Abstract
Rhodopsin activation is measured by the early receptor current (ERC), a conformation-associated charge motion, in human embryonic kidney cells (HEK293S) expressing opsins. After rhodopsin bleaching in cells loaded with 11-cis-retinal, ERC signals recover in minutes and recurrently over a period of hours by simple dark adaptation, with no added chromophore. The purpose of this study is to investigate the source of ERC signal recovery in these cells. Giant HEK293S cells expressing normal wild-type (WT)-human rod opsin (HEK293S) were regenerated by solubilized 11-cis-retinal, all-trans-retinal, or Vitamin A in darkness. ERCs were elicited by flash photolysis and measured by whole-cell recording. Visible flashes initially elicit bimodal (R1, R2) ERC signals in WT-HEK293S cells loaded with 11-cis-retinal for 40 min or overnight. In contrast, cells regenerated for 40 min with all-trans-retinal or Vitamin A had negative ERCs (R1-like) or none at all. After these were placed in the dark overnight, ERCs with outward R2 signals were recorded the following day. This indicates conversion of loaded Vitamin A or all-trans-retinal into cis-retinaldehyde that regenerated ground-state pigment. 4-butylaniline, an inhibitor of the mammalian retinoid cycle, reversibly suppressed recovery of the outward R2 component from Vitamin A and 11-cis-retinal–loaded cells. These physiological findings are evidence for the presence of intrinsic retinoid processing machinery in WT-HEK293S cells similar to what occurs in the mammalian eye.
Keywords: retinoids, retinal pigments, rhodopsin, pigment epithelium of eye, cultured cells
INTRODUCTION
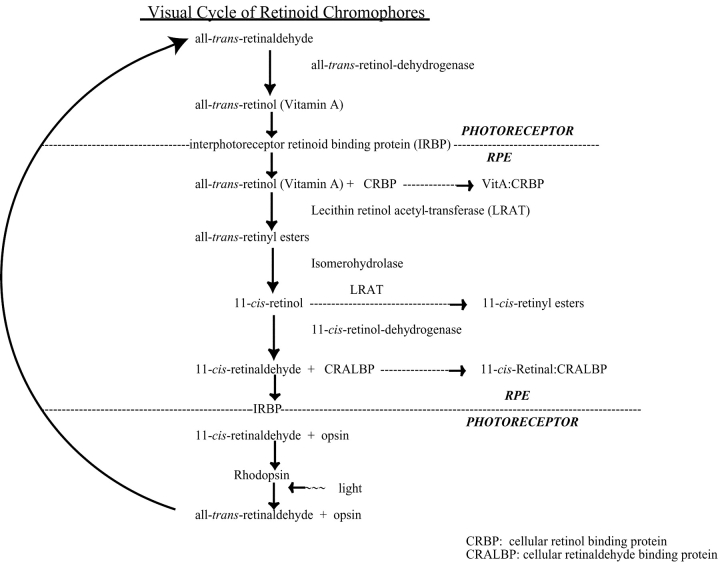
The outer segments of vertebrate rod and cone photoreceptors are closely embraced by microvilli of the retinal pigment epithelium (RPE).* The RPE is responsible for the enzymatic and chemical conversion of all-trans-retinol (Vitamin A) to 11-cis-retinal and the recovery of 11-cis-retinal from all-trans-retinal that is produced in photoreceptors by rhodopsin bleaching (Fig. 1). The visual chromophore cycle is essential to the maintenance of vertebrate rod and cone vision.
Figure 1.
The visual cycle of retinoid chromophores in the vertebrate eye. The chromophores, enzymes, binding proteins, and cell types are indicated for the major components of the cycle. Vitamin A in the serum or from photoreceptors with bleached pigment is transferred across the RPE plasma membrane and binds to CRBP-I, whereupon it is esterified to membrane lipids by LRAT (Barry et al., 1989). Exergonic hydrolysis of the all-trans-retinyl ester is coupled to endogonic isomerization that forms 11-cis-retinol, which is either esterified by LRAT or oxidized by 11-cis-retinol dehydrogenase (11cRDH) to yield 11-cis-retinal (Rando, 1991, 1992). The isomerase and the hydrolase are hypothesized to form a membrane-associated multiprotein/enzymatic complex called the IH (Bernstein et al., 1987; Barry et al., 1989; Rando, 1992) that engages LRAT (Rando, 1991) and possibly other regulatory units (e.g., RPE65). 11-cis-retinal in the RPE cytoplasm is solubilized by binding to CRALBP, which stores and traffics the ligand to partition into the RPE plasma membrane (Crabb et al., 1998). 11-cis-retinal complexes with abundant interstitial retinoid binding protein (IRBP) and albumin in the extracellular space between the apical RPE microvilli and outer segment plasma membranes of photoreceptors (Adler and Edwards, 2000). In photoreceptors, 11-cis-retinal forms a covalent protonated Schiff base linkage with a conserved lysine in the seventh transmembrane domain of the rod or cone opsin apoprotein to regenerate the respective rhodopsin visual pigments. Hydrolyzed from bleached pigment, all-trans-retinal is converted back to Vitamin A in photoreceptors by all-trans-retinol dehydrogenase (tRDH) (Haeseleer et al., 1998; Saari et al., 1998) before being released into the extracellular space for reuptake by the RPE and conversion back into 11-cis-retinal.
We recently demonstrated that fast, electrically active conformation changes of normal and mutant human rod rhodopsins can be recorded upon photolysis in a live-cell expression system (Sullivan and Shukla, 1999; Shukla and Sullivan, 1999; Sullivan et al., 2000). Rhodopsin charge motions are known as the early receptor current (ERC), a signal similar to the conformation-dependent charge motions of ionic channel gating currents. Human embryonic kidney 293S cells (HEK293S) that express wild-type (WT) or mutant human opsin apoproteins (Sullivan and Satchwell, 2000) are fused to form giant cells with abundant plasma membrane opsin. These are exposed in the dark to 11-cis-retinal solubilized in complex with BSA. After primary pigment regeneration, the BSA and retinal are washed out and ERC signals are recorded upon intense flash photolysis. Serial flash photolysis leads to ERC signal extinction. However, without exogenous 11-cis-retinal, simple dark adaptation promotes secondary visual pigment regeneration and ERC signal recovery. Subsequent photolysis bleaches visual pigment and extinguishes ERC signals, yet dark adaptation promotes recurrent signal recovery without fresh retinal. The single initial and transient 11-cis-retinal exposure supports recurrent regeneration of plasma membrane visual pigment after bleaching over hours without secondary addition of retinal. The action spectrum of giant WT-HEK293S cell R2 ERC signal on recurrent bleaches is fit by the absorption spectrum of rhodopsin purified from WT-HEK293S cells loaded with 11-cis-retinal (Shukla and Sullivan, 1999). This finding is evidence that formation of the normal ground state of human rod rhodopsin (11-cis-retinylidene chromophore) is the predominant pigment responsible for the recurrent bleach/recoveries of the ERC R2 signal in WT-HEK293S giant cells. However, a contribution of a small amount of isorhodopsin (9-cis-retinylidene chromophore) to the ERC action spectrum cannot be ruled out. Regeneration of ground state chromophore(s) during postbleach dark adaptation is supported by observations that the pigment formed has a normal photosensitivity comparable to human rod rhodopsin in photoreceptors (Shukla and Sullivan, 1999; Sullivan et al., 2000). The source of regenerating chromophore during dark adaptation is internal to the cell being recorded and long-lived bleaching intermediates do not contribute to the R2 signal (Shukla and Sullivan, 1999).
We are using the ERC approach to investigate the molecular biophysics of rapid electrically active conformational events in mutant and analogue chromophore visual pigments (Shukla and Sullivan, 1999; Brueggemann and Sullivan, 2001; unpublished data). Therefore, understanding the process of recurrent bleaching/recovery in the HEK293S opsin expression system is important. To explain the recurrent regeneration of bleached visual pigment, we proposed previously that solubilization of hydrophobic 11-cis-retinal by BSA leads to efficient retinoid transfer across the surface membrane and overloading into an intracellular membranous compartment. In this model a retinoid pool forms that is sufficient to support recurrent regeneration of bleached opsin by repeated plasma membrane partitioning of the hydrophobic chromophore (Shukla and Sullivan, 1999). If our previous model was correct, progressive run down of ERC charge or slowing of regeneration rate over successive bleach/recovery cycles is expected. This outcome is anticipated since the quantity of intracellular 11-cis-retinal substrate is fixed after initial overloading, and is irreversibly expended by each recurrent bleaching/regeneration cycle. We discovered, however, that the process of ERC signal bleaching and dark-adaptive signal recovery can be repeated on single giant cells in experiments lasting >4 h and with up to 18 complete bleach/recovery cycles without added 11-cis-retinal. Both the quantity of regenerated pigment repetitively recovered (total ERC charge), and the apparent rate of recovery, did not decrease or slow, respectively, over successive bleaching/dark adaptation cycles. This casts doubt upon the initial model of 11-cis-retinal overloading.
These observations lead us to propose an alternative hypothesis that WT-HEK293S cells have intrinsic metabolic retinoid machinery that maintains a steady load of cis-retinaldehydes as substrate available for pigment regeneration. Such machinery would oppose the progressive loss of chromophore acquired by serial bleaching. In the current work, experiments were designed to test, with physiological methods, if giant WT-HEK293S cells possess retinoid processing machinery that converts the all-trans-retinal released from bleached pigment back to a cis-retinaldehyde chromophore (e.g., 11-cis, 9-cis), which regenerates ground state pigment (rhodopsin, isorhodopsin). The ERC R2 charge motion serves as a sensitive and time-resolved assay of forward bleaching from ground state pigments to Metarhodopsin-II (Meta-II). A critical observation is that ground state pigment, and hence cis-retinaldehyde chromophore, is synthesized in WT-HEK293S cells after loading with Vitamin A (all-trans-retinol). Vitamin A cannot react with opsin to form a visual pigment and yields no initial ERC, but it provides the retinoid substrate backbone necessary for cis-retinaldehyde synthesis. Another critical observation is that an agent that short-circuits the retinoid visual cycle in the live mammalian eye can reversibly block recurrent bleaching/recovery cycles in WT-HEK293S giant cells. We conclude that HEK293S cells have a functional retinoid processing machinery that can generate cis-retinaldehydes from either Vitamin A, the native retinoid substrate in the RPE, or all-trans-retinal, which results from bleached visual pigment. To our knowledge, this study is the first to demonstrate a physiologically active retinoid metabolism capable of recurrent visual pigment regeneration in a cultured cell line. A preliminary report of these findings has been made (Brueggemann and Sullivan, 2000).
MATERIALS AND METHODS
Materials
HPLC-purified 11-cis-retinal was a gift from Dr. Rosalie Crouch (Medical College of South Carolina, Charleston, SC) and the National Eye Institute. All-trans-retinal, 9-cis-retinal, 13-cis-retinal, all-trans-retinol (Vitamin A), α-D-tocopherol (Vitamin E), and essentially fatty acid–free (FAF) BSA were obtained from Sigma-Aldrich. 4-butyl-aniline (4BA) was obtained from Sigma-Aldrich. All other chemicals were reagent grade. Cell culture media, sera, and antibiotics were obtained from Life Technologies.
Cell Culture and Fusion
WT-HEK293S cells constitutively express WT human rod opsin from stably integrated transgenes under control of the strong cytomegalovirus promoter (Nathans et al., 1989). HEK293S cells are the parent strain of WT-HEK293S cells and are an environmentally adapted clone capable of growing in suspension (Stillman and Gluzman, 1985). HEK293S cells do not express detectable opsin protein by immunocytochemistry or immunoblotting (Sullivan and Satchwell, 2000). Cells are grown on poly-L-lysine (average molecular weight, 120 kD) –coated glass coverslips at 37°C and 5% CO2 (in air) in DMEM/F12 containing 10% (vol/vol) heat-inactivated calf serum and supplemented with antibiotics (penicillin G and streptomycin sulfate both at 110 U/ml) and 2 mM glutamine. As described previously (Sullivan and Shukla, 1999), cells were chemically fused by transient exposure (5 min) to 50% (wt/vol) polyethylene glycol (average molecular weight 1,500 g/mole) in 75 mM HEPES (pH 8.0) (Boehringer). Giant cells were used for ERC recording between several hours and days following fusion.
Cellular Chromophore Loading and Rhodopsin Regeneration
Coverslips with attached giant cells were placed in a polystyrene dish in a light-tight container at room temperature (21–23°C) in regeneration buffer (in mM): 140 NaCl, 5.4 KCl, 1.8 CaCl2, 1.0 MgCl2, 10 glucose, 10 HEPES-NaOH, pH 7.2, and 2% (wt/vol) FAF-BSA (290 μM) (Sigma-Aldrich). This concentration of FAF-BSA is sufficient to solubilize the retinoids used in these experiments as determined by optical clarity in regeneration buffer. Spectrophotometrically quantified ethanolic stocks of 11-cis-retinal (extinction coefficient (in ethanol unless otherwise noted): 26.4 mM−1·cm−1 at 377 nm), all-trans-retinal (45.4 mM−1·cm−1), 9-cis-retinal (39.5 mM−1·cm−1 in hexane), 13-cis-retinal (35.6 mM−1·cm−1), and all-trans-retinol (Vitamin A) (52.77 mM−1· cm−1) were added to separate giant cell samples at a final concentration of 25 μM or 50 μM, and 0.025% (vol/vol) α-D-tocopherol (Vitamin E) was added as an antioxidant in all cases. Prior to ERC recording, cells were loaded with chromophore to regenerate pigment in the dark for 40 min at room temperature, the temperature at which ERC experiments were conducted, or overnight at 4°C. The use of 4°C suppresses detachment of cells from the coverslip over time in the absence of serum, and suppresses bacterial growth during overnight incubation that could confound cell stability and vitality and contribute to nonspecific cis-retinal synthesis (Rotmans et al., 1972; Ostapenko and Furayev, 1973; Bridges, 1977). Coverslips with regenerated cells were washed extensively in bath recording buffer without retinoids and transferred in darkness into the same buffer in the recording chamber. Cells were not exposed again to exogenous retinoids unless otherwise stated. In visual cycle short-circuiting experiments 4BA was added into regeneration buffer to a final concentration of 500 μM from an ethanolic stock (0.5 M).
