Abstract
The roles played by ATP binding and hydrolysis in the complex mechanisms that open and close cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels remain controversial. In this work, the contributions made by ATP and Mg2+ ions to the gating of phosphorylated cardiac CFTR channels were evaluated separately by measuring the rates of opening and closing of single channels in excised patches exposed to solutions in which [ATP] and [Mg2+] were varied independently. Channel opening was found to be rate-limited not by the binding of ATP alone, but by a Mg2+-dependent step that followed binding of both ATP and Mg2+. Once a channel had opened, sudden withdrawal of all Mg2+ and ATP could prevent it from closing for tens of seconds. But subsequent exposure of such an open channel to Mg2+ ions alone could close it, and the closing rate increased with [Mg2+] over the micromolar range (half maximal at ∼50 μM [Mg2+]). A simple interpretation is that channel closing is stoichiometrically coupled to hydrolysis of an ATP molecule that remains tightly associated with the open CFTR channel despite continuous washing. If correct, that ATP molecule appears able to reside for over a minute in the catalytic site that controls channel closing, implying that the site must entrap, or have an intrinsically high apparent affinity for, ATP, even without a Mg2+ ion. Such stabilization of the open-channel conformation of CFTR by tight binding, or occlusion, of an ATP molecule echoes the stabilization of the active conformation of a G protein by GTP.
Keywords: single channels, gating kinetics, nucleotide binding domains, ATP binding, free [Mg2+]
INTRODUCTION
Cystic fibrosis transmembrane conductance regulator (CFTR)* Cl− channels, like other members of the large ATP binding cassette (ABC) protein family, contain cytoplasmic nucleotide binding domains thought to bind and hydrolyze ATP. In prokaryotic ABC proteins, such as the histidine or maltose permeases, as well as eukaryotic ABC proteins, like P-glycoprotein or multidrug resistance–related protein (MRP), cycles of conformational changes driven by binding and hydrolysis of MgATP are believed to alternate access to the substrate transport sites from one side of the membrane to the other, in concert with alterations of substrate binding affinity (for reviews see Senior et al., 1995; Holland and Blight, 1999; Chang and Roth, 2001). Analogous cycles of MgATP binding and hydrolysis at the nucleotide binding domains in CFTR might reasonably be anticipated to control the conformational changes that open and close the gate to its anion-selective pore. However, despite numerous experiments designed to test that expectation, the complex mechanisms of CFTR channel gating, and their regulation by events at the nucleo-tide binding domains, remain unclear (for reviews see Gadsby and Nairn, 1999; Sheppard and Welsh, 1999).
A large body of evidence has established that, before a CFTR channel can be opened by MgATP (Anderson et al., 1991), the channel must first be phosphorylated by protein kinase A (Cheng et al., 1991; Picciotto et al., 1992), and probably also by protein kinase C (Jia et al., 1997), on multiple serines within the regulatory (R) domain (and possibly elsewhere; Csanády et al., 2000). Early studies showed that phosphorylated CFTR channels in excised, inside-out patches could be opened readily by low concentrations of a variety of hydrolyzable nucleotide triphosphates, but not by poorly hydrolyzable ATP analogues such as AMPPNP (Anderson et al., 1991; Nagel et al., 1992; Carson and Welsh, 1993; but see also Aleksandrov et al., 2000). This led to the suggestion that CFTR channel opening requires ATP hydrolysis (Anderson et al., 1991; Gadsby and Nairn, 1994; Hwang et al., 1994). Subsequent measurements using purified reconstituted protein confirmed that CFTR can indeed hydrolyze ATP (Li et al., 1996; Ram-jeesingh et al., 1999), but, like all other nucleoside triphosphatases (e.g., Higashijima et al., 1987b; John et al., 1993; Urbatsch et al., 1994; Weber et al., 1998), it needs Mg2+ ions (or other divalent cations) to do so (Li et al., 1996).
Previous tests of the suggested requirement of ATP hydrolysis for CFTR channel opening have included omission of Mg2+ ions, or chelating them with EDTA. But interpretation of the results has been equivocal because, although withdrawal of Mg2+ did impair CFTR channel opening by ATP, some gating, characterized by reduced rates of channel opening and closing, persisted (Anderson et al., 1991; Carson et al., 1995; Gunderson and Kopito, 1995; Schultz et al., 1996; Aleksandrov et al., 2000; Ikuma and Welsh, 2000). In addition, it has been suggested that an alternative explanation for the observed impairment of ATP-dependent channel opening caused by omitting Mg2+ ions might be consequent interference with ATP binding (Ikuma and Welsh, 2000). However, a strong challenge to a strict dependence of channel opening on MgATP hydroly-sis at either of CFTR's nucleotide binding domains (NBDs) has come from findings with channels bearing engineered mutations at the conserved Walker A lysine residues (K464 in the N-proximal NBD, NBD1; K1250 in the C-proximal NBD, NBD2), mutations shown to abolish MgATP hydrolysis, and/or vanadate-induced trapping of nucleotide in other ABC proteins, e.g., P-glycoprotein (Loo and Clarke, 1994; Müller et al., 1996; Urbatsch et al., 1998) and MRP1 (Gao et al., 2000; Hou et al., 2000). Thus, although ATPase activity is diminished 10–20-fold in mutant K464A CFTR, and practically abolished in K1250A CFTR (Ramjeesingh et al., 1999), channel opening rate at millimolar [MgATP] has been reported to be reduced only 2–4-fold in K464A and somewhat more severely (5–10-fold) in K1250A CFTR (Carson et al., 1995; Gunderson and Kopito, 1995; Ramjeesingh et al., 1999); and gating persists even in double mutant K464A/K1250A CFTR channels (Carson et al., 1995).
Hydrolysis of MgATP has also been proposed to play a critical role in orchestrating the closure of a CFTR channel from an open burst, but this too remains controversial. Thus, highly phosphorylated wild-type CFTR channels exposed to MgATP mixed with a poorly hydrolyzable analogue like MgAMPPNP, or with polyphosphates, like PPi or PPPi, may become “locked open” in prolonged open burst states (Gunderson and Kopito, 1994; Hwang et al., 1994; Carson et al., 1995), reminiscent of the extremely prolonged active state of G proteins occupied by MgGMPPNP in place of MgGTP (e.g., Bourne et al., 1991). These findings on the CFTR channel were interpreted as suggesting that the open burst state became prolonged when MgAMPPNP replaced MgATP at a catalytic site at which hydrolysis of MgATP normally prompted channel closure. The finding that K1250A CFTR channels, mutated within the NBD2 catalytic site, when exposed to millimolar MgATP alone displayed prolonged open bursts comparable to those elicited by MgAMPPNP in wild-type channels, suggested that NBD2 comprises the active site that controls normal termination of open bursts (Carson et al., 1995; Gunderson and Kopito, 1995). That simple picture is complicated, however, by the finding that the average burst duration of a group of wild-type CFTR channels exposed to millimolar MgATP can vary with phosphorylation status, reflecting varying contributions of two distinct populations of bursts; this implies the existence of two different mechanisms for termination of open bursts (Hwang et al., 1994; Csanády et al., 2000). A possibly related finding is that the open burst duration of K1250A CFTR channels, though prolonged at millimolar MgATP, has been reported to be brief at micromolar MgATP (Zeltwanger et al., 1999; Ikuma and Welsh, 2000). In addition, analyses of the temperature dependence of the gating of wild-type CFTR channels exposed to MgATP have been interpreted either as supporting a role for nucleotide hydrolysis in channel closing (Mathews et al., 1998b; Csanády et al., 2000), or as indicating that open and closed states of CFTR channels are in thermal equilibrium and, hence, that CFTR channels (like ligand-gated channels) close by a diffusion-limited process (Aleksandrov and Riordan, 1998; Aleksandrov et al., 2000).
To help clarify the steps that determine the rates of opening and closing of wild-type CFTR channels, in the present work we have examined the dependence on [Mg2+] of the opening and closing rates of individual native CFTR channels in inside-out patches of membrane excised from mammalian cardiac myocytes. The results argue strongly that, in highly phosphorylated CFTR, channel opening is rate-limited by a Mg2+-requiring step that follows, and is distinct from, ATP binding. They also suggest that the open burst state is stabilized by tight binding of ATP, and that hydrolysis of that tightly held ATP is stoichiometrically coupled to channel closure. Our results further indicate that ATP and Mg2+ ions may bind independently to a CFTR channel, both at the catalytic site that controls channel opening and at the site that controls closing.
MATERIALS AND METHODS
Isolation of Myocytes
Single ventricular myocytes were isolated by collagenase digestion of guinea pig hearts, as previously described (Hwang et al., 1993), and used within 24 h. Briefly, guinea pigs of either sex were deeply anesthetized with pentobarbital (50–100 mg/kg, intraperitoneal), the heart quickly excised, its aorta cannulated, and retrograde coronary perfusion begun with oxygenated Tyrode's solution at 36°C. After 2–3 min, the perfusate was switched to nominally Ca2+-free Tyrode's solution until contraction stopped, and then for ∼10 min to a solution containing 0.1–0.3 mg/ml Yakult, or 0.5–1.0 mg/ml Worthington (type 2), or 0.5–1.0 mg/ml Sigma-Aldrich (type 1) collagenase, or a mixture of the former with either of the latter. The enzyme solution was then washed out with a high K+, low Ca2+ medium (Isenberg and Klöckner, 1982) first at 36°C, then at room temperature, and the partially digested heart was cut into small chunks and filtered through nylon mesh; the resulting myocyte suspension was stored at 4°C in the same solution.
Excised Patch Recording
Giant (8–20-μm tip diameter) patch pipettes fabricated from borosilicate glass (N51A; Drummond Scientific) made hydrophobic at the tips with a hydrocarbon mixture (Hilgemann, 1995) were tightly sealed (resistance ≥20 GΩ) with light suction to large spherical sarcolemmal blebs (Collins et al., 1992) produced by storing myocytes in a Ca2+-free, high K+ solution. After excision, the inside-out patch was placed in a miniature flow chamber in which solutions could be exchanged rapidly (∼1 s) by switching electric valves (General Valve Corp.). Patch current was recorded with a List EPC-7 amplifier (List Electronics), filtered at 200 Hz, monitored directly on a chart recorder (Kipp and Zonen), and stored on videotape for later analysis using custom Asyst software. All recordings were made at room temperature (22–24°C).
