ i actions can capture the detailed single-channel kinetics. If not, then further analysis would be needed. It would also be useful to obtain rate constants for the various steps in the gating mechanism to supplement the equilibrium constants obtained in these studies. Models that have been developed to account for the gating of BK channels often assume that the various allosteric modulators are independent of one another, acting on a common step of open-closed equilibrium. Although these assumptions have proven successful in describing the data and are especially useful for simplifying the analysis, it would be worthwhile to determine to what extent these assumptions are valid using more detailed analysis of the kinetics of the single-channel data and macroscopic currents.
The present studies were concerned with Ca2+ i and Mg2+ i, whereas Oberhauser et al. 1988 have shown that many different divalent cations can activate and/or modulate the activity of BK channels. It would be of interest to extend the present studies to include additional divalent cations to further characterize the sites and their actions. More precise location of the low affinity site could give insight into its mechanism of action, and it is not entirely clear whether the calcium bowl accounts for all of the effects of the high affinity site (Braun and Sy 2001). Finally, it will be necessary to see how many additional allosteric modulating sites and/or mechanisms are involved in the gating of BK channels, leading to further expansion of the kinetic models.
The 250-state kinetic models for the voltage- and Ca2+-dependent gating of BK channels and their modulation by Mg2+ i appear complex due to the large numbers of states, but it needs to be remembered that these models follow directly from the simple underlying assumptions of a tetrameric channel with three allosteric activators (voltage, Ca2+, and Mg2+) that bias the closed-open transitions. These complex models provide a means, perhaps the only means, to describe the combined actions of the allosteric activators on the gating. How then does complexity lead to simplicity? Once one has the complex model, it is a simple matter to predict the intricate effects of the allosteric activators on the gating of BK channels.
References
- Adelman J.P., Shen K.Z., Kavanaugh M.P., Warren R.A., Wu Y.N., Lagrutta A., Bond C.T., North R.A. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- Atkinson N.S., Robertson G.A., Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. doi: 10.1126/science.1857984. [DOI] [PubMed] [Google Scholar]
- Barrett J.N., Magleby K.L., Pallotta B.S. Properties of single calcium-activated potassium channels in cultured rat muscle. J. Physiol. 1982;331:211–230. doi: 10.1113/jphysiol.1982.sp014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian S., Favre I., Moczydlowski E. Ca2+-binding activity of a COOH-terminal fragment of the Drosophila BK channel involved in Ca2+-dependent activation. Proc. Natl. Acad. Sci. USA. 2001;98:4776–4781. doi: 10.1073/pnas.081072398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A.F., Sy L. Contribution of potential EF hand motifs to the calcium-dependent gating of a mouse brain large conductance, calcium-sensitive K+ channel. J. Physiol. 2001;533:681–695. doi: 10.1111/j.1469-7793.2001.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler A., Tsunoda S., McCobb D.P., Wei A., Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Cox D.H., Aldrich R.W. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J. Gen. Physiol. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D.H., Cui J., Aldrich R.W. Allosteric gating of a large conductance Ca-activated K+ channel. J. Gen. Physiol. 1997;110:257–281. doi: 10.1085/jgp.110.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Aldrich R.W. Allosteric linkage between voltage and Ca2+-dependent activation of BK-type mslo1 K+ channels. Biochemistry. 2000;39:15612–15619. doi: 10.1021/bi001509+. [DOI] [PubMed] [Google Scholar]
- Cui J., Cox D.H., Aldrich R.W. Intrinsic voltage dependence and Ca2+ regulation of mslo large conductance Ca-activated K+ channels. J. Gen. Physiol. 1997;109:647–673. doi: 10.1085/jgp.109.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz L., Meera P.J., Amigo E., Stefani E., Alvarez O., Toro L., Latorre R. Role of the S4 segment in a voltage-dependent calcium-sensitive potassium (hSlo) channel. J. Biol. Chem. 1998;273:32430–32436. doi: 10.1074/jbc.273.49.32430. [DOI] [PubMed] [Google Scholar]
- Eigen M. New looks and outlooks on physical enzymology. Q. Rev. Biophys. 1968;1:3–33. doi: 10.1017/s0033583500000445. [DOI] [PubMed] [Google Scholar]
- Ferguson W.B. Competitive Mg2+ block of a large-conductance, Ca2+-activated K+ channel in rat skeletal muscle. Ca2+, Sr2+, and Ni2+ also block. J. Gen. Physiol. 1991;98:163–181. doi: 10.1085/jgp.98.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch J., Kirkwood A., Miller C. Allosteric effects of Mg2+ on the gating of Ca2+-activated K+ channels from mammalian skeletal muscle. J. Exp. Biol. 1986;124:5–13. doi: 10.1242/jeb.124.1.5. [DOI] [PubMed] [Google Scholar]