Flash Photolysis
Visual pigments in giant cells are activated with a monochromatic intense microbeam flash apparatus (Sullivan, 1998). The flash intensity in the microscope specimen plane was ∼4 × 108 photons/μm2 at peak energy, and was band limited by a three-cavity interference filter centered at 500 nm and with a bandwidth of 70 nm (full-width half maximum). Flash energy delivery is parafocal with the imaged giant cell and the diameter of the microbeam spot was ∼250 μm in these experiments. Flash duration (≈14 μs is sufficiently short such that the critical Metarhodopsin-I ⇔ Meta-II equilibrium, which yields the ERC R2 signal, is not perturbed by photoregeneration (see Sullivan and Shukla, 1999). Given the measured maximum photosensitivity of rhodopsin in this system (8.5 × 10−9 μm2; Shukla and Sullivan, 1999) a single flash of this intensity (4 × 108 photons/μm2) is sufficient to activate 97% of the rhodopsin molecules in a single giant cell at least once, as determined by crude estimates from the Poisson distribution (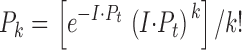 ), where k is the number of photons absorbed per molecule (k = 0, 1, 2, … ), I is the flash intensity, P
t is the photosensitivity, and P
k is the fraction of molecules absorbing k photons. There is sufficient intensity for multiple absorptions by a single rhodopsin molecule in a single flash. An odd number of absorptions by a single rhodopsin molecule can lead to pigment bleaching with high quantal efficiency. Even numbers of absorptions promote photoregeneration of the ground state pigment at substantial quantal efficiency from short-lived bathorhodopsin and lumirhodopsin intermediates with lifetimes overlapping the flash duration (≈14 μs) and absorption spectra overlapping the spectral energy band of the stimulus (see Sullivan and Shukla, 1999). Photoregeneration of the ground state by short-lived spectral intermediates limits the amount of bleaching possible in a single flash to no more than 50% (Hagins, 1955; Williams, 1964, 1965, 1974), which is why multiple flashes are needed to extinguish ERC charge, even though sufficient photons are present in each single flash to activate nearly all pigment molecules.
), where k is the number of photons absorbed per molecule (k = 0, 1, 2, … ), I is the flash intensity, P
t is the photosensitivity, and P
k is the fraction of molecules absorbing k photons. There is sufficient intensity for multiple absorptions by a single rhodopsin molecule in a single flash. An odd number of absorptions by a single rhodopsin molecule can lead to pigment bleaching with high quantal efficiency. Even numbers of absorptions promote photoregeneration of the ground state pigment at substantial quantal efficiency from short-lived bathorhodopsin and lumirhodopsin intermediates with lifetimes overlapping the flash duration (≈14 μs) and absorption spectra overlapping the spectral energy band of the stimulus (see Sullivan and Shukla, 1999). Photoregeneration of the ground state by short-lived spectral intermediates limits the amount of bleaching possible in a single flash to no more than 50% (Hagins, 1955; Williams, 1964, 1965, 1974), which is why multiple flashes are needed to extinguish ERC charge, even though sufficient photons are present in each single flash to activate nearly all pigment molecules.
Whole-Cell ERC Recording
The recording setup and procedures have been extensively described (Sullivan and Shukla, 1999; Sullivan et al., 2000). Briefly, retinoid-regenerated giant cells were imaged under infrared light (>830 nm) in a bath solution containing (in mM): 140 tetramethylammonium hydroxide (TMA-OH), 140 2-[N-morpholino]ethanesulfonic acid (MES-H), 2.0 CaCl2, 2.0 MgCl2, 5.0 HEPES-NaOH, pH 7.0 (E-1). Borosilicate pipettes yielding low series access resistance (2–4 megaohm) in cells were coated with Black Sylgard (#173; Dow Corning) and readily formed giga-ohm order seals with giant cell surfaces when filled with (in mM): 70 TMA-OH, 70 MES-H, 70 TMA-fluoride, 10 EGTA-CsOH, 10 HEPES-CsOH, pH 6.5. Replacing the bulk of permeant ions in these solutions decreases whole-cell current noise, in part from ionic channel shot noise, and improves signal-to-noise for recording low-level plasma membrane ERC capacitative signals. After seal formation, patch/seal capacitance was compensated, and whole-cell recording was achieved by suction. Series resistance errors should not inhibit accurate recording of small ERC signals at constant membrane voltage. Internal pH (6.5) was chosen to bias the Meta-I ⇔ Meta-II equilibrium strongly in favor of Meta-II (>90%) at room temperature (Parkes and Liebman, 1984). ERC signals were recorded with a patch clamp instrument (Axopatch 1C with a CV-4–resistive feedback headstage; Axon Instruments, Inc.) in voltage clamp mode (holding potential at 0 mV) through an 8-pole Bessel filter tuned at the panel (cutoff frequency 5 kHz) and digitized at 200 μs/point to obtain a full 100 ms of ERC R2 signal as described previously (Sullivan and Shukla, 1999). This undersamples the normal R1 kinetics which cannot be kinetically resolved under cellular electrophysiology, yet allows high fidelity recording of the entire R2 signal which has a slow relaxation tail (<100 ms). The true cutoff frequency (−3 dB) of a Bessel filter having a gradual roll-off characteristic is about one half the programmed panel frequency (Colquhoun and Sigworth, 1983). In these experiments, the Bessel −3 dB cutoff frequency is ∼2.5 kHz and the digitization of the data occurred at 5 kHz or just at the Nyquist criterion. Little, if any, aliasing should occur under these conditions. High intensity microbeam flashes were controlled and data acquired using pCLAMP 5.51 (CLAMPEX; Axon Instruments, Inc.) and a Scientific Solutions data interface as described (Sullivan, 1998). Whole-cell membrane capacitance (Cmem) (in picoFarads [pF]) was measured by integrating the current response to 20-mV depolarizing step pulses from a holding potential of −80 mV. ERC data traces were acquired from CLAMPEX using Origin (Microcal Software) and displayed after subtraction of baseline current. The R2 current waveform was integrated to obtain the charge in a single R2 signal (Qi). To obtain the total R2 charge (Q∞) from all regenerated rhodopsin, a series of identical 500-nm flashes was given in rapid sequence (∼0.1 Hz) to extinguish the ERC R2 charge motion. The individual R2 currents were separately integrated to obtain Qi values for each response and Q∞ was obtained by summing the Qi values over the entire bleach extinction series (Q∞ = ΣQi). Q∞ was normalized by plasma membrane surface area with Cmem as estimate (1μF/cm2). This normalization compensates for the known linear effect of giant cell size (surface area or volume) on the R2 signal (Sullivan and Shukla, 1999). As giant cell size increases more plasma membrane opsin results but opsin membrane density remains constant. Giant cells used for ERC studies ranged from ∼50–200 μm in diameter, so there was substantial variation in surface area estimated by Cmem. Attempts to isolate giant cells into defined size classes were not effective.
BSA Endocytosis Studies
Single or fused WT-HEK293S and HEK293S cells were exposed to regular regeneration buffer (containing 2% wt/vol FAF-BSA) or with regeneration buffer where 0.1% (wt/vol) of the total FAF-BSA was replaced with FITC-BSA. To simulate the normal chromophore loading conditions, incubations occurred at room temperature in darkness for 1–1.5 h. FITC-BSA was then removed and cells were observed through an FITC dichroic/filter cube on an Olympus IM2 microscope equipped for Hoffman contrast and epifluorescence and photographed with Kodak EliteChrome slide film (ASA 200).
RESULTS
cis-Retinaldehydes Are Required for the ERC R2 Signal
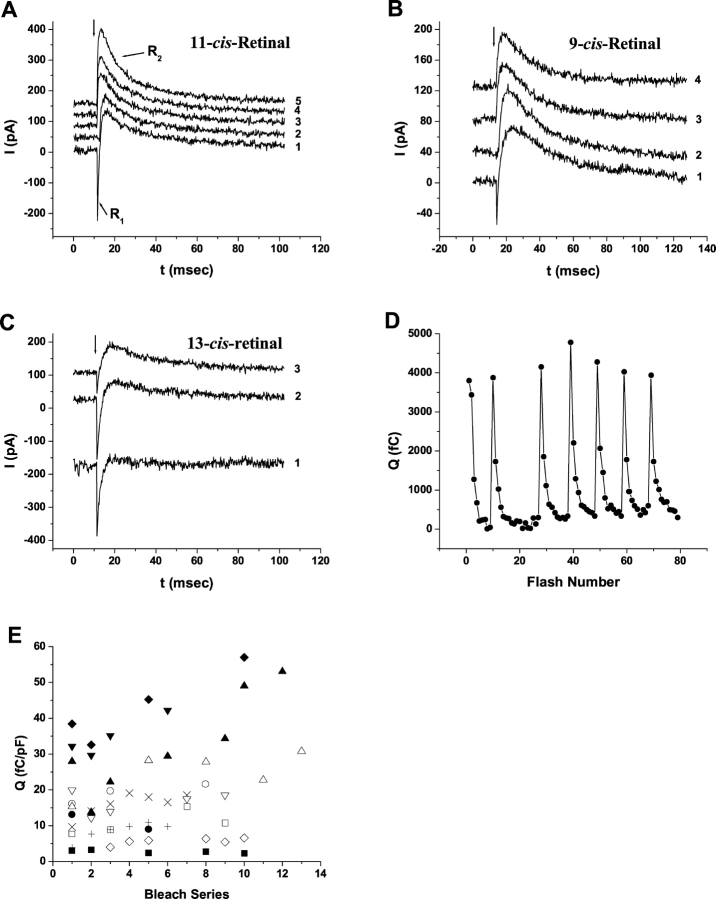
Retinoids are required to obtain ERC signals. Once retinoids are loaded and removed giant WT-HEK293S cells were not subsequently exposed to retinoids, unless explicitly stipulated. Visual pigment recovery after bleaching must occur from available retinoid substrates initially loaded into cells. WT-HEK293S cells regenerated with naturally occurring cis-retinaldehydes 11-cis-retinal (Fig. 2 A), 9-cis-retinal (Fig. 2 C), or 13-cis-retinal (Fig. 2 E) all yield whole-cell ERCs having both bimodal inward (R1) and outward (R2) currents on the initial 500-nm flash photolysis series. The R2 charge motion associates with formation of the Meta-II conformational states during forward bleaching of rhodopsin (Spalink and Stieve, 1980; Makino et al., 1991; Sullivan and Shukla, 1999; Shukla and Sullivan, 1999; unpublished data). All signals extinguish by serial flash photolysis. WT-HEK293S cells loaded with either 11-cis- or 9-cis-retinal regenerate pigments yielding large, inward R1 and outward R2 charge motions upon initial flash photolysis similar to ERCs of rod and cone photoreceptors (Makino et al., 1991; Sullivan et al., 2000). These chromophores regenerate stable ground state pigments, rhodopsin and isorhodopsin, respectively, which bleach by an identical series of conformational intermediates that promote charge motions (Kliger et al., 1984). In contrast, 13-cis-retinal, the chromophore of dark-adapted bacteriorhodopsin, regenerates a small but definitive R2 signal upon the initial bleach series despite a large R1 signal.
Figure 2.