Solutions
Pipettes were filled with a high [Cl−] (extracellular) solution containing (in mM): 145 N-methyl-D-glucamine (NMG+), 5 Cs+, 2 Ba2+, 2.3 Mg2+, 0.5 Cd2+, 159.6 Cl−, and 10 HEPES (pH 7.4 with NMG). The standard, ATP-free bath (cytoplasmic) solution contained (in mM): 140 NMG+, 20 tetraethylammonium, 3.2 Mg2+, 6.4 Cl−, 140 aspartate, 10 HEPES, 2 trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid (CDTA) (pH 7.4 with NMG). Pipette and bath solutions and the ≥0-mV holding potential were designed to minimize currents through Ca2+, Na+, and K+ channels. Osmolality of all solutions was ∼300 mOsmol/kg. To determine the [MgATP] dependence of channel open probability, Po (the average fraction of time a channel spends open), total [ATP] was varied by adding MgATP, while keeping free [Mg2+] (calculated with the program MAXC; Bers, 1994) constant at 1.2 mM. For other experiments, total [ATP] was kept constant at either 0 or 2 mM (added as Tris-ATP), [CDTA] was held constant at 2 mM, and free [Mg2+] was adjusted by varying added Mg2+: CDTA was chosen for its high stability constant for Mg2+, ∼1011 M−1 at 20°C (Martell and Smith, 1989). Only in the experiment of Fig. 2 B (see results) was CDTA replaced by 10 mM EGTA (stability constant ∼105 M−1 at 20°C) so as to weakly chelate contaminant Mg2+ ions (assumed ∼5 μM). At the outset of every experiment, PKA catalytic subunit, prepared as described (Kaczmarek et al., 1980) and diluted at ∼100 nM into bath solution containing 2 mM ATP and 1.2 mM free Mg2+, was applied to the patch to activate CFTR channels; unless otherwise indicated, the PKA was withdrawn before collection of the data reported here. To impede CFTR channel deactivation by dephosphorylation (Hwang et al., 1993, 1994), bath solutions included 0.4 μM microcystin-LR to inhibit phosphatases 1 and 2A (Honkanen et al., 1990).
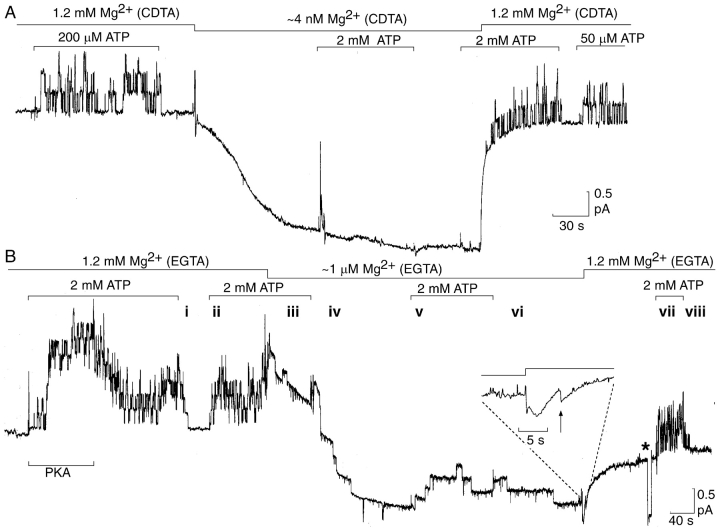
Figure 2.
At constant (2 mM) total [ATP], free [Mg2+] governs both opening and closing rates of cardiac CFTR channels. (A) Complete removal of Mg2+ (assuming contaminating total [Mg2+] ∼5 μM, 2 mM CDTA gives free [Mg2+] ∼4 nM) temporarily suppressed opening by 2 mM ATP of all prephosphorylated CFTR channels (at least three) in the patch. Free [Mg2+] and total [ATP] levels are indicated above the records; all solutions included 2 mM CDTA; Vh = 20 mV. The reversible shifts of holding current following large changes in free [Mg2+] (records in A and B; see also Figs. 3, B and C, and 5 A) correlated with changes of seal resistance, and likely reflected leaching of Mg2+ ions from the membrane–glass interface. (B) Low free [Mg2+] (∼5 μM contaminant Mg with 10 mM EGTA would give free [Mg2+] ∼0.2 μM) delays (slows) opening and closing. Lines indicate free [Mg2+] levels and exposures to 2 mM total [ATP]; periods i–viii are described in the text; in this experiment only, all solutions included 10 mM EGTA; Vh = 0 mV; a 20-mV test pulse (*), applied just before the last exposure to ATP, shows seal resistance was 20 GΩ; (inset) expanded record showing closure of final open channel (arrow) upon arrival of Mg2+ ions.
Measurement of Po and of Open and Closed Times
Average Po was determined by dividing the area under open channel currents by that expected if the largest number of simultaneously observed channels were all open. Closed (interburst) times were measured only in patches that showed no simultaneous openings at 2 mM [MgATP] and 1.2 mM free [Mg2+] (average Po = 0.41 ± 0.04 for the 15 single-channel patches of Fig. 1 C); open burst times were predominantly taken from single-channel patches, but were occasionally (at low [MgATP]) measured in patches containing two or three channels, though only during periods devoid of simultaneous openings. Burst and interburst durations were measured directly from amplified high-speed chart recordings using the half-amplitude criterion to identify openings and closings; control recordings of rectangular test pulses showed that openings or closings ≥35 ms long could be detected, i.e., for burst durations, the method is equivalent to suppressing brief “flickery” closures <35 ms (compare Winter et al., 1994; Zeltwanger et al., 1999; Csanády et al., 2000).
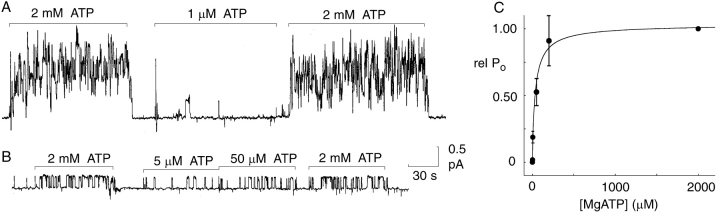
Figure 1.
At constant high (1.2 mM) free [Mg2+], [ATP] (≈ [MgATP]) determines opening rate of cardiac CFTR channels. (A and B) Records of unitary currents in two representative inside-out patches (after PKA washout) containing ≥5 (A) phosphorylated CFTR channels (Vh = 20 mV), or only one (B; Vh = 0 mV), respectively. Standard ATP-free bath (cytoplasmic) solution was replaced by one containing a test [MgATP] (1, 5, 50 μM), between exposures to the reference [MgATP] (2 mM), as indicated; solution was exchanged within ∼1 s. Relative Po was determined in each patch as the ratio of that at the test [MgATP] to the mean of the two bracketing Po values at 2 mM MgATP. (C) Summary of relative Po (rel Po) at test [MgATP] of 0, 1, 5, 50, and 200 μM, averaged from 5–20 measurements at each [MgATP] of phosphorylated channels but after withdrawal of PKA. The curve shows the least-squares fit to the Hill equation: rel Po = Po([MgATP])/Po(2 mM) = rel Pomax/{1+(K0.5/[ATP])n}, where n = 0.9 ± 0.2, K0.5 = 35 ± 11 μM, and rel Pomax = 1.04 ± 0.06.
Distributions of Open and Closed Dwell Times
To analyze the influence of [Mg2+] on gating kinetics, single-channel opening and closing rates (closures from bursts) were estimated, respectively, from exponential fits to distributions of closed-state and open-burst dwell times. For these experiments, closed times were measured in single-channel patches as latencies from the time of addition of 2 mM ATP until the channel first opened (e.g., Fig. 3, below). Correspondingly, open burst durations were measured from the time of addition of the test free [Mg2+] (or, for free [Mg2+] ≤5 μM, from the washout of ATP) until the channel closed (e.g., Fig. 5, below). At the usual 20-mV holding potential, channel closure (e.g., Fig. 5) or unlocking (e.g., Fig. 7) in the absence of nucleotide was signaled unequivocally by an abrupt ∼0.4-pA inward current shift that was followed by a sojourn of several seconds at the new current level. The relatively small numbers of measurements generally precluded construction and fitting of histograms. Instead, individual dwell times were ranked by duration in descending order (Baukrowitz et al., 1994; Csanády et al., 2000), plotted against rank, and fitted with a single exponential function by nonlinear least squares; the reasonable fits demonstrate the existence, in each case, of a single kinetically defined closed or open (burst) state. Normalization by the zero-time intercept yielded survivor functions of the form P(t) = e -kt, where P(t) is the probability that a channel remained closed (open) for an interval >t, given that it was closed (open) at t = 0 (P(0) = 1), and k gives the mean opening (closing) rate of the channel. In the case of channel opening at 1.2 mM [Mg2+], the 103 measurements were enough to distribute in 27 1-s bins, each containing 0–23 points which, when fit with a single exponential (ignoring the first bin), yielded a mean opening rate of 0.28 ± 0.03 s−1, not very different from that (0.22 ± 0.01 s−1) estimated as above from the survivor plot. Data are given as mean ± SEM.
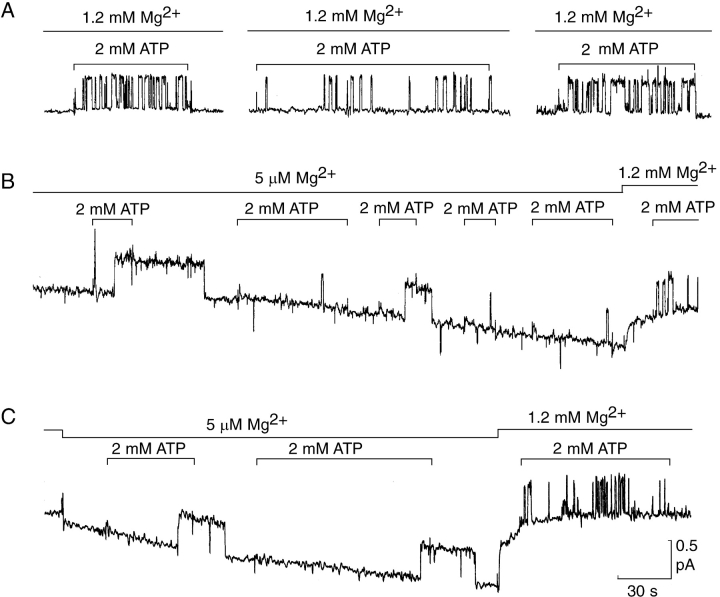
Figure 3.