- Horrigan F.T., Aldrich R.W. Allosteric voltage gating of potassium channels II. mSlo channel gating charge movement in the absence of Ca2+ J. Gen. Physiol. 1999;114:305–336. doi: 10.1085/jgp.114.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan F.T., Cui J., Aldrich R.W. Allosteric voltage gating of potassium channels I mSlo ioniccurrents in the absence of Ca2+. J. Gen. Physiol. 1141999. 277 304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A.J., Lewis R.S. Kinetic analysis of voltage- and ion-dependent conductances in saccular hair cells of the bull-frog, Rana catesbeiana . J. Physiol. 1988;400:237–274. doi: 10.1113/jphysiol.1988.sp017119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski G.J., Knaus H.G., Leonard R.J., McManus O.B., Garcia M.L. High-conductance calcium-activated potassium channelsstructure, pharmacology, and function. J. Bioenerg. Biomembr. 1996;28:255–267. doi: 10.1007/BF02110699. [DOI] [PubMed] [Google Scholar]
- Latorre R. Molecular workings of large conductance (maxi) Ca2+-activated K+ channels. In: Peracchia C., editor. Handbook of Membrane ChannelsMolecular and Cellular Physiology. Academic Press; New York: 1994. pp. 79–102. [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981;291:497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- McManus O.B., Magleby K.L. Accounting for the Ca2+-dependent kinetics of single large-conductance Ca2+-activated K+ channels in rat skeletal muscle. J. Physiol. 1991;443:739–777. doi: 10.1113/jphysiol.1991.sp018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meera P., Wallner M., Song M., Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0-S6), an extracellular N terminus, and an intracellular (S9-S10) C terminus. Proc. Natl. Acad. Sci. USA. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod J., Wyman J., Changeux J.-P. On the nature of allosteric transitionsa plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Nimigean C.M., Magleby K.L. Functional coupling of the β1 subunit to the large conductance Ca2+-activated K+ channel in the absence of Ca2+increased Ca2+ sensitivity from a Ca2+-independent mechanism. J. Gen. Physiol. 2000;115:719–734. doi: 10.1085/jgp.115.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhauser A., Alvarez O., Latorre R. Activation by divalent cations of a Ca2+-activated K+ channel from skeletal muscle membrane. J. Gen. Physiol. 1988;92:67–86. doi: 10.1085/jgp.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B.S., Magleby K.L. Gating kinetics of single-large-conductance Ca2+-activated K+ channels in high Ca2+ suggest a two-tiered allosteric gating mechanism. J. Gen. Physiol. 1999;114:95–124. doi: 10.1085/jgp.114.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B.S., Magleby K.L. Voltage and Ca2+ activation of single large-conductance Ca2+-activated channels described by a two tier allosteric gating mechanism. J. Gen. Physiol. 2000;116:75–99. doi: 10.1085/jgp.116.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M., Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys. J. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber M., Wei A., Yuan A., Gaut J., Saito M., Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J. Biol. Chem. 1998;273:3509–3516. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- Schreiber M., Yuan A., Salkoff L. Transplantable sites confer calcium sensitivity to BK channels. Nat. Neurosci. 1999;2:416–421. doi: 10.1038/8077. [DOI] [PubMed] [Google Scholar]
- Shen K.-Z., Lagrutta A., Davies N.W., Standen N.B., Adelman J.P., North R.A. Tetraethylammonium block of slowpoke calcium-activated potassium channels expressed in Xenopus oocytesevidence for tetrameric channel formation. Pflügers Arch. 1994;426:440–445. doi: 10.1007/BF00388308. [DOI] [PubMed] [Google Scholar]
- Shi J., Cui J. Intracellular Mg2+ enhances the function of BK-type Ca2+-activated K+ channels. J. Gen. Physiol. 2001;589–605 doi: 10.1085/jgp.118.5.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukder G., Aldrich R.W. Complex voltage-dependent behavior of single unliganded calcium-sensitive potassium channels. Biophys. J. 2000;78:761–772. doi: 10.1016/S0006-3495(00)76634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei A., Solaro C., Lingle C., Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 1994;13:671–681. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Wu Y.C., Art J.J., Goodman M.B., Fettiplace R. A kinetic description of the calcium-activated potassium channel and its application to electrical tuning of hair cells. Prog. Biophys. Mol. Biol. 1995;63:131–158. doi: 10.1016/0079-6107(95)00002-5. [DOI] [PubMed] [Google Scholar]
- Zhang X., Solaro C.R., Lingle C.J. Allosteric regulation of BK channel gating by Ca2+ and Mg2+ through a nonselective low affinity divalent cation site. J. Gen. Physiol. 2001;607–635 doi: 10.1085/jgp.118.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]