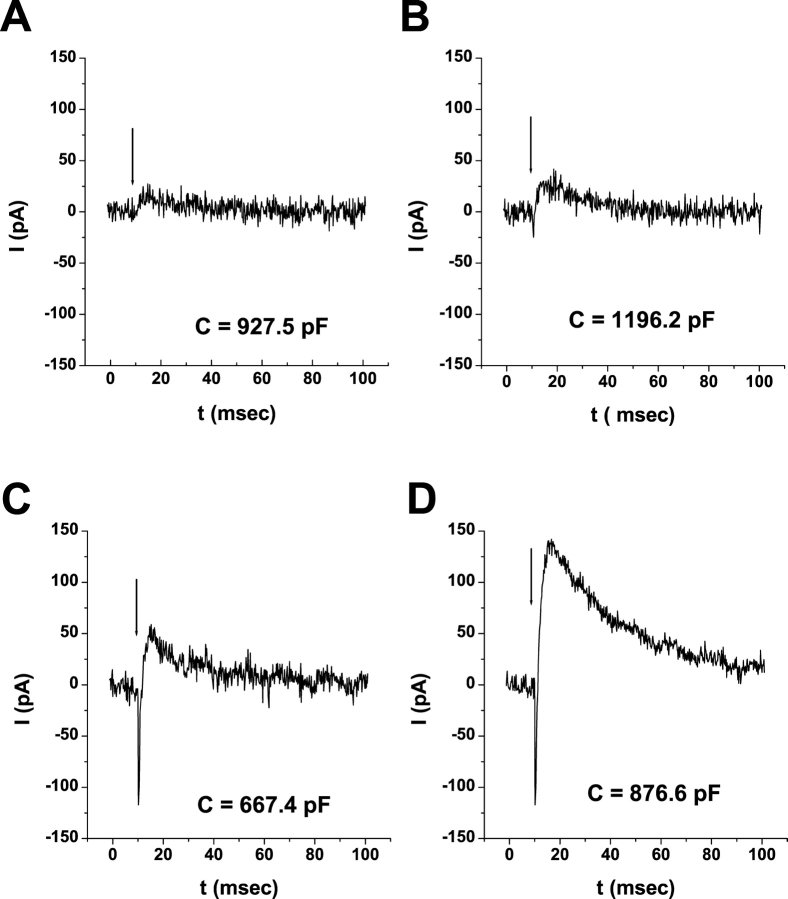
ERC signals from WT-HEK293S cells regenerated with different cis-retinaldehydes. Fused WT-HEK293S giant cells were loaded with 25 μM 11-cis-retinal or 9-cis-retinal or 50 μM 13-cis-retinal complexed to FAF-BSA. ERC signals on the first 500-nm flash during the primary bleaching extinction (A, C, and E) and secondary bleaching extinctions (B, D, and F) are shown for 11-cis-retinal– (A and B), 9-cis-retinal– (C and D), and 13-cis-retinal– (E and F) loaded representative cells. Membrane capacitances of the cells are indicated. The arrow indicates the timing of the flash stimulus. Responses from each single cell are representative of larger populations of cells regenerated with 11-cis-retinal (n = 54), 9-cis-retinal (n = 5), and 13-cis-retinal (n = 4).
After extinguishing ERC charge, a measure of ground state pigment bleaching, 10 min of dark adaptation allowed the second flash photolysis series to elicit pure R2 signals lacking R1 in both 11-cis- (Fig. 2 B) and 9-cis-retinal (Fig. 2 D) –loaded cells. Bleaching of the pure R2 signals and subsequent dark adaptation leads to the recovery again of pure R2 signals, which continue to recur on serial bleach/recovery cycles (Fig. 3). We did not attempt to distinguish the ERC R2 waveforms in recurrent bleach cycles of WT-HEK293S giant cells initially loaded with 11-cis-retinal or 9-cis-retinal. The action spectrum data of the pure R2 ERC charge motion on second and recurrent bleach/recovery series of cells initially loaded with 11-cis-retinal is fit by the absorption spectrum of human rod rhodopsin, which indicates that 11-cis-retinal is the predominant regenerating chromophore (Shukla and Sullivan, 1999). We did not utilize R2 action spectra to attempt to measure the relative amounts of 11-cis-retinal (rhodopsin) or 9-cis-retinal (isorhodopsin) synthesized because the spectra, absorption maxima of rhodopsin (493 nm) and isorhodopsin (483 nm), and their bandwidths are so similar (Crescitelli, 1985). In contrast, giant WT-HEK293S cells loaded with 13-cis-retinal (Fig. 2 F) have a bimodal ERC signal (R1 plus small R2) on the second bleach cycle and by the third bleach cycle the R1 signal has mostly disappeared, leading to essentially pure R2 signals otherwise similar to 11-cis-retinal– and 9-cis-retinal–loaded cells on successive bleach/recoveries (Fig. 3, A–C). This suggests that the loaded 13-cis-retinal chromophore can eventually be processed into cis-retinaldehydes (i.e., 11-cis, 9-cis) that yield large R2 charge motions. This may occur by bleaching of formed pigment to all-trans-retinal and subsequent thermal or enzymatic conversion reactions. The pure R2 waveform and kinetics from all three cis-retinals are qualitatively similar. This data indicates that the photoisomerization of the chromophores of rhodopsin (11-cis-retinylidene) and isorhodopsin (9-cis-retinylidene) and, for the first time, a 13-cis-retinal–regenerated pigment with the WT human rod opsin apoprotein, result in comparable state-dependent charge motions. This is strong evidence that the outward millisecond-order R2 signal is characteristic of electrically active conformation changes associated with forward bleaching of ground state rhodopsins with cis-retinaldehyde chromophores. When cells are regenerated with 11-cis-retinal (50 μM) at room temperature and then kept overnight at 4°C, bimodal ERCs are also recorded, indicating stability of the human rod rhodopsin ground state visual pigment in these cells after routine regeneration conditions. A similar behavior occurs with 9-cis-retinal. Overnight regenerations with 13-cis-retinal were not tested. The major observable differences between the cis-retinaldehydes are that 13-cis-retinal has a smaller R2 signal and persistent R1 signal on secondary extinctions. We did not test 7-cis-retinal which, to our knowledge, is not a natural ligand in rhodopsin pigments. All naturally occurring cis-retinaldehydes (11-, 9-, 13-cis) support ERCs with R2 components on initial and subsequent bleach series. In contrast, all-trans-retinal and all-trans-retinol (Vitamin A) generate no R2 responses on initial flash photolysis (see below). R2 is a signature of Meta-II formation starting from a stable ground state.
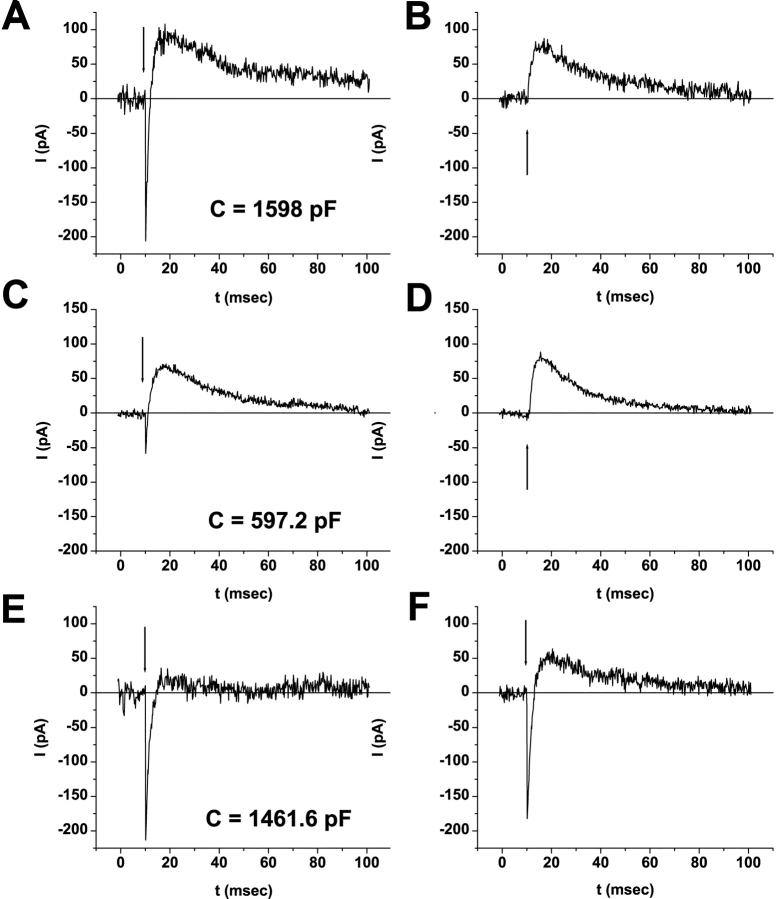
Figure 3.
ERC signal recovery during serial bleaches and dark adaptation. Fused WT-HEK293S giant cells were loaded with 25 μM 11-cis-retinal overnight at 4°C (A), 25 μM 9-cis-retinal for 40 min at room temperature (B), or 50 μM 13-cis-retinal for 40 min at room temperature (C). Single ERC traces recorded upon the first flash in each bleach series are shown. The number adjacent to the trace indicates the bleach series. Membrane capacitance was 340 pF (A), 597.2 pF (B), and 1461.6 pF (C). A criterion of 10 min of dark adaptation occurred between each bleach series. A labels the R1 and R2 signals. D shows an example of repetitive bleach/recoveries on a WT-HEK293S giant cell (635.2 pF) loaded with 25 μM 11-cis-retinal for 40 min at room temperature. The R2 charges per flash (Qi) are shown versus flash number. Seven sequential bleaches are displayed. The vertical line drawn between charge extinction (near 0 fC) and the next maximum indicates the criterion period of 10 min of dark adaptation before the next flash series. E shows the R2 Q∞ levels across serial bleaches under identical conditions for a set of cells (n = 12) loaded with 11-cis-retinal for 40 min at room temperature or overnight at 4°C. Gaps in the series for a single cell (each with unique symbol) indicate bleaches occurring under nonsymmetrical conditions of pH or with transmembrane voltage at nonzero values and are not included. Cells ranged in size from 93.5 to 577 pF.
After flash photolysis, cells loaded with 11-cis-retinal recurrently recover bleachable rhodopsin and R2 charge by simple dark adaptation. Fig. 3 D shows a representative experiment where an 11-cis-retinal–loaded WT-HEK293S giant cell was bleached to R2 extinction by serial flash photolysis. Dark adaptation consistently promotes R2 charge recovery. Bleaching followed by recovery was followed over seven cycles in this cell without evidence of R2 signal decrement or evidence of change in photosensitivity. Recurrent bleach/recovery of the R2 signal occurs with consistent levels of total integrated R2 charge (Q∞) recovered (Fig. 3 E). Q∞ remains essentially constant or increases slightly (1–2×) over many bleach/recovery cycles. To date a maximum of 18, 9, or 4 serial bleach/recovery cycles have been measured in 11-cis-, 9-cis-, or 13-cis-retinal–loaded cells with the longest experiments (11-cis) lasting >4 h.
All-Trans-Retinal and Vitamin A Are Retinoid Substrates for Rhodopsin Regeneration in WT-HEK293S Cells
WT-HEK293S giant cells loaded for 40 min at room temperature with 50 μM all-trans-retinal have purely negative (unimodal) ERCs with no outward R2 component (Fig. 4 A). These pure R1-like signals result from specific protonated Schiff base (PSB) formation of all-trans-retinal in a ligand binding pocket in opsin and a contribution of nonspecific PSBs formed by reaction of all-trans-retinal with lipids or nonopsin proteins in giant HEK293S cell plasma membranes (Brueggemann and Sullivan, 2001). The waveform and kinetics of the R1-like charge motion generated by all-trans-retinal suggests that it arises from biophysical processes distinct from the R2 signal.
Figure 4.
ERC evidence for retinoid conversions in giant WT-HEK293S cells. Cells were loaded with 50 μM chromophore complexed to FAF-BSA in all cases. ERCs were obtained upon the first 500-nm flash from cells regenerated for 40 min at room temperature in darkness (A and C) or overnight at 4°C in darkness (B and D). Each panel is from a single cell. Cells were regenerated with all-trans-retinal (A and B) or Vitamin A (C and D). Cells are representative of larger populations of cells examined (all-trans-retinal, 40 min: n = 43, overnight at 4°C: n = 7; Vitamin A, 40 min: n = 2 cells, overnight at 4°C: n = 23 cells).
After incubation in darkness (overnight at 4°C or hours at room temperature), all-trans-retinal–loaded cells yield bimodal (R1 + R2) ERC signals upon 500-nm flash photolysis (Fig. 4 B) that are comparable to first bleach cycle signals in giant WT-HEK293S cells regenerated with 11-cis- or 9-cis-retinal (Fig. 2, A and C). The large R2 signals obtained by lengthy dark incubation in all-trans-retinal–loaded cells suggests that WT-HEK293S cells used this retinoid substrate to synthesize 11-cis- or 9-cis-retinal that regenerated rhodopsin or isorhodopsin, respectively. 13-cis-retinal is unlikely to be a product of all-trans-retinal metabolism since it does not initially yield large R2 signals. Nonphotochemical synthesis of 7-cis-retinal is not known to occur in vivo. The large bimodal signals recovered from all-trans-retinal–loaded cells are bleached by flash photolysis. Recurrent bleach/recovery cycles demonstrate that the R1 signal disappears and a large R2 signal becomes manifest as in 11-cis-retinal–, 9-cis-retinal–, and 13-cis-retinal–loaded cells. These results suggest that WT-HEK293S giant cells, in the absence of light, convert all-trans-retinal into a cis-retinaldehyde product (11-cis- and 9-cis-retinal) that regenerates ground state pigment(s).