Influence of free [Mg2+] on opening rate of prephosphorylated channels at constant 2 mM total [ATP]. (A) Representative records from three single-channel patches at 1.2 mM free [Mg2+]. Bars mark sudden addition and removal of 2 mM ATP solution, which included 2 mM CDTA, 5.1 mM total Mg, 1.9 mM MgATP complex, 0.1 mM free ATP; Vh = +20 mV. (B and C) Response of two representative single-channel patches to lowering free [Mg2+] to 5 μM; lines indicate free [Mg2+] and exposures to 2 mM ATP solution which, at 5 μM free [Mg2+], included 2 mM CDTA, 1.6 mM total Mg, 0.1 mM MgATP complex, 1.9 mM free ATP; Vh = 20 mV.
Figure 5.
Mg2+ ions alone prompt closing of CFTR channels from open bursts initially prolonged by withdrawal of Mg2+ along with nucleotide. (A–D) Representative records from four patches containing one (A, C, and D) or two (B) channels, showing shorter delays to channel closure (arrows) at higher free [Mg2+]; all solutions contained 2 mM CDTA. Lines above records indicate free [Mg2+] levels and timing of exposures to 2 mM total [ATP]: prephosphorylated channels in all patches were first opened with 2 mM ATP at 5 μM free Mg2+, whereupon ATP was withdrawn and free [Mg2+] kept at 5 μM or lowered to zero; times to channel closure were measured at those free [Mg2+] levels (C and D), or after raising free [Mg2+] to 40 μM (B) or 1.2 mM (A).
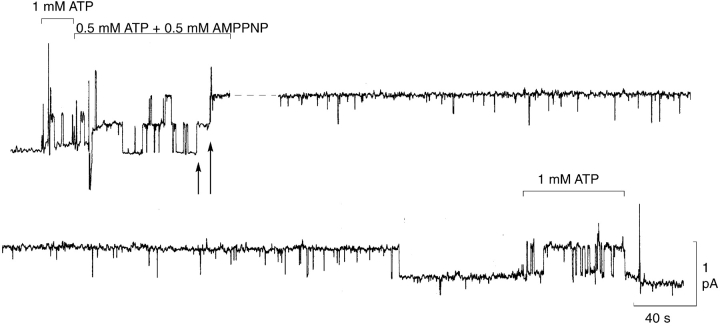
Figure 7.
The poorly hydrolyzable analogue MgAMPPNP, added in the presence of MgATP, can lock prephosphorylated cardiac CFTR channels open in a prolonged burst state. Representative current trace from a patch containing ≤3 channels; Vh = 20 mV. 125 nM PKA was applied with MgATP for 40 s immediately before the start of the record. Lines mark exposure to 1 mM MgATP, or a mixture of 0.5 mM MgATP and 0.5 mM MgAMPPNP, all at constant 1.2 mM free [Mg2+]. Arrows mark channel-locking events; dashed line signifies a 4.5-min break in the record, during which time the two channels remained stably locked. 13 min after removal of all nucleotides, one of the two locked channels finally closed (leaving one locked open), after which that channel apparently opened and closed normally in the presence of 1 mM MgATP (judging from the substantial channel activity at 1 mM MgATP after unlocking, in comparison to that before locking).
RESULTS
Influence of [ATP] at High (1.2 mM) Free [Mg2+] on Cardiac CFTR Channel Gating
Recordings of unitary CFTR Cl− channel currents in inside-out patches that contain several channels (Fig. 1 A) or just one (Fig. 1 B), excised from guinea-pig ventricular myocytes, confirm that, once phosphorylated, cardiac CFTR channels need MgATP to sustain gating, because the channels all shut within seconds of washing away MgATP (Fig. 1, A and B). Also, as shown previously for recombinant human epithelial CFTR channels expressed in mammalian cells or Xenopus oocytes and examined in excised patches (Venglarik et al., 1994; Winter et al., 1994; Zeltwanger et al., 1999; Csanády et al., 2000) or in planar bilayers (Gunderson and Kopito, 1994; Li et al., 1996), the average frequency of opening (i.e., the reciprocal of the mean interval between open bursts) of these native myocyte CFTR channels (Nagel et al., 1992; Hwang et al., 1994) was low, but not zero, at 1 μM MgATP and increased as the MgATP concentration (free [Mg2+] was kept at 1.2 mM, so that [MgATP] ≈ total [ATP]) was raised over the μM-mM range (Fig. 1, A and B). Consequently, average single-channel Po increased with [MgATP] (at constant 1.2 mM free [Mg2+]) along an approximately hyperbolic curve (Fig. 1 C), and was half maximal at 35 ± 11 μM MgATP. Measurements of mean open (burst) and closed (interburst) dwell times at each test MgATP concentration, in single-channel patches, revealed substantial patch-to-patch variation, likely reflecting differences in channel phosphorylation status (Hwang et al., 1994; Winter and Welsh, 1997; Mathews et al., 1998a; Csanády et al., 2000). To minimize the influence of this variability, mean dwell times were compared at low and high [MgATP] for each channel. In twelve patches, the average ratio of mean burst durations at low Po (1 μM ≤ [MgATP] ≤ 50 μM) to those at high Po ([MgATP] = 2 mM) was 0.9 ± 0.1 (n = 12), indicating that burst duration varies little over the [MgATP] range of 1 μM to 2 mM. In contrast, the corresponding ratio of mean interburst times at the same low and high [MgATP] in the seven patches containing a single channel averaged 6.5 ± 2.0 (n = 7). These results demonstrate that, at a fixed free [Mg2+] of 1.2 mM, varying [MgATP] principally influences the closed interburst times or, in other words, the opening rate of cardiac CFTR channels, as established previously for epithelial CFTR channels (Gunderson and Kopito, 1994; Venglarik et al., 1994; Winter et al., 1994; Li et al., 1996; Zeltwanger et al., 1999; Csanády et al., 2000).
Lowering Free [Mg2+] Slows CFTR Channel Gating at Constant 2 mM Total [ATP]
As the influence of Mg2+ withdrawal on gating of epithelial CFTR channels is somewhat controversial (compare Anderson et al., 1991; Gunderson and Kopito, 1995; Li et al., 1996; Schultz et al., 1996; Ikuma and Welsh, 2000), we examined the ability of cardiac CFTR channels to open when exposed to supramaximal [ATP], 2 mM, in the nominal absence of free Mg2+ ions (estimated free [Mg2+] ∼4 nM), ensured by the presence of 2 mM CDTA. A 90-s exposure to 2 mM ATP failed to elicit even a solitary channel opening in a patch containing at least three channels (Fig. 2 A). Nor were any openings observed during a second application of 2 mM ATP until, after 15 s, Mg2+ ions were restored (signaled by the sudden change of seal current), when channels quickly began to open (Fig. 2 A). In experiments on six patches (including that in Fig, 2A), containing an average of 2 channels each, that comprised 750 s of recording during exposure to Mg2+-free solution with 2 mM ATP, we observed a total of no more than five channel openings; this is equivalent to an opening rate of ≤0.004 s−1. Evidently, cardiac CFTR channels require the simultaneous presence of ATP and Mg2+ to open at a rate ≥2% of the maximal rate (∼0.2 s−1, see below), indicating that Mg2+ ions are crucial either for the binding of ATP at the site that controls channel opening, or for some step that follows ATP binding, or for both.
If such a Mg2+-requiring step were involved only in opening a CFTR channel, a sudden drop of the free [Mg2+] to low (μM) levels would be expected to reduce average channel current. But, on the contrary, average patch current initially increased when, at constant 2 mM [ATP], free [Mg2+] was lowered to ∼1 μM (by the weaker chelator EGTA; Fig. 2 B, iii). Evidently, at low free [Mg2+] the channels not only opened slowly when ATP was added (Fig. 2 B, v), but they also closed slowly when ATP was removed (Fig. 2 B, iv and vi; also in the presence of ATP, Fig. 2 B, v). Because at 1.2 mM free Mg2+ the same channels opened and closed promptly, within just a few seconds of adding (Fig. 2 B, ii and vii) or withdrawing (Fig. 2 B, i and viii) ATP (as well as in the presence of ATP, Fig. 2 B, ii and vii), these delays at ∼1 μM free Mg2+ argue that the rates of both opening and closing of the CFTR channels in Fig. 2 B were controlled by [Mg2+]-dependent steps. The following experiments further characterize these steps.
At Low Free [Mg2+], Competition between Free ATP and MgATP Slows CFTR Channel Opening
The strong effects of lowered free [Mg2+] on CFTR channel gating are clearly seen in records from single-channel patches. At 5 μM free Mg2+ (Fig. 3, B and C), channel opening on sudden addition of 2 mM ATP was consistently delayed by many seconds when compared with observations at 1.2 mM free Mg2+ (Fig. 3, A–C), even when the two conditions were imposed consecutively on the same channel (Fig. 3, B and C). In many instances (though not all), channel closing on ATP withdrawal was also markedly delayed at 5 μM free Mg2+ (Fig. 3, B and C). Each delay from the time of ATP addition until the time of channel opening (i.e., the dwell time in the closed interburst state at 2 mM ATP) was measured, and the individual values from numerous trials are summarized in the semilogarithmic survivor plots, one for each of the two free [Mg2+] levels, of Fig. 4. The plots reveal an approximately exponential distribution of these dwell times both at 5 μM and at 1.2 mM free Mg2+. The slopes of these distributions yield mean opening rates for CFTR channels of 0.03 s−1 and 0.22 s−1, at 5 μM and 1.2 mM free Mg2+, respectively, in the presence of 2 mM total [ATP].