Dietary Vitamin A is the ultimate source of retinoid substrate for the visual cycle. If WT-HEK293S cells have a retinoid metabolism similar to that in the eye, then Vitamin A should be able to support the synthesis of cis-retinaldehydes needed to form ground state rhodopsins that yield large R2 signals. When WT-HEK293S cells are loaded with 50 μM all-trans-retinol (Vitamin A) at room temperature for 40 min, there is typically no initial ERC upon flash photolysis, even in very large cells (Fig. 4 C). This is not surprising, as Vitamin A cannot form the covalent PSB with opsin needed for pigment regeneration. After 40 min regeneration with Vitamin A, occasional cells do show a small purely negative R1-like signal similar in waveform, but smaller in amplitude, when compared with ERCs of cells regenerated with equivalent concentrations of all-trans-retinal. R1-like charge motions likely result from all-trans-retinal generated as a result of nonphotochemical solution oxidation of Vitamin A to all-trans-retinal or its synthesis from Vitamin A in cells by ubiquitous cellular dehydrogenases. When Vitamin A–loaded cells are incubated overnight in the dark at 4°C, bimodal ERCs result the following day (Fig. 4 D) that are not distinguishable from those resulting from loading and regeneration with 11-cis-retinal or 9-cis-retinal. R2 signals first appear within a few hours when Vitamin A loading is conducted at room temperature in darkness. Although initial R1-like signals in Vitamin A were not unexpected because of the paths available for synthesis of all-trans-retinal, large R2 signals were quite surprising because they require the presence of a cis-retinaldehyde to react with the opsin apoprotein, and none was present in the Vitamin A stock as assayed by a complete lack of initial R2 charge motion in Vitamin A–loaded cells. The synthesis of 11-cis- or 9-cis-retinaldehyde could only result from both the oxidation of Vitamin A and an isomerization around either the 11=12 or 9=10 polyene bonds. These results indicate that WT-HEK293S cells, using Vitamin A as the sole metabolic substrate, have the capacity to synthesize 11-cis- or 9-cis-retinaldehydes that regenerate ground state visual pigments in the dark. After overnight regeneration in Vitamin A the resultant ERC signals bleach and recover recurrently as we find with WT-HEK293S cells initially loaded with 11-cis-retinal or 9-cis-retinal. That is, loading WT-HEK293S giant cells with Vitamin A in the dark leads to a preparation indistinguishable by ERC assay from cells loaded for 40 min with either 11-cis-retinal or 9-cis-retinal. In control experiments, giant WT-HEK293S cells transferred from growth medium to regeneration buffer without Vitamin A show no signal at all the following day. Therefore, WT-HEK293S cells do not obtain or synthesize retinoids de novo from simple substrates in culture medium or later from the raw regeneration buffer (containing FAF-BSA). When regeneration buffer is loaded with Vitamin A and kept in the dark overnight at 4°C without WT-HEK293S cells and then the next day naïve WT-HEK293S cells are placed in this conditioned buffer, at best, small negative charge motions are seen after 40 min incubation. This is consistent with overnight generation of a small amount of all-trans-retinaldehyde by thermochemical oxidation of Vitamin A in aqueous buffer that is freely exchangeable with ambient oxygen during the incubation. These control experiments rule out contribution to cis-retinal synthesis of thermal or chemical oxidation/isomerization reactions occurring in solution, or the cellular metabolism of any low-level contaminants that might be present in the Vitamin A stocks (98–99% pure by HPLC per Sigma-Aldrich).
When WT-HEK293S cells are regenerated with equivalent levels of Vitamin A palmitate (all-trans-retinol esterified to palmitic acid) at either 4°C or room temperature, no R1 or R2 signals are generated upon subsequent flash photolysis (Fig. 5). The rationale for testing this substrate is that it can be used in the formation of 9-cis-retinal by nonenzymatic thermochemical retinoid conversion reactions, but is not processed to 11-cis-retinoids by the isomerohydrolase (IH) complex of the RPE (Rando and Chang, 1983; Bernstein et al., 1987).
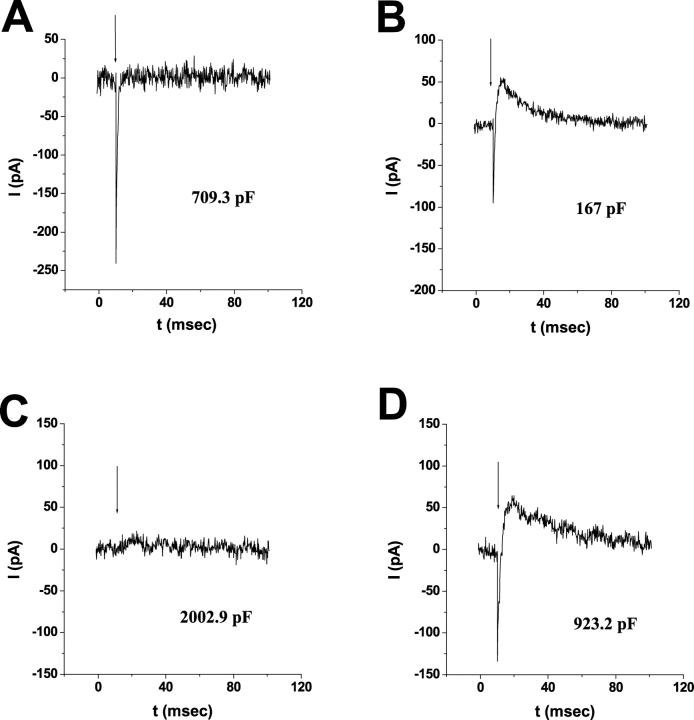
Figure 5.
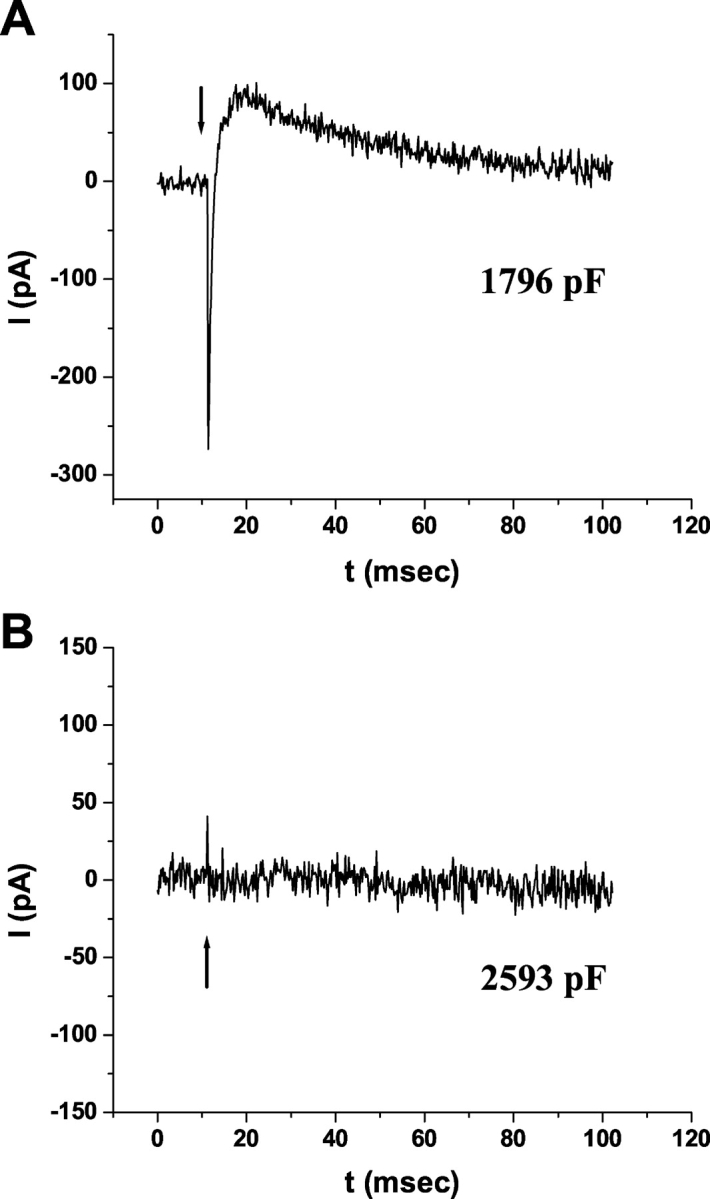
Vitamin A palmitate is not metabolized to cis-retinaldehydes in giant WT-HEK293S cells. Giant cells were regenerated with 50 μM Vitamin A (A) or 50 μM Vitamin A palmitate (B) overnight at 4°C in darkness and ERCs recorded upon the first 500-nm flash the following day. Membrane capacitances of the representative cells are indicated. Responses shown are representative of a larger population of Vitamin A palmitate–regenerated cells (n = 5).
Short-circuiting Pigment Regeneration in WT-HEK293S Cells
To begin probing into the nature of the retinoid conversion reactions in WT-HEK293S cells we sought metabolic inhibitors that block retinoid metabolism in the RPE. The aromatic amine, 4-butyl-aniline, is a proven and potent agent that short circuits the chromophore visual cycle in the intact mammalian retina/RPE by preventing new synthesis of 11-cis-retinal either from Vitamin A or from all-trans-retinal that results from rhodopsin bleaching (Bernstein et al., 1986a,b). Established or putative mechanisms of action of 4BA include: (a) its chemical capacity to form Schiff bases with any retinaldehyde, hence covalently trapping chromophores into 4BA-adducts that prevent visual pigment formation (Bernstein et al., 1986a,b), and/or (b) a direct inhibition of enzymes responsible for retinoid conversions in the intracellular milieu (e.g., IH and/or lecithin retinyl acetyl transferase [LRAT] activities). If 4BA short-circuits recurrent retinoid conversion reactions in WT-HEK293S cells, then it should prevent the appearance of R2 signals from Vitamin A or all-trans-retinal or the recurrent signal, for example, in 11-cis-retinal–loaded cells. When all-trans-retinal (50 μM) (Fig. 6 A) or Vitamin A (50 μM) (Fig. 6 B) are loaded into WT-HEK293S cells overnight at 4°C or at room temperature in the presence of 4BA, ERC signals the next day were uniformly small. Some cells had purely negative small signals, some had small bimodal signals with both R1 and R2 components, and some had small unimodal signals with only R2. Overall, 84% of cells had small R2 signals (n = 32), but the measured quantity of R2 charge was markedly suppressed in comparison to cells without 4BA (see Fig. 7). The small amount of visual pigment formed (10–25%) in WT-HEK293S cells loaded with either substrate and 4BA rapidly extinguish by sequential 500-nm flashes and after 10 min of dark recovery, subsequent flash photolysis yields little, if any, ERC charge movement. As in the mammalian eye, 4BA antagonizes retinoid conversion reactions in WT-HEK293S cells when present at 10-fold molar excess over Vitamin A substrate leaving some leakage synthesis of cis-retinaldehyde.
Figure 6.
4BA inhibits retinoid processing in giant WT-HEK293S cells. ERCs were obtained upon the first 500-nm flash from giant WT-HEK293S cells after overnight loading/regeneration in 50 μM all-trans-retinal with 0.5 mM 4BA (A) or 50 μM Vitamin A with 0.5 mM 4BA (B) and 1 h after overnight exposure of Vitamin A with 4BA followed by washout of chromophore and 4BA (C), or exposure of Vitamin A plus 4BA overnight-regenerated cells to 11-cis-retinal (50 μM) for 30 min (D). Responses are representative of larger cell populations studied (all-trans-retinal with 4BA, n = 12 cells; Vitamin A with 4BA, n = 31 cells).
Figure 7.
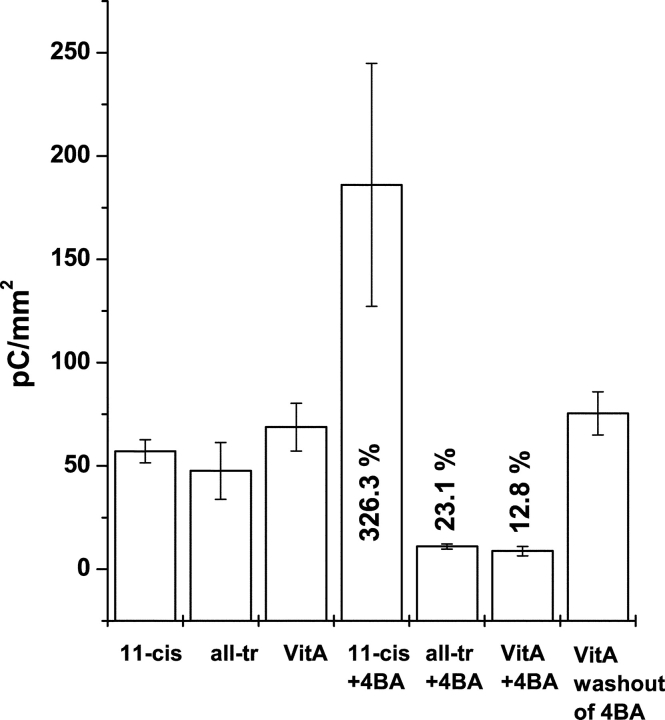
Effect of 4BA on the quantity of ERC R2 charge motion. Giant WT-HEK293S cells were regenerated with 50 μM 11-cis-retinal (11cis, n = 9 cells), all-trans-retinal (allTr, n = 4), or Vitamin A (VitA, n = 13) as controls and with 0.5 mM 4BA inhibitor (+4BA: 11cis, n = 4; allTr, n = 4; VitA, n = 7 cells), and for Vitamin A after washout of the 4BA (VitA washout, n = 15 cells). The total extinguishable ERC R2 charge (Q∞) obtained from each cell was normalized to the cell area (mm2) to compensate for the effects of cell size on opsin levels (Sullivan and Shukla, 1999). Means ± standard error of mean are plotted. The additional numbers inside certain bars are the percent difference of the means of each retinoid with 4BA versus the mean in each retinoid alone. All statistical tests were conducted at the P < 0.05 level of confidence. Mean levels of R2 charge were significantly different across the entire set of experimental conditions (one-way analysis of variance [ANOVA], P = 1.8e−6). Mean levels of R2 charge were not statistically different between 11cis, allTr, VitA controls (ANOVA, P = 0.484). Mean levels of R2 charge were not statistically different for VitA and VitA after washout of 4BA (t test, P = 0.669). Levels of R2 charge were statistically different between 11cis versus 11cis+4BA (t test, P = 0.006), for allTr versus allTr+4BA (t test, P = 0.038), and VitA versus VitA+4BA (t test, P = 0.0015).