Figure 4.
Survivor plots summarizing delays to first channel opening (i.e., closed-state dwell times following sudden introduction of 2 mM total [ATP]) from single-channel patches like those in Fig. 3. The closed times (23 delays from 9 patches at 5 μM Mg2+; 103 delays from 42 patches at 1.2 mM Mg2+) were ranked, fitted with a single exponential, and normalized to yield probability estimates (see materials and methods) plotted here on semilogarithmic axes against dwell time. Dashed lines show least-squares fits, yielding channel opening rates of 0.22 ± 0.003 s−1 at 1.2 mM Mg2+ (•), and 0.03 ± 0.003 s−1 at 5 μM Mg2+ (▴).
Could this ∼7-fold slower opening of CFTR channels at 5 μM free Mg2+ be accounted for by the expected reduced concentration of MgATP complex available for binding at the NBDs? Under the conditions of these experiments, the concentration of the MgATP complex is calculated to be ∼100 μM at 5 μM free [Mg2+] and 2 mM total [ATP]. For comparison, Fig. 1 C shows that, at 1.2 mM free [Mg2+], channel Po (and hence opening rate) was already ∼75% of its maximal value when [MgATP] was 100 μM (compare Venglarik et al., 1994; Zeltwanger et al., 1999; Csanády et al., 2000). In other words, at high free [Mg2+] a reduction of [MgATP] from 2 mM to 100 μM would not be expected to appreciably lower the rate of channel opening. The greatly slowed opening of the CFTR channels observed at 5 μM free Mg2+ cannot, therefore, be attributed to diminished availability of MgATP complex per se. Nor can it be attributed to reduced availability of uncomplexed ATP because, on the contrary, when [MgATP] was ∼100 μM in the experiments of Fig. 3 (5 μM free Mg2+) the free [ATP] was far higher (∼1.9 mM) than it was in the 100 μM [MgATP] solution used in the experiments of Fig. 1 (free [Mg2+] 1.2 mM, free [ATP] <10 μM). The simplest interpretation, then, is that at 5 μM free [Mg2+] and 2 mM total [ATP] competition between the ∼100 μM MgATP and the ∼1.9 mM free ATP results in the NBDs being mostly occupied by ATP without a Mg2+ ion, a condition under which channel opening is demonstrably impaired (Fig. 2 A). We therefore conclude that the speed of CFTR channel opening is controlled not by the probability of ATP binding at the responsible site, but by the probability of that site (or sites) being occupied simultaneously by ATP and a Mg2+ ion. The severalfold slowing of channel opening (Figs. 3 and 4) attributed to this competition between MgATP and the almost 20-fold more plentiful free ATP further implies that free ATP may not only bind at the active site that controls opening of CFTR channels, but may do so nearly as well as MgATP. Thus, the K0.5(Po) of ∼40 μM (Fig. 1 C) implies (Csanády et al., 2000) an apparent affinity, K0.5(rCO), of ∼60 μM for MgATP binding at the site that controls opening rate (rCO), from the relation K0.5(rCO) = K0.5(Po) × (1+[rCOmax/rOC]) using maximal opening rate, rCOmax, of ∼0.2 s−1 (Fig. 4), and closing rate, rOC, of ∼0.3 s−1 (see Fig. 6 B, below). The ∼7-fold reduction in opening rate then suggests an apparent affinity of ∼170 μM for competitive binding of free ATP at the opening site. This concurs with the moderately (∼10-fold) reduced apparent affinity for binding of 8-azidoATP without Mg2+ to CFTR at ∼0°C compared with that for binding of Mg-8-azidoATP (Travis et al., 1993; cf. Aleksandrov et al., 2002).
Figure 6.
Influence of free [Mg2+] on rate of channel closing from prolonged open bursts. (A) Semilog survivor plots of probability channel stayed open, summarizing measurements from records like those in Fig. 5. Dashed lines show least-squares fits, yielding mean channel closing rates of 0.29 ± 0.01 s−1 at 1.2 mM Mg2+ (▴), 0.21 ± 0.01s−1 in 200 μM Mg2+ (▿), 0.16 ± 0.01 s−1 in 40 μM Mg2+ (♦), 0.05 ± 0.001 s−1 in 5 μM Mg2+ (○), and 0.03 ± 0.002 s−1 in 0 μM Mg2+ (•). (B) Closing rates (kobs) from data in A plotted against free [Mg2+], showing fit to kobs = k0 + {kMg[Mg2+]/([Mg2+] + K0.5)}, with k0 (closing rate in 0 Mg2+) set at 0.03, yielding kMg = 0.26 ± 0.02 s−1, and K0.5 = 51 ± 17 μM. (Inset) Semilog survivor plot of 60 open-state dwell times (○) before channel closure after sudden ATP withdrawal at constant 1.2 mM free [Mg2+] from 33 patches; single exponential fit (dashed line) gives closing rate of 0.26 ± 0.01 s−1 (○ in main graph).
Mg2+ Ions Can Close CFTR Channels Previously Trapped in Prolonged Open State by Withdrawal of Mg2+ from Open Channels
In the experiment of Fig. 2 B, in the presence of ∼1 μM free [Mg2+], only three of the four channels observed to open on addition of 2 mM ATP (Fig. 2 B, v) were seen to close by the end of the ensuing 140-s interval after withdrawal of the ATP. The downward jump in the current record (arrow, Fig. 2 B, inset) within 5 s of readmitting solution containing 1.2 mM free [Mg2+] but no ATP, suggests that closing of that last channel was prompted by the arrival of Mg2+ ions. We systematically repeated this kind of experiment (Fig. 5) by adding various concentrations of Mg2+ to patches containing a single open CFTR channel (or two open channels; Fig. 5 B). We first opened a channel with 2 mM total [ATP] at 5 μM free [Mg2+], temporarily trapped the channel in the open state by sudden withdrawal of all (or most) Mg2+ ions (and of ATP to prevent further opening), and then exposed the open channel to a solution containing a known concentration of Mg2+ but without any ATP. We found that, on average, the channel closed more quickly the higher the free [Mg2+] we added back (Fig. 5). The measured delays before channel closure at each free [Mg2+] approximated an exponential distribution (Fig. 6 A) whose slope provides a measure of the average rate of channel closing at that [Mg2+]. The results show that Mg2+ ions alone saturably accelerated closing of open CFTR channels, half-maximally at 51 ± 17 μM free [Mg2+] and to a maximal rate of 0.29 s−1 at 1.2 mM free [Mg2+] (Figs. 6, A and B); a Hill fit gave a Hill coefficient of 0.8 ± 0.3, not different from 1.0, justifying the Michaelis fit shown in Fig. 6 B. An independent estimate of the maximal closing rate at high [Mg2+], obtained by measuring delays to closure of individual CFTR channels following withdrawal of ATP, with free [Mg2+] held constant at 1.2 mM, gave a comparable value, 0.26 s−1 (Fig. 6 B, ○ and inset).
Because this acceleration of CFTR channel closing by increases in free [Mg2+] at micromolar levels echoes the activation by similar micromolar concentrations of free Mg2+ of ATP hydrolysis by other ABC transporters, like Pgp (e.g., Urbatsch et al., 1994), or by ion-motive ATPases such as Na,K-ATPase (Covarrubias and De Weer, 1991; Campos and Beaugé, 1992), a possible interpretation is that ATP hydrolysis underlies this Mg2+-mediated closure of CFTR channels. This is consonant with earlier conclusions that ATP hydrolysis prompts channel closure, based on extreme stabilization of the open state of WT CFTR channels exposed to MgATP plus a poorly hydrolyzable analogue (like MgAMPPNP; see below), or of K1250A mutant channels exposed to just MgATP (Hwang et al., 1994; compare with Gunderson and Kopito, 1994, 1995; Carson et al., 1995). But, in all of the experiments summarized in Figs. 5 and 6, unbound ATP had been continuously rinsed away for many seconds before arrival of the Mg2+ ions that caused channel closure. For readdition of Mg2+ alone to catalyze ATP hydrolysis and close a channel, an ATP molecule must have remained tightly bound at a catalytic site on CFTR, thereby keeping the channel open, apparently for up to 2 min in the absence of Mg2+ (Fig. 6 A). If an open CFTR channel cannot close until that residual ATP molecule becomes hydrolyzed or dissociates, then the minimal channel closing rate with no Mg2+, 0.03 s−1, provides an upper estimate for the dissociation rate of ATP. This slow release implies that ATP is bound tightly at that catalytic site, even in the absence of Mg2+ ions.
Kinetics of AMPPNP-induced Locking and of Unlocking of Cardiac CFTR Channels
An alternative means of interfering with CFTR channel closure is to expose the channels to the poorly-hydrolyzable analogue AMPPNP (Gunderson and Kopito, 1994; Hwang et al., 1994; Carson et al., 1995; Mathews et al., 1998b; Csanády et al., 2000). Fig. 7 illustrates the extreme stabilization of the CFTR channel open state caused by MgAMPPNP in the presence of MgATP, with free [Mg2+] held constant at 1.2 mM. Exposure to a mixture of 0.5 mM MgATP plus 0.5 mM MgAMPPNP caused, after ∼80 s of relatively normal opening and closing, first one and then a second channel to become locked in the open state (Fig. 7, arrows). Both channels remained open long after all nucleotides had been washed from the bath until, eventually, 13 min after nucleotide washout, one of the channels became unlocked, and closed. Reapplication of 1 mM MgATP then resulted in normal opening and closing of at least one channel, almost certainly including the one just unlocked (from comparison of the patterns of gating in 1 mM MgATP before locking and after unlocking). As cardiac CFTR channels do not open at a measurable rate during exposure to 0.5 mM MgAMPPNP alone (Nagel et al., 1992; Hwang et al., 1994), and become locked by MgAMPPNP only in the presence of MgATP (Hwang et al., 1994), the locking appears to involve an interaction of CFTR with MgAMPPNP that occurs only when MgATP is also present to open the channel. To determine the average rate of that interaction, we measured the cumulative dwell time in the open-channel state, in the presence of MgATP plus MgAMPPNP, that preceded each individual channel locking event (compare Baukrowitz et al., 1994; Mathews et al., 1998a,b). The resulting survivor plot (Fig. 8 A) shows an approximately exponential distribution of these cumulative open times, indicating an average locking rate of open channels of 0.076 ± 0.003 s−1 at 0.5 mM MgAMPPNP (with 0.5 mM MgATP). Collected measurements of the durations of individual dwell times in the locked-open state (from records like that in Fig. 7) are presented in the semilogarithmic plot of Fig. 8 B. These dwell times are also reasonably well fitted by a single exponential, and they yield an average unlocking rate of 0.0018 ± 0.0001 s−1, equivalent to a mean locked-open duration of >9 min.