In contrast, when giant WT-HEK293S cells are loaded with Vitamin A and 4BA overnight in the dark at room temperature and then washed and placed in ERC recording buffer (E-1) for 1 h with neither Vitamin A nor 4BA before ERC recording, large bimodal ERCs were found upon flash photolysis (Fig. 6 C). The rapid recovery from 4BA block to yield large R2 signals indicates that this agent neither impedes primary Vitamin A loading into WT-HEK293S cells nor permanently impairs the subsequent intracellular reactions that convert this substrate into cis-retinaldehydes. The 4BA antagonism in Vitamin A–loaded WT-HEK293S cells is reversible such that significant amounts of 11-cis-retinal and/or 9-cis-retinal are synthesized within 1 h to regenerate ground state photopigments supportive of robust bimodal ERC signals with large R2 signals. This suggests that 4BA functions as a noncovalent antagonist. If the hydrophobic 4BA, in significant molar excess over loaded retinoids (500 μM vs. 50 μM), had chemically reacted to form Schiff bases with loaded or newly formed retinaldehydes in WT-HEK293S cells, that fraction of substrate molecules would have been irreversibly trapped as covalent adducts that cannot form PSBs with opsin and could not have participated in the prompt regeneration and recovery of bleachable pigment that was observed upon 4BA removal. Prompt recovery from 4BA antagonism in Vitamin A–loaded cells to levels of R2 charge statistically comparable to controls indicates that 4BA does not act by covalent trapping of cis-retinaldehyde. When WT-HEK293S cells were regenerated in Vitamin A plus 4BA overnight at 4°C, and then 11-cis-retinal was added (25 μM) to the regeneration buffer in the dark for 40 min with Vitamin A and 4BA still present, normal bimodal ERCs were observed (Fig. 6 D). This indicates that 4BA does not prevent 11-cis-retinal regeneration with human rod opsin to form rhodopsin in WT-HEK293S membranes. Therefore, even at 20-fold molar excess over equilibrated 11-cis-retinal levels, 4BA does not act by conversion of 11-cis-retinal into adducts incapable of pigment regeneration. Similarly, when 11-cis-retinal and 4BA are added together to regeneration buffer and cells are incubated overnight at 4°C, the next day normal bimodal ERCs are recorded. 4BA does not block regeneration of the ground state of rhodopsin with 11-cis-retinal in WT-HEK293S giant cells. Finally, 4BA inhibits the amplitude of both R1 and R2 signals resulting from overnight all-trans-retinal regeneration. It appears that the presence of 4BA in Vitamin A and all-trans-retinal–loaded WT-HEK293S cells antagonizes retinoid-processing pathways that are essential to convert all-trans-retinoid isomeric states into cis-retinals that form stable ground state pigments with large R2 signals.
We quantified the efficacy of 4BA to antagonize the ability of WT-HEK293S giant cells to regenerate cis-retinylidene visual pigments when loaded with various retinoid substrates (Vitamin A, all-trans-retinal, 11-cis-retinal). Total R2 charge motion (Q∞) was measured in giant WT-HEK293S cells under each condition and divided by the cell surface area (from Cmem) for each cell before averaging. This normalizes for the linear effects of cell size on plasma membrane opsin quantity and ERC R2 levels (Sullivan and Shukla, 1999). The mean and standard error of total R2 charge motion during the first bleach cycle for 11-cis-retinal, all-trans-retinal, and Vitamin A (all at 50 μM) are shown for WT-HEK293S giant cell recordings after overnight incubation at 4°C in the absence and presence of 4BA and after 4BA washout for Vitamin A (Fig. 7). The null hypothesis (no effect of 4BA and all initial substrates equivalently regenerate R2 charge) that the sample means across all seven experimental conditions were the same was refuted (ANOVA, 0.05 level, P = 1.81 × 10−6). Subset statistical tests indicated the nature of the differences in means. Without 4BA all experimental conditions (11-cis-retinal, all-trans-retinal, Vitamin A, and Vitamin A + 4BA + Washout) generate statistically comparable mean R2 charges (ANOVA, P = 0.44915). All-trans-Retinal and Vitamin A lead to approximately the same quantity of ground state visual pigment when compared with 11-cis-retinal, which can immediately form rhodopsin. With overnight incubation, cells convert all-trans-retinal or Vitamin A substrates to generate sufficient levels of cis-retinaldehyde to load equivalent numbers of opsin molecules and yield similar levels of bleachable ground state pigment when compared with direct regeneration with 11-cis-retinal. This experiment does not indicate whether retinoid metabolism or the number of opsin molecules is limiting to the levels of pigment formed, however. 4BA suppresses R2 charge motion in both all-trans-retinal (mean 76.9% decrease, t test, P = 0.038) and Vitamin A (mean 87.3% decrease, t test, P = 0.0015) versus equivalent samples without 4BA. Surprisingly, in cells loaded with 11-cis-retinal and 4BA there is a marked increase in the quantity of R2 charge (mean 326%) on the first bleach cycle than in cells loaded with 11-cis-retinal alone (t test, P = 0.006), in obvious contrast to the inhibitory effect of 4BA on pigment regeneration with the other substrates. This initial stimulatory effect occurs only on the initial bleach cycle (see below). ERC R2 waveforms of cells loaded with either all-trans-retinal or Vitamin A and 4BA are comparable to those observed with 11-cis-retinal and 4BA, except that the signals are larger in the later case. The effect of 4BA is reversible and after a 1–2-h washout, as shown for Vitamin A, complete recovery occurs. 4BA has an antagonistic effect on pigment regeneration in WT-HEK293S cells loaded with the two naturally occurring trans-retinoid substrates, but an agonistic effect with respect to the native cis-retinal substrate.
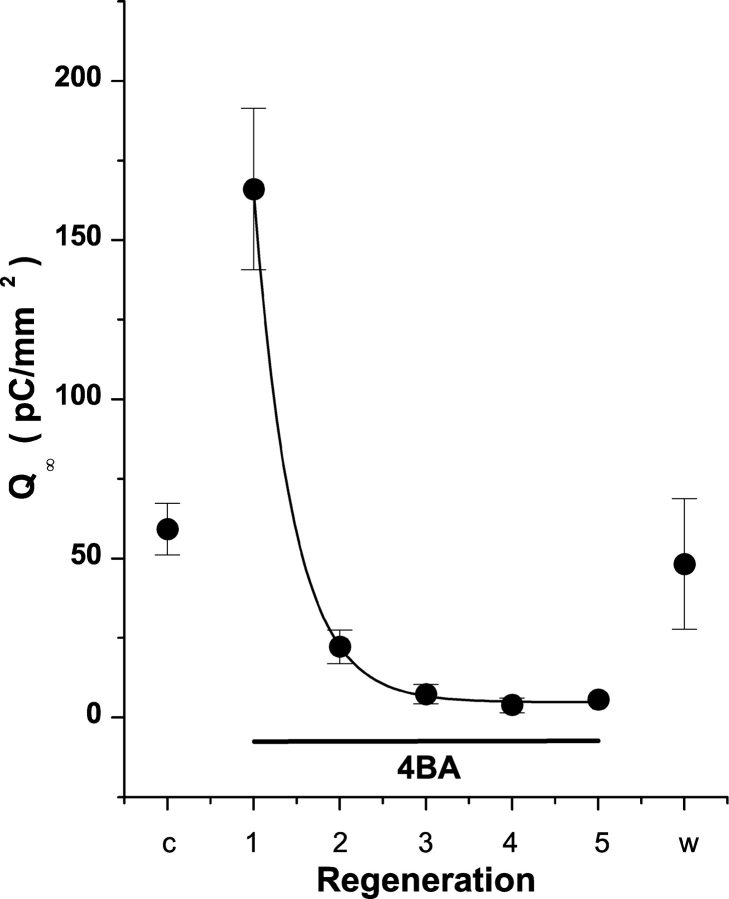
To further investigate the agonistic effect of 4BA on levels of rhodopsin regeneration in 11-cis-retinal–loaded cells we examined its impact on the quantity of R2 charge in serial bleaches. WT-HEK293S cells were regenerated with 11-cis-retinal (without 4BA) for a control bleach to measure the quantity of R2 charge or visual pigment initially present after routine 40 min regeneration (Fig. 8). After the bleach and during the next 10 min of dark adaptation, 4BA (500 μM in recording buffer plus BSA) was perfused outside giant cells. On the next R2 photolytic extinction and pigment bleaching there is a large increase in R2 charge (280.6%, t test, P = 0.0017) relative to the control extinction without 4BA. This increase is similar to observations after overnight regenerations with 11-cis-retinal in the presence of 4BA (Fig. 7) and indicates that transient 4BA exposure in WT-HEK293S giant cells preloaded with 11-cis-retinal also promotes a significant and rapid increase in the quantity of regenerated visual pigment after the first test bleach. Since 4BA does not influence the chemistry of visual pigment regeneration (see Fig. 6 D), and on this time scale could not influence the levels of opsin protein expression, the initial increase in regenerated visual pigment seen as enhanced R2 charge is an indicator of transiently increased levels of 11- or 9-cis-retinaldehyde chromophore available for prompt visual pigment formation. This implies that a fraction of available opsin apoprotein had not reacted with 11-cis-retinal before 4BA presentation, and that free cis-retinal levels in the cell are indeed limiting to visual pigment formation. During the subsequent bleach cycles the amount of R2 charge decreases exponentially (e-fold change/0.45 bleach cycle) to very low but nonzero mean levels (≈5% of control) by the third bleach/recovery cycle and remain essentially constant over the next three bleach cycles (Fig. 8). This later antagonistic response to transient 4BA exposure in 11-cis-retinal–loaded WT-HEK293S giant cells is similar to the effects of overnight 4BA exposure in Vitamin A and all-trans-retinal–regenerated cells. There are little stored reserves of cis-retinal that can continue to maintain pigment regeneration in 4BA and continuous retinoid metabolism is needed. Likewise, the subsequent washout of 4BA with no subsequent 11-cis-retinal exposure allows visual pigment and R2 charge motion to regenerate to levels comparable to control levels before 4BA infusion. 4BA neither traps retinoid precursors nor irreversibly inactivates participating retinoid processing enzymes.
Figure 8.
4BA inhibits dark adaptation in 11-cis-retinal–regenerated WT-HEK293S cells. Giant cells were regenerated with 50 μM 11-cis-retinal and cell surface area–normalized R2 charge motion obtained for the first bleach cycle (Control, c). 4BA complexed to FAF-BSA was then perfused through the chamber and the amount of R2 charge motion obtained in successive bleach cycles determined (1Æ5). Recovery time between bleach cycles was 10 min in all cases and flash stimulation was 500 nm. After the fifth bleach cycle, 4BA was washed out of the chamber and the recovery of R2 charge determined (washout, w). Control and washout conditions were not statistically different. The decay of R2 charge motion in 4BA (0.5 mM) was reliably fit with a single exponential model with a decay constant of 0.45 ± 0.01 cycles (P < 0.05). Data are presented as mean ± SE (n = 7 cells).
BSA Is Unlikely to Serve as Retinoid Binding Protein During Cellular Retinoid Conversions
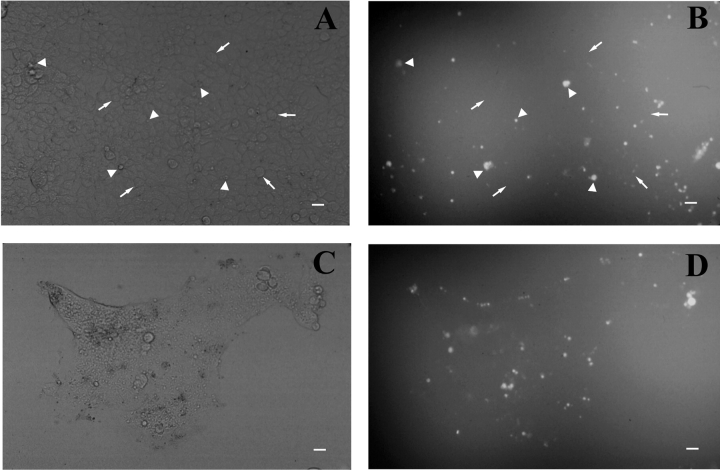
BSA supports retinoid conversion reactions in RPE cell membranous extracts in vitro as an enzymatic cofactor and can functionally replace cellular retinol binding protein (CRBP) or cellular retinaldehyde binding protein (CRALBP) in in vitro reactions (Das and Gouras, 1988; Das et al., 1990; Noy and Xu, 1990). It was important to determine if BSA was transferred into HEK293S cells during initial chromophore loading (Malaba et al., 1995). Single (unfused) (Fig. 9, A and B) and giant (fused) (Fig. 9, C and D) WT-HEK293S cells on either poly-l-lysine coated coverslips or polystyrene dishes were exposed to regeneration buffer containing 1.9% (wt/vol) BSA and 0.1% FITC-BSA and showed essentially no FITC-BSA uptake. Scattered foci of fluorescent staining in the unfused cell preps appeared to be swollen cells, cell fragments, or debris, whereas intact cells demonstrated little, if any, FITC-BSA uptake. Similarly, the cytoplasm of fused giant WT-HEK293S cells demonstrates no apparent staining, whereas some associated membranous blebs or debris produced fluorescent foci. There was little, if any, observed uptake of FITC-BSA into giant cells when membrane permeability was uncompromised. Single and fused HEK293S cells (without opsin) behave in a similar manner. No fluorescence is seen in single or giant cells exposed to regeneration buffer without FITC-BSA. Hence, healthy viable cells do not appear to be capable of active pinocytotic uptake of FITC-BSA. These findings make it unlikely that large amounts of BSA serve as a retinoid binding protein inside the WT-HEK293S giant cell cytoplasm after initial retinoid loading. We cannot exclude, however, small amounts of BSA uptake beyond the limits of detection of fluorescence microscopy.