Figure 8.
Rates of channel locking by 0.5 mM MgAMPPNP plus 0.5 mM MgATP (A) and of unlocking after nucleotide withdrawal (B). (A) Cumulative dwell time in the open state before each locking event was measured for locking of 31 channels in 17 patches, and the times plotted as a survivor function: the slope gives a mean locking rate for open channels of 0.076 ± 0.003 s−1. (B) Summary of dwell times in the locked open state, stabilized by MgAMPPNP, of 19 channels in 12 patches. Times from nucleotide washout to channel closure are shown as a survivor plot: its slope (dashed line) gives the average channel unlocking rate of 0.0018 ± 0.0001 s−1.
The fact that, once unlocked, a channel can again open and close normally when exposed to MgATP alone (e.g., Fig. 7) implies that AMPPNP is then no longer associated with the channel, because if it were still bound the channel could become locked again whenever MgATP next opened it. Thus, unlocking likely signals dissociation from a CFTR channel of an AMPPNP molecule that had remained bound, on average, for almost 10 min. This, in turn, implies that the catalytic site that controls channel closing retains AMPPNP in the presence of Mg2+ substantially longer (Fig. 8 B) than it holds ATP without a Mg2+ ion (Figs. 5 and 6). To assess whether this difference in apparent residence time might be attributable to the presence of Mg2+ throughout the experiments with AMPPNP, in a few experiments with AMPPNP we tried withdrawing all Mg2+ (as well as all nucleotides) once a channel had become locked open. Instability of the seal in the total absence of divalent cations (see baseline shifts in Figs. 2, 3, and 5 A) precluded completion of most prolonged recordings, but in four patches it was possible to lock open a single channel with 0.5 mM MgAMPPNP plus 0.5 mM MgATP (with 1.2 mM free [Mg2+]), remove all nucleotides after, on average, 74 s, and then, an average of 37 s later still, switch to Mg2+-free solution (with 2 mM CDTA). In one of those patches the channel unlocked and closed 107 s after that switch, but the three other channels were still locked open when, after 134, 470, and 560 s in the Mg2+-free solution, respectively, seal failure precluded further recording. That these four channels remained locked open for an average of at least 5.3 min (≥318 s) after washout of Mg2+ suggests that the mere absence of Mg2+ cannot explain the much shorter mean open dwell time (0.6 min) of channels believed to harbor ATP after removal of Mg2+ (Fig. 6 A).
DISCUSSION
The major findings of these experiments, on phosphorylated CFTR channels in inside-out patches excised from cardiac myocytes, are that channel opening is rate-limited by a Mg2+-dependent step that follows nucleotide binding, that the open burst is terminated by a [Mg2+]-sensitive step (with a rate that is half maximal at ∼50 μM [Mg2+]), and that ATP and Mg2+ ions appear able to bind independently at the catalytic site that controls channel opening and probably also at the site that controls channel closing. These results further establish the qualitative similarity between the gating mechanisms of cardiac and epithelial isoforms of CFTR, and they place important constraints on the molecular nature of those mechanisms.
ATP may Bind Independently of Mg2+ at the Site that Controls Channel Opening
As in these experiments on cardiac CFTR channels, previous investigations of the influence of Mg2+ ions on the gating of epithelial-type CFTR channels found that virtual removal of Mg2+ ions, or drastic reduction of free [Mg2+], while keeping total [ATP] constant, caused a substantial prolongation of interburst closed times (Gunderson and Kopito, 1995; Schultz, et al., 1996; Aleksandrov et al., 2000; Ikuma and Welsh, 2000). This was generally accompanied by a similarly substantial prolongation of open burst times (Gunderson and Kopito, 1995; Schultz, et al., 1996; Aleksandrov et al., 2000), which, in at least one study, was sufficient to cause an obvious increase in channel Po (at room temperature; Aleksandrov et al., 2000), comparable to our findings with cardiac CFTR (Fig. 2 B). Such examples of an intervention having similar, but offsetting, deleterious effects on both opening and closing rates underscore the shortcomings of measures of Po, or of macroscopic currents, for probing CFTR channel gating mechanisms.
The mechanism underlying the slowing of channel opening at extremely low free [Mg2+] was suggested to be the consequent reduction of [MgATP] in the solution (Gunderson and Kopito, 1995) or impaired binding of ATP to CFTR (Schultz et al., 1996; Ikuma and Welsh, 2000). The data presented here, however, show that the severalfold slowing of channel opening (Figs. 3 and 4) caused by lowering free [Mg2+] from 1.2 mM to 5 μM, while keeping total [ATP] constant at 2 mM, cannot be attributed to the concomitant lowering of the concentration of the MgATP complex to ∼100 μM. This is because direct measurements show that channel opening rate is still ∼75% of maximal when [MgATP] is 100 μM and free [Mg2+] is 1.2 mM (compare Fig. 1 with Fig. 2 A). The greatly reduced opening rate observed with 100 μM MgATP plus ∼1.9 mM free ATP must therefore be attributed to the presence of the excess free ATP. The extremely weak ability of free ATP by itself to open CFTR channels is demonstrated in Fig. 2 A. Thus, the ready ability of free ATP to inhibit channel opening by MgATP implies that free ATP competes with MgATP for binding at the catalytic site that controls channel opening. The conclusion that free ATP seems able to bind at that site in the absence of Mg2+ ions need not be surprising. Fluorescence assays have amply demonstrated binding of GTPγS or GTP to G-protein α subunits (Higashijima et al., 1987a,b) of ATP or ADP to the F1-ATPase (Weber et al., 1998), and of ATPγS, ATP, or ADP to MalK, the nucleotide binding domain of a prokaryotic ABC protein (Schneider et al., 1994), all in the absence of Mg2+ ions. Similarly, in CFTR itself, nucleotide binding, assayed at ∼0°C by photolabeling with [α-32P]8-azidoATP, occurred to about the same extent with or without Mg2+ ions, although half-maximal labeling was obtained at ∼10 μM 8-azidoATP in the presence of Mg2+, but ∼100 μM 8-azidoATP in its absence (Travis et al., 1993).
Control of Channel Closing by Hydrolysis of ATP, Presumably at NBD2
The present results show that lowering [Mg2+] to μM levels also prolongs the burst duration of cardiac CFTR channels, i.e., slows channel closing, as reported for epithelial CFTR channels (Gunderson and Kopito, 1995; Schultz, et al., 1996). The burst duration was prolonged even further when all Mg2+ ions (together with all nucleotides) were withdrawn, or chelated by CDTA, once a channel had opened in the presence of MgATP (Figs. 5 and 6). That prolongation was sufficient to permit tests of the effect of readdition of Mg2+ ions alone, in solutions devoid of nucleotide. Although channels could eventually close in the absence of both Mg2+ and nucleotide, Mg2+ ions alone were able to accelerate closing, an effect that was half maximal at ∼50 μM [Mg2+]. What is the likely nature of this Mg2+-dependent step that rate limits closing of CFTR channels? Marked prolongation (locking) of open bursts caused by addition of MgAMPPNP to MgATP solutions (e.g., Figs. 7 and 8, above) in both cardiac (Hwang et al., 1994) and epithelial CFTR channels (Gunderson and Kopito, 1994,1995; Carson et al., 1995; Mathews et al., 1998b; Zeltwanger et al., 1999) has been interpreted as reflecting occupancy by the poorly hydrolyzable MgAMPPNP of a site which normally binds MgATP, and at which hydrolysis of that MgATP triggers closing of the channel. The observation that mutant K1250A CFTR channels show similarly prolonged bursts during exposure to MgATP alone suggested that NBD2 contains the catalytic site where hydrolysis leads to channel closure (Gunderson and Kopito, 1994, 1995; Carson et al., 1995; Zeltwanger et al., 1999). Analysis of the temporal asymmetry of changes in character of rapid current blocking events during open bursts of CFTR channels, and their modification by nucleotide analogues and by mutation of NBD2 (K1250A) but not NBD1 (K464A), provided additional evidence that hydrolysis of ATP tightly bound at NBD2 causes the channel to close (Gunderson and Kopito, 1995). The strong influence of increases in temperature to shorten CFTR channel burst duration (Q10 = 3.6; Mathews et al., 1998b; compare with Csanády et al., 2000) further supports the interpretation that closing from a burst is rate limited by a hydrolysis step. This interpretation is not weakened by the observation that the rate (obtained as the reciprocal of intraburst open times) of occurrence of the more frequent, brief, intraburst closures (referred to as flickery closures; Linsdell and Hanrahan, 1996; Ishihara and Welsh, 1997) is little affected by changes of temperature (Aleksandrov and Riordan, 1998); examples of such brief flickery closures, though predominantly filtered out by our chart recorder, are still evident within prolonged open bursts even after washout of all nucleotides (e.g., Figs. 2 B, 3, B and C, and 7).
Our finding that increasing [Mg2+] in the μM range accelerates termination of open bursts is also consistent with hydrolysis being the rate-limiting step for channel closing, as other ATPases are similarly activated by μM levels of [Mg2+] (e.g., Covarrubias and De Weer, 1991; Campos and Beaugé, 1992; Urbatsch et al., 1994). Indeed, Schultz et al. (1996) found that, at room temperature, in solutions comparable to ours, ATP hydrolysis by luciferase was accelerated by [Mg2+] with an apparent Km of ∼57 μM (at constant 1 mM total [ATP]). The fact that a CFTR channel eventually closes even in the absence of Mg2+ ions (e.g., Figs. 5 and 6, above) does not constitute a valid argument against ATP hydrolysis normally controlling channel closing (Schultz et al., 1996). Presumably, when hydrolysis is prevented, either by lack of Mg2+ ions (Li et al., 1996) or by NBD mutation (e.g., K1250A; Ramjeesingh et al., 1999), dissociation of nonhydrolyzed nucleotide from NBD2 becomes the rate-limiting step for the delayed channel closure. This, in turn, implies that channel closing is normally rate limited by a faster step, related to hydrolysis: closing could be triggered by a conformational change related to hydrolysis itself, or to dissociation of hydrolysis product(s) (Baukrowitz et al., 1994; Gadsby and Nairn, 1994, 1999; Hwang et al., 1994; Gunderson and Kopito, 1995; Sheppard and Welsh, 1999). Although activation of hydrolysis of an ATP molecule tightly bound to CFTR provides the most consistent interpretation of the channel closing initiated by reintroduction of just Mg2+ ions, we cannot rule out Mg2+-dependent clo-sure by some unknown, nucleotide-independent mechanism. However, channel closure by Mg2+-induced dissociation of tightly-bound nucleotide would seem unlikely, as Mg2+ ions are known to greatly enhance, rather than weaken, nucleotide binding to other nucleoside triphosphatases (e.g., John et al., 1993; Weber et al., 1998).
Mg2+ and Nucleotide Interactions with the Site that Controls Channel Closing
If our interpretation is correct, that a Mg2+ ion may indeed close an open CFTR channel by catalyzing hydrolysis of a tightly-bound ATP at NBD2, this finding admits four further conclusions. First, ATP must be able to remain bound at NBD2 of CFTR without the presence of a Mg2+ ion, as already concluded above for binding of ATP at the channel opening site. Binding of free nucleotide, without Mg2+, to other nucleoside triphosphatases has been discussed above, and independent binding and dissociation of Mg2+ ions to/from sites already occupied by nucleotide has long been known for α subunits of heterotrimeric G proteins (e.g., Higashijima et al., 1987a) as well as for the small G protein p21-ras (John et al., 1993). Second, although the Mg2+ may be freely exchangeable at the NBD2 active site, the ATP must be bound very tightly, even in the absence of any stabilizing influence of a Mg2+ ion. Our upper estimate for the dissociation rate from the open channel of that ATP, 0.03 s−1, would imply an extremely high intrinsic binding affinity for ATP without Mg2+ at NBD2 (e.g., dissociation constant ≤30 nM, if the binding constant were 106 M−1s−1, as it is for binding of GTP to p21-ras; John et al., 1993). MgAMPPNP seems to be bound to open CFTR channels with even higher apparent affinity than ATP, since the unlocking rate, 0.0018 s−1, of channels exposed to MgATP plus MgAMPPNP is presumed to reflect the dissociation rate of MgAMPPNP. As no comparably high affinity binding of ATP or MgAMPPNP to any site on closed CFTR channels (e.g., at 0°C) has been detected (Travis et al., 1993; but compare with Aleksandrov et al., 2001, 2002), it seems likely that some structural rearrangement causes the nucleotide at NBD2 to become occluded there only in the open channel conformation. Third, activation of channel closing by Mg2+ is reasonably well described by a Michaelis function (Fig. 6 A), consistent with action of a single Mg2+ ion. If that action is to catalyze hydrolysis of an occluded ATP, then we may conclude that hydrolysis of a single ATP molecule is stoichiometrically coupled to closing of a single channel. Although the quaternary structure is not known for any ABC protein, it has recently been suggested that CFTR might function as an obligate dimer (Zerhusen et al., 1999; Raghuram et al., 2001), although this remains controversial (Marshall et al., 1994; Wang et al., 2000; Ramjeesingh et al., 2001). Regardless of how many NBDs a single CFTR channel comprises, our data suggest that a single hydrolysis event is sufficient to gate that channel shut. Fourth, this stabilization of CFTR's open-burst channel conformation by tight binding of nucleotides resembles the stabilization of the active conformation of G proteins by GTP, and apparently CFTR, like G proteins, exploits hydrolysis of that nucleotide as a timing device to determine the lifetime of the active state (compare Gadsby and Nairn, 1994, 1999; Carson and Welsh, 1995; Gunderson and Kopito, 1995; Manavalan et al., 1995).
Which Nucleotide Binding Site Controls Channel Opening?
In light of the above discussion, what can we discern about the location and nature of the Mg2+-dependent step that rate limits channel opening? Our results indicate that at low free [Mg2+] but high total [ATP], channel opening is delayed by competition between free ATP and MgATP for occupancy of the appropriate site (or sites). Similarly, once MgATP succeeds in opening a CFTR channel, the longer burst durations seen at low free [Mg2+] (Figs. 2 and 3) compared with those at comparable [MgATP] but with high free [Mg2+] (e.g., 1–50 μM MgATP; Fig. 1), must also be explained by occupancy of the closing catalytic site (argued above to be NBD2) by Mg2+-free ATP instead of the MgATP needed for the (hydrolysis) step that leads to efficient channel closure. In other words, the ATP that remains bound at NBD2 while the channel is open must wait until a catalytic Mg2+ ion binds with it at the active site for long enough to effect channel closure. Gunderson and Kopito (1995) similarly explained the prolonged burst durations of epithelial CFTR channels at low free [Mg2+] as indicating that Mg2+ at the NBD2 catalytic site is in rapid equilibrium with the surrounding solution.
There are two ways to satisfy the dual requirements that MgATP must bind at the opening site, but then later ATP must remain bound at NBD2, without Mg, after Mg2+ ions have been rinsed from the open channel. One way is to have the MgATP complex responsible for channel opening reside at NBD1. The other possibility is that it is MgATP at NBD2 that prompts channel opening, immediately after which, at low or zero free [Mg2+], the Mg2+ dissociates leaving ATP still bound at NBD2 until arrival of a new Mg2+ ion permits its hydrolysis leading to channel closing. However, both of these alternatives are at odds with the interpretation given by Harrington et al. (1999) for their finding, in a CFTR channel exposed to 1 mM ATP with 0.2 mM Mg2+ plus 0.8 mM Ca2+, of two populations of burst durations, one brief and the other ∼20-fold prolonged. Because the brief one resembled the single population observed when the same channel was exposed to 1 mM ATP and 1 mM Mg2+ (with no Ca2+), the authors concluded that, once Ca2+ or Mg2+ induced a burst, there was no exchange and both cations remained tightly bound, resulting in the two distinct burst lengths. Those, at first sight, discrepant observations might be reconciled with our interpretation (compare Gunderson and Kopito, 1995) of the slowed closing seen at low free [Mg2+] if it is assumed that only Mg2+ ions, and not Ca2+ ions, are in rapid equilibrium at the NBD2 catalytic site. In that case, given the much shorter duration of bursts induced by Mg2+, dissociation of a Mg2+ ion during one of those bursts and its replacement (irreversibly) by a Ca2+ ion, would be expected to result in a burst imperceptibly longer, on average, than the mean burst duration measured in just Ca2+ solution.
The apparently much shorter dwell time at the closing site of nonhydrolyzed ATP (Figs. 5 and 6) in comparison to AMPPNP (Figs. 7 and 8), in both cases after Mg2+ withdrawal, offers a further clue. Unless NBD2 has a markedly higher intrinsic affinity at room temperature for AMPPNP than for ATP (which seems unlikely; Aleksandrov et al., 2001, 2002), these different dwell times are possibly attributable to the presence of high free [Mg2+] when the channels were opened with AMPPNP plus ATP (Fig. 7), but only 5 μM free Mg2+ in the case of ATP alone (Fig. 5). Given that in both instances channel opening required a Mg–nucleotide complex at the opening site, only if that site were NBD2 might subsequent washout of Mg2+ from NBD2 be reasonably anticipated to leave ATP bound there in a different manner than AMPPNP. The difference would in fact lie in the form of ATP bound at NBD1 which, in the experiment at high free [Mg2+] would be expected to be Mg–ATP complex, but at low free [Mg2+] might have been free ATP. Such an influence of NBD1 status on the stability of nucleotide bound at NBD2 is suggested by mutagenesis experiments (unpublished data).
Nature of the Step that Controls Channel Opening
Regardless of whether it occurs at NBD1 or NBD2, what is the likely nature of the Mg2+-dependent step that controls opening of a CFTR channel? An obvious candidate is ATP hydrolysis, which in intact CFTR occurs at a rate (≤1 s−1 at ∼30°C; Li et al., 1996) comparable to that of the rate-limiting steps for channel opening and closing. If CFTR can function as a monomer, and if a monomeric CFTR channel can close by hydrolyzing an ATP molecule that remains tightly bound at NBD2 throughout the open burst, then (absent nucleotide exchange on the open channel) any nucleotide hydrolysis associated with opening would presumably have to occur at NBD1. That isolated NBD1 is indeed capable of ATP hydrolysis is confirmed by recent measurements on full-length (compare Chan et al., 2000) NBD1 constructs, either His-tagged (Duffieux et al., 2000) or in tandem with GST-R domain (Howell et al., 2000), which yielded maximal Mg2+-dependent (Duffieux et al., 2000) ATPase rates (at 30°C) of 0.2 s−1 and 0.4 s−1, and K0.5 values for [MgATP] of ∼250 μM and ∼60 μM, respectively; these values are comparable to those (0.2 s−1, and 0.3–1 mM) reported for intact CFTR (Li et al., 1996). Moreover, Li et al. (1996) found that withdrawal of Mg2+ rendered ATPase activity immeasurable, and practically abolished CFTR channel opening, whereas we show here that channel opening rate was reduced >50-fold when CDTA was used to chelate Mg2+ ions (Fig. 2 A). Therefore, despite previous suggestions to the contrary (Reddy and Quinton, 1996; Schultz et al., 1996; Aleksandrov et al., 2000; Ikuma and Welsh, 2000), quantitative comparison of rates of channel gating and rates of ATP hydrolysis by wild-type CFTR provide no evidence to rule out ATP hydrolysis as the step that rate limits CFTR channel opening. Although high (e.g., 5 mM) concentrations of poorly hydrolyzable analogues like AMPPNP and ATPγS can open CFTR channels in the absence of ATP (Aleksandrov et al., 2000), they do so only poorly, at a rate ∼5% of the maximum attained with MgATP (unpublished data). These poorly hydrolyzable analogues are therefore comparable to free ATP (without Mg2+; Fig. 2 A) in their ability to open wild-type CFTR channels.