Figure 9.
Lack of bulk BSA uptake into single or giant WT-HEK293S cells. Single (A and B) or giant PEG-fused (C and D) WT-HEK293S cells were exposed to regeneration buffer containing 1.9% (wt/vol) FAF-BSA plus 0.1% FITC-BSA. Hoffman contrast images of representative fields of single (A) or PEG-fused (C) WT-HEK293S cells are shown beside fluorescence images from the same respective fields (B and D). Arrows in A and B indicate healthy single cells and arrowheads indicate unhealthy single cells and single cells or debris taking up FITC-BSA. Scale marker is 20 μm in all fields and approximates the size of a single unfused HEK293S cell.
DISCUSSION
Nature of the Visual Pigments and Retinoids Formed in WT-HEK293S Cells
In the current work we observed that the three naturally occurring cis-retinaldehydes (11-cis, 9-cis, 13-cis) promote bimodal (R1 + R2) ERC signals after the initial regeneration, unlike all-trans-retinal that yields a pure negative signal. The positive or outward-directed R2 current is therefore an indicator of visual pigment formation with a cis-retinaldehyde. The R2 signal has an established relationship to rhodopsin bleaching. It reflects the conformation-dependent charge motions that associate with the millisecond-order formation of the biochemically active Meta-II or R* structural states that bind and activate transducin (Cone, 1967; Ebrey, 1968; Spalink and Stieve, 1980; Shukla and Sullivan, 1999 ; Sullivan and Shukla, 1999; unpublished data). The R2 waveform is the same regardless of whether the rhodopsin or isorhodopsin ground states are photolysed to form Meta-II because each bleaches through common intermediates starting from the first and sole photochemically formed state, bathorhodopsin (Kliger et al., 1984). That is, measurement of R2 waveforms per se does not distinguish between 11-cis- or 9-cis-retinal–regenerated pigments.
WT-HEK293S giant cells regenerated with 50 μM 11-cis-retinal at room temperature and kept overnight at 4°C have bimodal ERCs the following day, indicating stability of cellular rhodopsin pigment. If the ground state 11-cis-retinylidene chromophore had spontaneously isomerized to all-trans-retinal, then we would expect only inward R1-like charge motions after overnight loading/regeneration. The outcome observed is not surprising since rod rhodopsin is unusually stable to activation by nonphotonic sources of energy at physiological temperatures (Aho et al., 1988). The rate of spontaneous activation of rod rhodopsins in darkness is so low that each rhodopsin molecule has a physiological half-life measured in hundreds of years, far beyond its real biological lifetime in the rod (≈14 d). On the time scale of these experiments, opsin functions as an irreversible chemical trap for retinals (Yau et al., 1979; Baylor et al., 1980). Opsin apoprotein in the plasma membrane of WT-HEK293S cells covalently traps cis-retinals capable of reacting to form ground state visual pigments that are stable in darkness.
Cells regenerated with 13-cis-retinal had both R1 and R2 ERC signals after both 40 min and overnight regeneration. ERC signal amplitudes were much smaller than those in similarly sized giant cells loaded with lower concentrations of 11-cis-retinal or 9-cis-retinal. Although visible pigment formation between bovine opsin and 13-cis-retinal was not detected previously by spectrophotometry (Hubbard and Wald, 1952; Matsumoto and Yoshizawa, 1978), ERC measurements demonstrate that a 13-cis-retinal pigment forms, but the small charge motions observed indicate that the yield is low even by this very sensitive assay. 13-cis-retinal chromophore is likely not as well accommodated into the human opsin retinal binding pocket due to structural constraints that slow pigment regeneration (Hubbard and Wald, 1952). We have not examined charge motions in cells regenerated with 7-cis-retinal because this ligand does not occur in the ground state of visual pigments in nature. Small R2 signals would be anticipated from this analogue pigment, relative to rhodopsin and isorhodopsin, because 7-cis-retinal is not likely to regenerate as well as naturally occurring ligands due to steric constraints, and because the peak absorbance of a 7-cis-retinal analogue human pigment would be <450 nm and substantially offset from the stimulus bandwidth (500 ± 35 nm) used in these experiments (DeGrip et al., 1976; Matsumoto and Yoshizawa, 1978; Liu et al., 1984). We therefore take the recurrent appearance of large R2 signals after bleaching to indicate the regeneration of only those stable ground state pigments (rhodopsin, isorhodopsin) that can be achieved by naturally occurring cis-retinal chromophores (11-cis-retinal, 9-cis-retinal).
Comparable waveforms of recovered large cis-retinal–dependent R2 signals are observed during sequential bleach/recovery cycles in 11-cis- or 9-cis-retinal–loaded cells (Fig. 2) and not distinguishable by the methods applied here. The most likely explanation for recurrent ERC signal recovery after serial bleaches in 11-cis- or 9-cis-retinal–loaded cells is that 11-cis- or 9-cis-retinaldehyde was consistently present to regenerate rhodopsin or some isorhodopsin from bleached pigment during dark adaptation. Similarly, although 13-cis-retinal regenerates a distinct charge motion compared with rhodopsin or isorhodopsin after initial loading, successive bleach/recoveries lead to loss of R1 and growth of large R2 signals which are then indistinguishable from those observed in cells loaded with 11-cis- or 9-cis-retinal. The large pure R2 ERC signals observed during recurrent bleach/recovery cycles of 11-cis-retinal–, 9-cis-retinal–, or 13-cis-retinal–loaded cells suggests that the chromophore(s) regenerating R2 are synthesized by a single pathway starting from a common substrate. All-trans-retinal is the product of both rhodopsin or isorhodopsin bleaching and would also likely appear after bleaching of 13-cis-rhodopsin. Synthesis of 11-cis- or 9-cis-retinal from all-trans-retinal in bleached cells loaded with a variety of retinoid substrates would explain the observed recurrent recovery of the R2 signal.
The R1 signal that extinguishes after the first photolytic bleach in both 11-cis-retinal– and 9-cis-retinal–loaded cells is likely due to some all-trans-retinal that was present as a contaminant in the chromophore stocks or that formed during regeneration. After chromophore loading, all-trans-retinal yields a pure negative R1 signal upon visible flash photolysis of WT-HEK293 cells (Brueggemann and Sullivan, 2001). The R1 signal results from specific PSB formation between all-trans-retinal and a ligand-binding pocket in human opsin, as well as nonspecific reaction with nonopsin proteins and membrane lipids. The opsin-specific all-trans-retinal pigment underlying R1 charge motion has an action spectrum that peaks at 463 ± 3 nm. The R1 signal indicates that all-trans-retinal is available to form visible absorbing pigments. Independent appearance of inward R1 and outward R2 signals would allow assay of the kinetics of formation of all-trans-retinal and 11-cis-retinal (or 9-cis-) in studies of retinoid metabolism in these cells. That Vitamin A generates little, if any, ERC charge motion after initial regeneration indicates that protonated Schiff base formation is essential to generate both R1 and R2 signals.
WT-HEK293S cells incubated in the dark at room temperature or 4°C with all-trans-retinal for longer times (2–24 h) have, in addition to the R1 ERC signal, an R2 charge motion upon 500-nm flash photolysis in the initial bleach. R1 persistence is an indicator of continued availability of some all-trans-retinal in cells or thermal stability of pigments initially formed with this ligand. The R2 component indicates that 11- or 9-cis-retinaldehyde was synthesized during the incubation period and reacted with opsin to form ground state pigments. When WT-HEK293S cells are loaded with Vitamin A in the dark, a similar regeneration of both R1 and R2 signals occurs over time. This surprising result suggests the formation of independent pigments due to synthesis of all-trans-retinal and 11-cis or 9-cis-retinals from Vitamin A. All-trans-retinal– and Vitamin A–loaded cells then continue to generate, over recurrent bleach/recovery cycles, a source of 11-cis- or 9-cis-retinaldehyde that forms ground state visual pigment.
Initial bleaching of bimodal (R1 plus R2) ERCs followed by dark adaptation of all-trans-retinal– or Vitamin A–loaded cells ultimately results in large R2 charge motions that are not distinguishable from those resulting in 11-cis-retinal–, 9-cis-retinal–, or 13-cis-retinal–loaded cells. This suggests that the loss of the R1-like signal in both cases is due to photolysis and hydrolysis of all-trans-retinal–regenerated pigments (with opsin, other membrane proteins, or lipids). Since opsin can trap both cis- and trans-retinaldehydes, bleaching and hydrolysis is necessary to clear the opsin-binding pocket to eventually achieve a uniform visible absorbing pigment with a single ligand that promotes a large R2 charge motion.
Is the chromophore regenerating R2 on recurrent bleach/recoveries 11-cis-retinal or 9-cis-retinal? In vitro exposure of crude extracts of bleached rod outer segments or membranes to exogenous all-trans-retinal leads to preferential generation of 9-cis-retinal and isorhodopsin in the dark (Rotmans et al., 1972; Ostapenko and Furayev, 1973; Futterman and Rollins, 1973; Futterman, 1974; Futterman and Futterman, 1974; Bridges, 1977). However, the conditions used in these studies are in stark contrast to those used in the ERC experiments reported. To form 9-cis-retinal bacterial, contamination of the extracts was essential, a strict reducing environment was necessary (no oxygen), certain nucleophiles were present, and long time periods were necessary (3–20 h). After Schiff base formation with phosphatidyl-ethanolamine lipids under reducing conditions in the dark, all-trans-retinal will isomerize to 13-cis-retinal, which does not yield robust ERC signals, while 9-cis-retinal is not formed (Groenendijk et al., 1980). Finally, thermochemical isomerizations of all-trans-retinal, 11-cis-retinal, or Vitamin A palmitate yield only modest amounts of 9-cis-retinal isomer at equilibrium (Rando and Chang, 1983). Yet, Vitamin A palmitate did not support ERC signal generation in WT-HEK293S cells (Fig. 5). It is unlikely that bulk 9-cis-retinal is forming spontaneously from all-trans-retinal or oxidized Vitamin A by thermochemical processes in our experiments (Bernstein et al., 1985). Moreover, small amounts of 9-cis-retinal that might form and regenerate isorhodopsin would bleach upon photolysis to form all-trans-retinal preventing net accumulation. Although there may be some potential for enzymatic formation of 9-cis-retinal through intracellular retinoid processing machinery (Romert et al., 1998), the recurrent recovery of large uniformly sized R2 signals in WT-HEK293S cells loaded with any chromophore, other than potentially 9-cis-retinal itself, is likely due to 11-cis-retinal as the regenerating ligand.
Some giant cells permitted ERC recording for >4 h with 10–20 bleach/recovery periods (maximum 18 in 11-cis-retinal–loaded cells). Total R2 charge is consistent across serial extinctions and does not decrease (Fig. 3 D). This indicates that the quantity of rhodopsin formed and the apparent regeneration rate are uniform over many bleach/recovery cycles. In some 11-cis-retinal–loaded giant WT-HEK293S cells the (integrated) R2 charge across a single bleach series is relatively constant over serial bleach/recoveries. In others it increases slowly over serial cycles, but does not exceed a level twofold greater than initially found (Fig. 3 E). Total R2 charge is a direct measure of the amount of bleachable plasma membrane rhodopsin in a giant cell (≈ 0.186e/R*) (Makino et al., 1991; Sullivan and Shukla, 1999). The ERC R2 waveform and photosensitivity to bleaching appear constant during the slow growth of Q∞. The slow growth of R2 charge suggests that more plasma membrane rhodopsin or isorhodopsin is regenerated over time. This can result from greater availability of free cis-retinal ligand to react with constant levels of plasma membrane opsin, greater opsin levels in the membrane with a fixed cis-retinal source, or both. We did not observe significant increases in plasma membrane capacitance over the course of long experiments, which would occur if large numbers of cytoplasmic opsin-containing vesicles were fusing with the surface membrane. This finding decreases the likelihood that slow growth of R2 charge represents increased availability of opsin apoprotein molecules. Rather, the initial 4BA enhancement effect in 11-cis-retinal–loaded cells indicates that some fraction of free opsin remains available in the plasma membrane after criterion 10 min of dark adaptation. This suggests that the rate-limiting step in forming ground state pigment is the availability of free cis-retinal during dark adaptation. The increase, albeit slight, in R2 charge over successive bleach/recovery cycles likely reflects increased synthesis of 11-cis- or 9-cis-retinal, which then regenerate larger quantities of plasma membrane ground state pigments. The slow increase in R2 charge appears to require prior light exposure, which suggests that the activity of one or more components of retinoid metabolism in these cells is regulated by prior light history (unpublished data).
These data provide convincing evidence that WT-HEK293S cells have the capacity to synthesize 11-cis-retinal or 9-cis-retinal from all-trans-retinol or all-trans-retinaldehyde. The presence in WT-HEK293S cells of enzymatic retinoid processing pathways is substantiated by several observations. First, the appearance and maintenance of strong R2 ERC signals after Vitamin A or all-trans-retinal loading can only result from cis-retinaldehydes actively synthesized by cellular machinery. Second, when all-trans-retinal or Vitamin A regenerated giant WT-HEK293S cells are kept in the dark at room temperature for only 2–3 h large R2 signals appear that are comparable in size to those occurring after overnight incubation at 4°C. The influence of temperature on rate suggests the contribution of cellular enzymatic machinery to retinoid conversion reactions. Third, retinoid conversion metabolism in WT-HEK293S cells shows substrate specificity. The lack of ERC signals after cellular loading of Vitamin A palmitate decrease the likelihood that 9-cis-retinal is generated by nonenzymatic thermochemical reactions to contribute to the recurrent regeneration of visual pigment (isorhodopsin). Retinoid conversion reactions in WT-HEK293S cells manifest functionally similar outcomes during dark adaptation to retinoid processing machinery in the RPE.
Short Circuiting the Bleach/Recovery Cycle in WT-HEK293S Cells
In the mammalian eye, the synthesis of 11-cis-retinal during dark adaptation is inhibited by 4BA, a “short-circuiting” antagonist of the visual cycle in the RPE (Bernstein et al., 1986a,b). In experiments on 11-cis-retinal–loaded cells, regenerated ground state visual pigment and R2 charge decline to nearly extinguished levels by the third bleach cycle under constant 4BA exposure. There are little reserves of free cis-retinaldehydes in WT-HEK293S giant cells available to recurrently regenerate visual pigment during exposure to a visual cycle antagonist. These results suggest that cellular retinoid metabolism maintains the levels of available cis-retinaldehyde needed to achieve consistent quantities of rhodopsin regeneration and R2 charge recovery found over many bleach/dark adaptation cycles and several hours of recording in the absence of added retinoids.
Previously, we proposed a simple overloading model for dark adaptive recovery of R2 signals. We postulated that a robust source of 11-cis-retinal, preloaded and solubilized into intracellular membranes, supported recurrent partitioning to the plasma membrane and regeneration of bleached opsin (Shukla and Sullivan, 1999). After steady-state loading of hydrophobic chromophore (25 μM) throughout internal cellular membranes, one expects ∼10–50-fold greater concentration of ligand than plasma membrane opsin receptor (Shukla and Sullivan, 1999; Sullivan and Shukla, 1999). This retinaldehyde concentration does not appear to be limiting to the second-order reaction of rhodopsin pigment formation, provided that all loaded chromophore is available to participate in the reaction. However, the initial 4BA enhancement effect indicates that the amount of free membrane-soluble 11-cis-retinal available to regenerate ground state visual pigment in giant WT-HEK293S cells is quite limited. The amount of rhodopsin formed at steady-state appears to be only ∼30–40% of the total amount of opsin apoprotein present. If a large fraction of loaded cis-retinal chromophore were freely soluble or readily accessible, then one would expect much higher levels of saturation of opsin molecules with ligands. Our prior model of a large store of chromophore immediately available to regenerate bleached pigment is inconsistent with the results. Partial pigment formation does not appear to result from limitations imposed by the rate of chemical reaction of cis-retinal with bleached opsin, because this process is rapid relative to the time scale used for loading or dark adaptation (Shukla and Sullivan, 1999; unpublished data). In the absence of 4BA the substoichiometric regeneration of rhodopsin in cells initially loaded with 11-cis-retinal suggests that cellular reactions somehow act to limit the quantity of initial and recurrent visual pigment formation. WT-HEK293S cells must rapidly convert 11-cis-retinal into a form where it is not freely accessible for pigment regeneration during the initial loading process. To refine our previous model, we now propose that the bulk of the loaded chromophore in WT-HEK293S cells is likely to be deposited in esterified storage forms (retinyl-esters) by an LRAT-like enzyme that lacks stereospecificity for the isomeric state (Fulton and Rando, 1987; Ruiz et al., 1999). LRAT-like enzyme activity is found in diverse cultured cell lines (Guo et al., 2000), including HEK293 cells (Ruiz et al., 1999). We further hypothesize that this storage element is later used and replenished during recurrent bleach/recovery and dark adaptation. Initially, some retinal will also react with plasma membrane opsin to form visual pigments that yield the ERC signals observed. Also, some retinal will be directly solubilized in membranes where it would tend to react rapidly with available primary amines (lysines in proteins or phosphatidyl-ethanolamine lipids). The later pathway is unproductive for recurrent regeneration, since Schiff base adducts of 11-cis-retinal with phosphatidyl-ethanolamine in synthetic membranes undergo rapid (tens of minutes) downhill thermal isomerization in the dark at physiological temperatures to form predominantly all-trans-retinal and some 13-cis-retinal, even under reducing conditions (Groenendijk et al., 1980). These chromophores do not yield initially robust R2 signals.
To maintain recurrent bleach/recovery cycles in WT-HEK293S cells, intracellular synthesis of cis-retinal must occur to replenish depleted stores after photolytic bleaching of rhodopsin or isorhodopsin and hydrolysis of all-trans-retinal. Otherwise, as evidenced by the 4BA effect in 11-cis-retinal–loaded cells, the ability to regenerate visual pigment and strong R2 signals after serial bleaches would run down and extinguish. The marked suppression of 4BA on resultant R2 and R1 charge motion after loading all-trans-retinal (77% decrease of R2) and Vitamin A (87% decrease of R2) suggests that this agent inhibits enzymes essential for generation of cis-retinaldehydes in giant WT-HEK293S cells. Bypass of the preexisting 4BA block (with Vitamin A) by 11-cis-retinal (Fig. 6 D) indicates that 4BA does not chemically trap exogenously added 11-cis-retinal into Schiff base adducts that do not form visual pigment, that it does not promote downhill isomerization of the 11-cis-retinal chromophore, and that it does not impair the ability of opsin to regenerate with chromophore in this system (see Bernstein et al., 1986a,b). Plasma membrane rhodopsin, once formed, is stable in these cells at least over a 16-h incubation period and chromophore exchange with other retinoids does not appear to occur. The large enhancement (>300%) of the R2 signal found only on the primary bleach in 11-cis-retinal/4BA–loaded WT-HEK293S cells is a direct indicator that more, rather than less rhodopsin regenerated in the presence of 4BA in comparison to controls. To form more rhodopsin with 4BA than without, more 11-cis-retinal had to be initially available during the first long pigment regeneration to drive the second order recombination reaction with opsin toward completion. The implication is that a substantial fraction of the opsin in the giant cell plasma membrane does not form visual pigment at the concentration of 11-cis-retinal commonly used (25 μM) during normal loading and regeneration without 4BA. After the initial bleach cycle in 11-cis-retinal/4BA–loaded cells, total recovered R2 charge declines exponentially (e-fold/0.45 bleach cycles) over subsequent bleach/regeneration cycles to achieve steady-state 4BA suppression levels (95%) lower than those observed in all-trans-retinal– (77%) and Vitamin A– (87%) loaded cells. This is additional evidence that the supply of 11-cis-retinal available to regenerate pigment during the criterion dark adaptation time (10 min) is indeed limited in giant WT-HEK293S cells. Over 60% (1- e−1) of the free or available cis-retinal pool is depleted after the first bleach and dark adaptation in 4BA. Only 4–5 bleach cycles are needed to nearly extinguish the entire (>95%) cis-retinal pool available for pigment regeneration and recoverable R2 charge in 4BA. Yet, WT-HEK293S giant cells can sustain at least 10–20 bleach/recovery cycles in the absence of this agent. This suggests that, without 4BA, the amount of retinoid turnover between bleach/recovery cycles must be small. The rapid recovery (10 min) of R2 signal after 4BA washout in Vitamin A or 11-cis-retinal–loaded cells suggests that 4BA inhibition is reversible and specific, as opposed to nonspecific effects of this agent on WT-HEK293S giant cell metabolism or physiology. As evidenced by similar mean R2 charge values during antagonism, 4BA blocks synthesis of cis-retinals and formation of visual pigment regardless of the initial retinoid substrates loaded in these experiments. 11-cis-retinal, all-trans-retinal and Vitamin A vary substantially in both isomeric state of the polyene as well as the reduction state of the reactive moiety of the molecule (aldehyde or alcohol). These substrates require different levels of processing to yield cis-retinals capable of ground state rhodopsin regeneration during the initial loading. It is unlikely that 4BA impacts retinoid conversion steps that treat these diverse retinoid substrates differentially, given the similar levels of regenerated R2 charge found without and with 4BA. Rather, these results suggest that 4BA antagonizes a common enzymatic step in retinoid processing machinery that is used during the conversion of all of the different substrates.
These results strongly suggest that 4BA reversibly blocks critical retinoid conversion step(s) in WT-HEK293S cells when presented with diverse retinoid substrates. Assuming a retinoid processing machinery in HEK293S cells similar to that in the photoreceptors/RPE, we can propose a hypothesis for the mechanism of 4BA inhibition of rhodopsin regeneration during dark adaptation. The 11-cis-retinal initially loaded into cells would complex with intracellular binding proteins before reduction to 11-cis-retinol by a ubiquitously expressed cisRDH. 11-cis-retinol is readily esterified with microsomal membrane lipids by an LRAT-like activity known to be present in these cells (see below) (Ruiz et al., 1999). 11-cis-retinyl esters could be used to generate new 11-cis-retinaldehyde during recurrent bleach/recovery cycles through ubiquitous esterase activity. We propose that the initial enhancement by 4BA of R2 signals in 11-cis-retinal–loaded cells suggests that 4BA is an antagonist of LRAT (Fulton and Rando, 1987). With less 11-cis-retinal being shuttled into ester storage forms in the presence of 4BA, more would be initially available to regenerate greater amounts of pigment and R2 charge. As LRAT esterifies all-trans-retinol into trans-retinyl esters that serve as substrate for an isomerase, the suppressive effect of 4BA on R2 in Vitamin A or all-trans-retinal–loaded cells can also be explained by LRAT inhibition. 4BA inhibition of LRAT would block formation of all-trans-retinyl esters used by the isomerase and would thus short-circuit initial and recurrent cis-retinal formation. The lower mean level of 4BA inhibition on R2 charge of all-trans-retinal– (77%) versus Vitamin A– (87%) loaded cells may reflect an additional enzymatic step needed to reduce all-trans-retinal to all-trans-retinol before esterification. An alternative model for 4BA action is that it inhibits the isomerase complex. If the marked 4BA-induced steady-state suppression of R2 charge after diverse substrate loading (11-cis-retinal, Vitamin A, all-trans-retinal) were due to isomerase inhibition, then the enzyme would need to be capable of reversible substrate flow. Recent studies suggest that isomerase product formation is reversible, driven by mass action and influenced by retinoid binding proteins that trap products (Winston and Rando, 1998; Stecher et al., 1999; McBee et al., 2000). The mechanism of inhibition of dark adaptation by 4BA or other retinoid cycle antagonists in WT-HEK293S cells is a problem for future study.
The Nature of Enzymatic Retinoid Processing Machinery in WT-HEK293S Cells
Could the adenoviral (E1A, E1B genes) -transformed human embryonic kidney HEK293S line (Graham et al., 1977) embody a retinoid processing machinery that is functionally homologous to that in photoreceptors and RPE (Fig. 1)? Oxidation/reduction reactions between the retinoid alcohol and aldehyde states are necessary. Ester formation with the alcohol (e.g., Vitamin A) is the common form of retinoid storage in diverse cell types, so acyl transferases appear necessary as well as ester hydrolases to recover stored retinols. An isomerase is essential to accomplish the conversion of all-trans to 11-cis chromophore states. Finally, intracellular retinoid binding proteins are needed to traffic hydrophobic retinoids between different enzyme activities. Normal kidney cells process Vitamin A and carry out diverse chemical conversions of a metabolic, endocrine, and xenobiotic nature, including alcohol/aldehyde oxidation/reduction reactions as well as ester transfer and hydrolase reactions (Lohr et al., 1998; Brenner, 2000). Given our physiological results and reports in the literature, many, but not all, of the enzymes and proteins known to be essential for the visual retinoid cycle, or functional homologues, are either known to be expressed in HEK293 cells (LRAT, RPE65, cRDH, isomerase) or likely to be expressed (CRBP-I, tRDH) (Table I). Molecular and biochemical experiments should be designed to test for these and other proteins (e.g., CRALBP) in HEK293 cells. The adenoviral oncogenic transformation of HEK293 cells is associated with induction of the human RPE65 gene, which is not expressed in normal human kidney cells (Ma et al., 1999). Could other components of a coordinated set of retinoid processing genes also be induced in HEK293 cells? Review of the literature found reports of induced expression of CRBP and cellular retinoic acid binding proteins in human tumor cells that were not expressed in cells from normal human tissues (Ong et al., 1975; Saari et al., 1978). Retinoid metabolism may be common to many cell types, reflecting the role of 9-cis-retinoids in development and differentiation (Romert et al., 2000).
TABLE I.
Visual Cycle Proteins in the Mammalian Eye and Kidney
| Photoreceptor/RPE | HEK293/kidney |
|---|---|
| IRBPa | BSAb |
| tRDHc | tRDH-liked |
| CRBP-Ie | CRBP-If |
| LRATg | LRAT-likeh |
| IHi | IH-likej |
| RPE65k | RPE65l |
| 11cRDHm | 11cRDHn |
| CRALBPo | ? |
Homologous functions expressed in kidney or transformed human embryonic kidney (HEK293) cells are indicated. In the case of BSA, this agent is known to replace or support the function of IRBP in delivering retinoids into the RPE.
Das et al., 1990; McDowell, 1993; Shukla and Sullivan, 1999; Sullivan and Shukla, 1999; Adler and Edwards, 2000.
Fulton and Rando, 1987; Barry et al., 1989; Ruiz et al., 1999.