Despite the usual caveats with mutants, a compelling argument against a requirement for ATP hydrolysis to open CFTR channels remains the results of mutagenesis studies. Thus, mutation of key catalytic site residues, K464 in NBD1 or K1250 in NBD2, lower the overall ATPase rate of purified, reconstituted CFTR by about one or two orders of magnitude, respectively (Ramjeesingh, et al., 1999); the same mutations, on the other hand, reduce the opening rate of the channels only <4-fold, and <10-fold, respectively (Carson et al., 1995; Gunderson and Kopito, 1995; Ramjeesingh et al., 1999). Nucleotide binding assays (at 0°C to prevent hydrolysis) using 8-azidoATP photolabeling show that binding occurs with the same micromolar apparent affinity in wild-type, mutant K1250M, and double mutant K464A/K1250A, CFTR (Carson et al., 1995). These findings, together with the fact that even double mutant K464A/K1250A CFTR channels open and close at measurable rates (Carson et al., 1995), make it seem unlikely that ATP hydrolysis at either NBD1 or NBD2 is a prerequisite for channel opening. We may conclude, then, that opening of a wild-type CFTR channel is rate limited by a Mg2+-requiring, highly temperature-dependent (Mathews et al., 1998b) step, which possibly occurs at NBD2 (compare Gunderson and Kopito, 1995), and which probably does not reflect hydrolysis of ATP. Perhaps, instead, that step represents formation of a prehydrolysis complex, or transition state (e.g., Mildvan, 1997; cf. Aleksandrov and Riordan, 1998; Chen et al., 2001).
Acknowledgments
We thank Drs. László Csanády and Paola Vergani for helpful discussions, and Peter Hoff and Atsuko Horiuchi for technical assistance.
This research was supported by National Institutes of Health grant NIH HL49907.
David Clapham served as guest editor.
Dr. Dousmanis' present address is Neurological Institute, 710 West 168th Street, New York, NY 10032.
Footnotes
Abbreviations used in this paper: ABC, ATP binding cassette; CDTA, 2 trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid; CFTR, cystic fibrosis transmembrane conductance regulator; MRP, multidrug resistance–related protein.
References
- Aleksandrov, A.A., X.-B. Chang, L. Aleksandrov, and J.R. Riordan. 2000. The non-hydrolytic pathway of cystic fibrosis transmembrane conductance regulator ion channel gating. J. Physiol. 528:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksandrov, L., A. Mengos, X.-B. Chang, A. Aleksandrov, and J.R. Riordan. 2001. Differential interactions of nucleotides at the two nucleotide binding domains of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 276:12918–12923. [DOI] [PubMed] [Google Scholar]
- Aleksandrov, L., A. Aleksandrov, X.-B. Chang, and J.R. Riordan. 2002. The first nucleotide binding domain of CFTR is a site of stable nucleotide interaction whereas the second is a site of rapid turnover. J. Biol. Chem. 277:15419–15425. [DOI] [PubMed] [Google Scholar]
- Aleksandrov, A.A., and J.R. Riordan. 1998. Regulation of CFTR ion channel gating by MgATP. FEBS Lett. 431:97–101. [DOI] [PubMed] [Google Scholar]
- Anderson, M.P., H.A. Berger, D.P. Rich, R.J. Gregory, A.E. Smith, and M.J. Welsh. 1991. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 67:775–784. [DOI] [PubMed] [Google Scholar]
- Baukrowitz, T., T.-C. Hwang, A.C. Nairn, and D.C. Gadsby. 1994. Coupling of CFTR Cl channel gating to an ATP hydrolysis cycle. Neuron. 12:473–482. [DOI] [PubMed] [Google Scholar]
- Bers, D.M. 1994. A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 40:3–29. [DOI] [PubMed] [Google Scholar]
- Bourne, H.R., D.A. Sanders, and F. McCormick. 1991. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 349:117–127. [DOI] [PubMed] [Google Scholar]
- Campos, M., and L. Beaugé. 1992. Effects of magnesium and ATP on pre-steady-state phosphorylation kinetics of the Na+,K+-ATPase. Biochim. et Biophys Acta. 1105:51–60. [DOI] [PubMed] [Google Scholar]
- Carson, M.R., and M.J. Welsh. 1993. 5′-adenylylimidodiphosphate does not activate CFTR chloride channels in cell-free patches of membrane. Am. J. Physiol. 265:L27–L32. [DOI] [PubMed] [Google Scholar]
- Carson, M.R., and M.J. Welsh. 1995. Structural and functional similarities between the nucleotide-binding domains of CFTR and GTP-binding proteins. Biophys. J. 69:2443–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, M.R., S.M. Travis, and M.J. Welsh. 1995. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J. Biol. Chem. 270:1711–1717. [DOI] [PubMed] [Google Scholar]
- Chan, K.W., L. Csanády, D. Seto-Young, A.C. Nairn, and D.C. Gadsby. 2000. Severed molecules functionally define the boundaries of CFTR's N-terminal nucleotide binding domain. J. Gen. Physiol. 116:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, G., and C.B. Roth. 2001. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science. 293:1793–1800. [DOI] [PubMed] [Google Scholar]
- Chen, J., S. Sharma, F.A. Quiocho, and A.L. Davidson. 2001. Trapping the transition state of an ATP-binding cassette transporter: Evidence for a concerted mechanism of maltose transport. Proc. Natl. Acad. Sci. USA. 98:1525–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, S.H., D.P. Rich, J. Marshall, R.J. Gregory, M.J. Welsh, and A.E. Smith. 1991. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 66:1027–1036. [DOI] [PubMed] [Google Scholar]
- Collins, A., A.V. Somlyo, and D.W. Hilgemann. 1992. The giant cardiac membrane patch method: stimulation of outward Na+-Ca2+ exchange current by MgATP. J. Physiol. 454:27–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias, Y.G.-M., and P. De Weer. 1991. Kinetics of magnesium interaction with Na,K-ATPase. The Sodium Pump: Recent Developments. The Rockefeller University Press, New York. 409–412.
- Csanády, L., K.W. Chan, D. Seto-Young, D.C. Kopsco, A.C. Nairn, and D.C. Gadsby. 2000. Severed channels probe regulation of gating of CFTR by its cytoplasmic domains. J. Gen. Phys. 116:477–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffieux, F., J.-P. Annereau, J. Boucher, E. Miclet, O. Pamlard, M. Schneider, V. Stoven, and J.-Y. Lalleman. 2000. Nucleotide-binding domain 1 of cystic fibrosis transmembrane conductance regulator. Eur. J. Biochem. 267:5306–5312. [DOI] [PubMed] [Google Scholar]
- Gadsby, D.C., and A.C. Nairn. 1994. Regulation of CFTR channel gating. Trends Biochem. Sci. 19:513–518. [DOI] [PubMed] [Google Scholar]
- Gadsby, D.C., and A.C. Nairn. 1999. Control of CFTR channel gating by phosphorylation and nucleotide hydrolysis. Physiol. Rev. 79:S77–S107. [DOI] [PubMed] [Google Scholar]
- Gao, M., H.-R. Cui, D.W. Loe, C.E. Grant, K.C. Almquist, S.P.C. Cole, and R.G. Deeley. 2000. Comparison of the functional characteristics of the nucleotide binding domains of multidrug resistance protein 1. J. Biol. Chem. 275:13098–13108. [DOI] [PubMed] [Google Scholar]
- Gunderson, K.L., and R.R. Kopito. 1995. Conformational states of CFTR associated with channel gating: the role of ATP binding and hydrolysis. Cell. 82:231–239. [DOI] [PubMed] [Google Scholar]
- Gunderson, K.L., and R.R. Kopito. 1994. Effects of pyrophosphate and nucleotide analogs suggest a role for ATP hydrolysis in cystic fibrosis transmembrane regulator channel gating. J. Biol. Chem. 269:19349–19353. [PubMed] [Google Scholar]
- Harrington, M.A., K.L. Gunderson, and R.R. Kopito. 1999. Redox reagents and divalent cations alter the kinetics of cystic fibrosis transmembrane conductance regulator channel gating. J. Biol. Chem. 274:27536–27544. [DOI] [PubMed] [Google Scholar]
- Higashijima, T., K.M. Ferguson, M.D. Smigel, and A.G. Gilman. 1987. a. The effects of GTP and Mg2+ on the GTPase activity and the fluorescent properties of Go. J. Biol. Chem. 262:757–761. [PubMed] [Google Scholar]
- Higashijima, T., K.M. Ferguson, P.C. Sternweis, E.M. Ross, M.D. Smigel, and A.G. Gilman. 1987. b. The effect of activating ligands on the intrinsic fluorescence of guanine nucleotide-binding regulatory proteins. J. Biol. Chem. 262:752–756. [PubMed] [Google Scholar]
- Hilgemann, D.W. 1995. The giant membrane patch. In Single Channel Recording, 2nd ed. B. Sakmann and E. Neher, eds. Plenum Press, New York. 303–327.
- Holland, I.B., and M.A. Blight. 1999. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J. Mol. Biol. 293:381–399. [DOI] [PubMed] [Google Scholar]
- Honkanen, R.E., J. Zwiller, R.E. Moore, S. Daily, B.S. Khatra, M. Dukelow, and A.L. Boynton. 1990. Characterization of mocrocystin-LR, a potent inhibitor of type 1 and type 2a protein phosphatases. J. Biol. Chem. 265:19401–19404. [PubMed] [Google Scholar]
- Hou, Y., L. Cui, J.R. Riordan, and X. Chang. 2000. Allosteric interactions between the two non-equivalent nucleotide binding domains of multidrug resistance protein MRP1. J. Biol. Chem. 275:20280–20287. [DOI] [PubMed] [Google Scholar]
- Howell, L.D., R. Borchardt, and J.A. Cohn. 2000. ATP hydrolysis by a CFTR domain: pharmacology and effects of G551D mutation. Biochem. Biophys. Res. Comm. 271:518–525. [DOI] [PubMed] [Google Scholar]
- Hwang, T.-C., M. Horie, and D.C. Gadsby. 1993. Functionally distinct phospho-forms underlie incremental acivation of protein kinase-regulated Cl− conductance in mammalian heart. J. Gen. Physiol. 101:629–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, T.-C., G. Nagel, A.C. Nairn, and D.C. Gadsby. 1994. Regulation of the gating of cystic fibrosis transmembrane conductance regulator Cl channels by phosphorylation and ATP hydrolysis. Proc. Natl. Acad. Sci. USA. 91:4698–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma, M., and M.J. Welsh. 2000. Regulation of CFTR Cl− channel gating by ATP binding and hydrolysis. Proc. Natl. Acad. Sci. USA. 97:8675–8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenberg, G., and U. Klöckner. 1982. Calcium tolerant ventricular myocytes prepared by preincubation in a “KB Medium”. Pflugers Arch. 395:6–18. [DOI] [PubMed] [Google Scholar]
- Ishihara, H., and M.J. Welsh. 1997. Block by MOPS reveals a conformation change in the CFTR pore produced by ATP hydrolysis. Am. J. Physiol. 273:C1278–C1289. [DOI] [PubMed] [Google Scholar]
- Jia, Y.L., C.J. Mathews, and J.W. Hanrahan. 1997. Phosphorylation by protein kinase C is required for acute activation of cystic fibrosis transmembrane conductance regulator by protein kinase A. J. Biol. Chem. 272:4978–4984. [DOI] [PubMed] [Google Scholar]
- John, J., H. Rensland, I. Schlichting, I. Vetter, G.D. Borasio, R.S. Goody, and A. Wittinghofer. 1993. Kinetic and structural analysis of the Mg2+-binding site of the guanine nucleotide-binding protein p21H-ras. J. Biol. Chem. 268:923–929. [PubMed] [Google Scholar]
- Kaczmarek, L.K., K.R. Jennings, F. Strumwasser, A.C. Nairn, U. Walter, F.D. Wilson, and P. Greengard. 1980. Microinjection of catalytic subunit of cyclic AMP-dependent protein kinase enhances calcium action potentials of bag cell neurons in cell culture. Proc. Natl. Acad. Sci. USA. 77:7487–7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C., M. Ramjeesingh, W. Wang, E. Garami, M. Hewryk, D. Lee, J.M. Rommens, K. Galley, and C.E. Bear. 1996. ATPase activity of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 271:28463–28468. [DOI] [PubMed] [Google Scholar]
- Linsdell, P., and J.W. Hanrahan. 1996. Flickery block of single CFTR chloride channels by intracellular anions and osmolytes. Am. J. Physiol. 271:C628–C634. [DOI] [PubMed] [Google Scholar]
- Loo, T.W., and D.M. Clarke. 1994. Reconstitution of drug-stimulated ATPase activity following co-expression of each half of human P-glycoprotein as separate polypeptides. J. Biol. Chem. 269:7750–7755. [PubMed] [Google Scholar]
- Manavalan, P., D.G. Dearborn, J.M. McPherson, and A.E. Smith. 1995. Sequence homologies between nucleotide binding regions of CFTR and G-proteins suggest structural and functional similarities. FEBS Lett. 366:87–91. [DOI] [PubMed] [Google Scholar]
- Marshall, J., S. Fang, L.S. Ostedgaard, C.R. O'Riordan, D. Ferrara, J.F. Amara, H. Hoppe, R.K. Scheule, M.J. Welsh, A.E. Smith, and S.H. Cheng. 1994. Stoichiometry of recombinant cystic fibrosis transmembrane conductance regulator in epithelial cells and its functional reconstitution into cells in vitro. J. Biol. Chem. 269:2987–2995. [PubMed] [Google Scholar]
- Martell, A.E., and R.M. Smith. 1989. Critical Stability Constants, Vol. 1. Plenum Press, New York. 469 pp.
- Mathews, C.J., J.A. Tabcharani, X.-B. Chang, T.J. Jensen, J.R. Riordan, and J.W. Hanrahan. 1998. a. Dibasic protein kinase A sites regulat bursting rate and nucleotide sensitivity of the cystic fibrosis transmembrane conductance regulator chloride channel. J. Physiol. 508:365–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, C., J.A. Tabcharani, and J.W. Hanrahan. 1998. b. The CFTR chloride channel: nucleotide interactions and temperature-dependent gating. J. Membr. Biol. 163:55–66. [DOI] [PubMed] [Google Scholar]
- Mildvan, A.S. 1997. Mechanisms of signaling and related enzymes. Proteins. 29:401–416. [PubMed] [Google Scholar]
- Müller, M., E. Bakos, E. Welker, A. Váradi, U.A. Germann, M.M. Gottesman, B.S. Morse, I.B. Roninson, and B. Sarkadi. 1996. Altered drug-stimulated ATPase activity in mutants of the human multidrug resistance protein. J. Biol. Chem. 271:1877–1883. [DOI] [PubMed] [Google Scholar]
- Nagel, G., T.-C. Hwang, K.L. Nastiuk, A.C. Nairn, and D.C. Gadsby. 1992. The protein kinase A-regulated cardiac Cl−channel resembles the cystic fibrosis transmembrane conductance regulator. Nature. 360:81–84. [DOI] [PubMed] [Google Scholar]
- Picciotto, M., J. Cohn, G. Bertuzzi, P. Greengard, and A.C. Nairn. 1992. Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 267:12742–12752. [PubMed] [Google Scholar]
- Raghuram, V., D.D. Mak, and J.K. Foskett. 2001. Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction. Proc. Natl. Acad. Sci. USA. 98:787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramjeesingh, M., C. Li, E. Garami, L.J. Huan, K. Galley, Y. Wang, and C.E. Bear. 1999. Walker mutations reveal loose relationship between catalytic and channel-gating activities of purified CFTR (cystic fibrosis transmembrane conductance regulator). Biochemistry. 38:1463–1468. [DOI] [PubMed] [Google Scholar]
- Ramjeesingh, M., C. Li, I. Kogan, Y. Wang, L.J. Huan, and C.E. Bear. 2001. A monomer is the minimum functional unit required for channel and ATPase activity of the cystic fibrosis transmembrane conductance regulator. Biochemistry. 40:10700–10706. [DOI] [PubMed] [Google Scholar]
- Reddy, M.M., and P.M. Quinton. 1996. Deactivation of CFTR-C1 conductance by endogenous phosphatases in the native sweat duct. Am. J. Physiol. 270:C474–C480. [DOI] [PubMed] [Google Scholar]
- Schneider, E., S. Wilken, and R. Schmid. 1994. Nucleotide-induced conformational changes of MalK, a bacterial ATP binding cassette transporter protein. J. Biol. Chem. 269:20456–20461. [PubMed] [Google Scholar]
- Schultz, B.D., R.J. Bridges, and R.A. Frizzell. 1996. Lack of conventional ATPase properties in CFTR chloride channel gating. J. Membr. Biol. 151:63–75. [DOI] [PubMed] [Google Scholar]
- Senior, A.E., M.K. Al-Shawi, and I.L. Urbatsch. 1995. The catalytic cycle of P-glycoprotein. FEBS Lett. 377:285–289. [DOI] [PubMed] [Google Scholar]
- Sheppard, D.N., and M.J. Welsh. 1999. Structure and function of the CFTR chloride channel. Physiol. Rev. 79:S23–S45. [DOI] [PubMed] [Google Scholar]
- Travis, S.M., M.R. Carson, D.R. Ries, and M.J. Welsh. 1993. Interaction of nucleotides with membrane-associated cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 268:15336–15339. [PubMed] [Google Scholar]
- Urbatsch, I.L., L. Beaudet, I. Carrier, and P. Gros. 1998. Mutations in either nucleotide-binding site of P-glycoprotein (Mdr3) prevent vanadate trapping of nucleotide at both sites. Biochemistry. 37:4592–4602. [DOI] [PubMed] [Google Scholar]
- Urbatsch, I., M.K. Al-Shawi, and A.E. Senior. 1994. Characterization of the ATPase activity of purified Chinese hamster P-glycoprotein. Biochemistry. 33:7069–7076. [DOI] [PubMed] [Google Scholar]
- Venglarik, C.J., B.D. Schultz, R.A. Frizzell, and R.J. Bridges. 1994. ATP alters current fluctuations of cystic fibrosis transmembrane conductance regulator: evidence for a three-state activation mechanism. J. Gen. Physiol. 104:123–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., H. Yue, R.B. Derin, W.B. Guggino, and M. Li. 2000. Accessory protein facilitated CFTR-CFTR interaction, a molecular mechanism to potentiate the chloride channel activity. Cell. 103:169–179. [DOI] [PubMed] [Google Scholar]
- Weber, J., S.T. Hammond, S. Wilke-Mounts, and A.E. Senior. 1998. Mg2+ coordination in catalytic sites of F1-ATPase. Biochemistry. 37:608–614. [DOI] [PubMed] [Google Scholar]
- Winter, M.C., D.N. Sheppard, M.R. Carson, and M.J. Welsh. 1994. Effect of ATP concentration on CFTR Cl− channels. Biophys. J. 66:1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, M.C., and M.J. Welsh. 1997. Stimulation of CFTR activity by its phosphorylated R. domain. Nature. 389:294–296. [DOI] [PubMed] [Google Scholar]
- Zeltwanger, S., F. Wang, G.T. Wang, K.D. Gillis, and T.-C. Hwang. 1999. Gating of cystic fibrosis transmembrane conductance regulator chloride channels by adenosine triphosphate hydrolysis. J. Gen. Physiol. 113:541–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerhusen, B., J. Zhao, J. Xie, P.B. Davis, and J. Ma. 1999. A single conductance pore for chloride ions formed by two cystic fibrosis transmembrane conductance regulator molecules. J. Biol. Chem. 274:7627–7630. [DOI] [PubMed] [Google Scholar]