Ruiz et al., 1999; Guo et al., 2000.
Bernstein et al., 1987; Cañada et al., 1990; Rando, 1991, 1992; Winston and Rando, 1998.
Shukla and Sullivan, 1999; results from this study.
Ma et al., 1999.
Gamble et al., 1999; Wang et al., 1999; Jang et al., 2000; Romert et al., 2000.
Potential Utility of the Opsin-HEK293S Expression System
The HEK293S system is an emerging resource for biophysical and structure/function studies of visual pigment activation in a live cell environment. It has significant potential for investigation of the impact of human rod opsin mutations on pigment regeneration and dark adaptation (e.g., rod versus cone). Once the molecular biology and biochemistry of retinoid isomerization reactions are developed in this simple cell culture model system, heterologous expression of human cDNAs for the visual cycle could be used to investigate their ability to enhance visual pigment recovery during dark adaptation. This system may be useful to investigate remaining challenges of the visual retinoid cycle, such as protein–protein interactions and its regulation. Human hereditary retinal degenerations associate with mutations in visual cycle genes (RDH5, RPE65, CRALBP, LRAT) (Gu et al., 1997; Marlhens et al., 1997; Maw et al., 1997; Morimura et al., 1998; Cideciyan et al., 2000; Hirose et al., 2000; Nakamura et al., 2000; Thompson et al., 2001). The intrinsic retinoid processing machinery in HEK293S cells might be useful to investigate the impact of expressed mutant gene products on the physiological and biochemical processes of the chromophore visual cycle pathway. Finally, if a single cis-retinal (11- or 9-) is synthesized by HEK293 cells, then there is distinct potential for structural biology studies (Okada et al., 2000; Palczewski et al., 2000) of expressed WT and mutant pigments crystallized from bioreactor level cell populations that are regenerated with a single inexpensive precursor, Vitamin A.
Acknowledgments
We thank Dr. Jeremy Nathans (Johns Hopkins School of Medicine, Baltimore, MD) for providing the WT-HEK293S cell line, Dr. Bruce Stillman (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY) for providing the HEK293S cell line, Dr. Rosalie K. Crouch (Medical University of South Carolina, Charleston, SC) and NEI for providing 11-cis-retinal, Dr. Mary E. Pierce (SUNY Upstate Medical University) for allowing access to a fluorescence microscope, and John Misasi for assistance with Photoshop®. We appreciate the critical read of the manuscript prior to submission by Dr. Kenneth W. Foster (Syracuse University). We also appreciate the critiques by the reviewers that helped to shape a refined presentation of our work.
This work was funded entirely by the National Eye Institute (EY 11384 to J.M. Sullivan).
Footnotes
Abbreviations used in this paper: CRALBP, cellular retinaldehyde binding protein; CRBP, cellular retinol binding protein; 11cRDH, 11-cis-retinol dehydrogenase; ERC, early receptor current; FAF, fatty acid–free; 4BA, 4-butyl-aniline; HEK293S, human embryonic kidney 293 culture line, suspension adapted; IH, isomerohydrolase; IRBP, interstitial retinoid binding protein; LRAT, lecithin retinyl acetyl transferase; Meta-II, metarhodopsin-II; PSB, protonated Schiff base; tRDH, all-trans-retinol dehydrogenase; RPE, retinal pigment epithelium; WT, wild-type.
References
- Adler, A.J., and R.B. Edwards. 2000. Human interphotoreceptor matrix contains serum albumin and retinol-binding protein. Exp. Eye Res. 70:227–234. [DOI] [PubMed] [Google Scholar]
- Aho, A.-C., K. Donner, C. Hydén, L.O. Larsen, and T. Reuter. 1988. Low retinal noise in animals with low body temperature allows high visual sensitivity. Nature. 334:348–350. [DOI] [PubMed] [Google Scholar]
- Barry, R.J., F.J. Cañada, and R.R. Rando. 1989. Solubilization and partial purification of retinyl ester synthetase and retinoid isomerase from bovine ocular pigment epithelium. J. Biol. Chem. 264:9231–9238. [PubMed] [Google Scholar]
- Baylor, D.A., G. Matthews, and K.-W. Yau. 1980. Two components of electrical dark noise in toad retinal rod outer segments. J. Physiol. 309:591–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein, P.S., B.S. Fulton, and R.R. Rando. 1986. a. Mechanism of action of aromatic amines that short-circuit the visual cycle. Biochemistry. 25:3370–3377. [DOI] [PubMed] [Google Scholar]
- Bernstein, P.S., W.C. Law, and R.R. Rando. 1987. Biochemical characterization of the retinoid isomerase system of the eye. J. Biol. Chem. 262:16848–16857. [PubMed] [Google Scholar]
- Bernstein, P.S., J.R. Lichtman, and R.R. Rando. 1985. Nonstereospecific synthesis of 11-cis-retinal in the eye. Biochemistry. 24:487–492. [DOI] [PubMed] [Google Scholar]
- Bernstein, P.S., J.R. Lichtman, and R.R. Rando. 1986. b. Short-circuiting the visual cycle with retinotoxic aromatic amines. Proc. Natl. Acad. Sci. USA. 83:1632–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner, B.M. 2000. The Kidney. 6th ed. W.B. Saunders, Philadelphia, PA. 2653 pp.
- Bridges, C.D. 1977. Rhodopsin regeneration in rod outer segments: utilization of 11-cis-retinal and retinol. Exp. Eye Res. 24:571–580. [DOI] [PubMed] [Google Scholar]
- Brueggemann, L., and J.M. Sullivan. 2001. all-trans-Retinal forms a visible-absorbing pigment with human rod opsin. Biochemistry. 40:4446–4453. [DOI] [PubMed] [Google Scholar]
- Brueggemann, L., and J.M. Sullivan. 2000. HEK293S cells have functional retinoid processing machinery. Invest. Ophthalmol. Vis. Sci. 41:S616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañada, F.J., W.C. Law, and R.R. Rando. 1990. Substrate specificities and mechanism in the enzymatic processing of Vitamin A into 11-cis-retinol. Biochemistry. 29:9690–9697. [DOI] [PubMed] [Google Scholar]
- Cideciyan, A.V., F. Haeseleer, R.N. Fariss, T.S. Aleman, G.F. Jang, C.L.M.J. Verlinde, M.F. Marmor, S.G. Jacobson, and K. Palczewski. 2000. Rod and cone visual cycle consequences of a null mutation in the 11-cis-retinol dehydrogenase gene in man. Vis. Neurosci. 17:667–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun, D., and F.J. Sigworth. 1983. Fitting and statistical analysis of single-channel records. Single Channel Recording. B. Sakmann and E. Neher, editors. Plenum Press, New York, N.Y. 191–263.
- Cone, R.A. 1967. Early receptor potential: photoreversible charge displacement in rhodopsin. Science. 155:1128–1131. [DOI] [PubMed] [Google Scholar]
- Crabb, J.W., Z. Nie, Y. Chen, J.D. Hulmes, K.A. West, J.T. Kapron, S.E. Ruuska, N. Noy, and J.C. Saari. 1998. Cellular retinaldehyde-binding protein ligand interactions. Gln-210 and Lys-221 are in the retinoid binding pocket. J. Biol. Chem. 273:20712–20720. [DOI] [PubMed] [Google Scholar]
- Crescitelli, F. 1985. Some properties of solubilized human rhodopsin. Exp. Eye Res. 40:521–535. [DOI] [PubMed] [Google Scholar]
- Das, S.R., N. Bhardwaj, and P. Gouras. 1990. Synthesis of retinoids by human retinal epithelium and transfer to outer segments. Biochem. J. 268:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das, S.R., and P. Gouras. 1988. Retinoid metabolism in cultured human retinal pigment epithelium. Biochem. J. 250:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrip, W.J., R.S.H. Liu, V. Ramamurthy, and A. Asato. 1976. Rhodopsin analogues from highly hindered 7-cis isomers of retinal. Nature. 262:416–418. [DOI] [PubMed] [Google Scholar]
- Ebrey, T.G. 1968. The thermal decay of the intermediates of rhodopsin in situ. Vision Res. 8:965–982. [DOI] [PubMed] [Google Scholar]
- Fulton, B.S., and R.R. Rando. 1987. Biosynthesis of 11-cis-retinoids and retinyl esters by bovine pigment epithelium membranes. Biochemistry. 26:7938–7945. [DOI] [PubMed] [Google Scholar]
- Futterman, S. 1974. Recent studies on a possible mechanism for visual pigment regeneration. Exp. Eye Res. 18:89–96. [DOI] [PubMed] [Google Scholar]
- Futterman, A., and S. Futterman. 1974. The stability of 11-cis-retinal and reactivity toward nucleophiles. Biochim. Biophys. Acta. 337:390–394. 4832626 [Google Scholar]
- Futterman, S., and M.H. Rollins. 1973. The catalytic isomerization of all-trans-retinal to 9-cis-retinal and 13-cis-retinal. J. Biol. Chem. 248:7773–7779. [PubMed] [Google Scholar]
- Gamble, M.V., E. Shang, R. Piantedosi-Zott, J.R. Mertz, D.J. Wolgemuth, and W.S. Blaner. 1999. Biochemical properties, tissue expression, and gene structure of a short chain dehydrogenase/reductase able to catalyze cis-retinol oxidation. J. Lipid Res. 40:2279–2292. [PubMed] [Google Scholar]
- Graham, F.L., J. Smiley, W.C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59–74. [DOI] [PubMed] [Google Scholar]
- Groenendijk, G.W.T., W.M. Jacobs, S.L. Bonting, and F.J.M. Daemen. 1980. Dark isomerization of retinals in the presence of phosphatidylethanolamine. Eur. J. Biochem. 106:119–128. [DOI] [PubMed] [Google Scholar]
- Gu, S.-M., D.A. Thompson, C.R. Srisailapathy Srikumari, B. Lorenz, U. Finckh, A. Nicoletti, K.R. Murthy, M. Rathmann, G. Kumaramanickavel, M.J. Denton, and A. Gal. 1997. Mutations in RPE65 cause autosomal recessive childhood onset severe retinal dystrophy. Nat. Genet. 17:194–197. [DOI] [PubMed] [Google Scholar]
- Guo, X., A. Ruiz, R.R. Rando, D. Bok, and L.J. Gudas. 2000. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin:retinol acyltransferase in carcinoma lines. Carcinogenesis. 21:1925–1933. [DOI] [PubMed] [Google Scholar]
- Haeseleer, R., J. Huang, L. Lebioda, J.C. Saari, and K. Palczewski. 1998. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J. Biol. Chem. 273:21790–21799. [DOI] [PubMed] [Google Scholar]
- Hagins, W.A. 1955. The quantum efficiency of bleaching of rhodopsin in situ. J. Physiol. 129:22P. [PubMed] [Google Scholar]
- Herr, F.M., E. Li, R.B. Weinberg, V.R. Cook, and J. Storch. 1999. Differential mechanisms of retinoid transfer from cellular retinol binding proteins types I and II to phospholipid membranes. J. Biol. Chem. 274:9556–9563. [DOI] [PubMed] [Google Scholar]
- Hirose, E., Y. Inoue, H. Morimura, N. Okamoto, M. Fukada, S. Yamamoto, T. Fujikado, and Y. Tano. 2000. Mutations in the 11-cis retinol dehydrogenase gene in Japanese patients with fundus albipunctatus. Invest. Ophthalmol. Vis. Sci. 41:3933–3935. [PubMed] [Google Scholar]
- Hubbard, R., and G. Wald. 1952. Cis-trans isomers of vitamin A and retinene in the rhodopsin system. J. Gen. Physiol. 36:269–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, G.-F., J.K. McBee, A.M. Alekseev, F. Haeseleer, and K. Palczewski. 2000. Stereoisomeric specificity of the retinoid cycle in the vertebrate retina. J. Biol. Chem. 275:28128–28138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliger, D.S., J.S. Horwitz, J.W. Lewis, and C.M. Einterz. 1984. Evidence for a common BATHO-intermediate in the bleaching of rhodopsin and isorhodopsin. Vision Res. 24:1465–1470. [DOI] [PubMed] [Google Scholar]
- Liu, R.S.H., H. Matsumoto, A. Kini, A.E. Asato, M. Denny, A. Kropf, and W.J. DeGrip. 1984. Seven new hindered isomeric rhodopsins. A reexamination of the stereospecificity of the binding site of bovine opsin. Tetrahedron. 40:473–482. [Google Scholar]
- Lohr, J.W., G.R. Willsky, and M.A. Acara. 1998. Renal drug metabolism. Pharmacol. Rev. 50:107–141. [PubMed] [Google Scholar]
- Ma, J.X., D.C. Zhang, M. Laser, N.A. Brownlee, G.G. Re, D.J. Hazen-Martin, T.M. Redmond, and R.K. Crouch. 1999. Identification of RPE65 in transformed kidney cells. FEBS Lett. 452:199–204. [DOI] [PubMed] [Google Scholar]
- Makino, C.L., W.R. Taylor, and D.A. Baylor. 1991. Rapid charge movements and photosensitivity of visual pigments in salamander rods and cones. J. Physiol. 442:761–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaba, L., S. Smeland, H. Senoo, K.R. Norum, T. Berg, R. Blomhoff, and G.M. Kindberg. 1995. Retinol-binding protein and asialo-orosomucoid are taken up by different pathways in liver cells. J. Biol. Chem. 270:15685–15692. [DOI] [PubMed] [Google Scholar]
- Marlhens, F., C. Bareil, J.M. Griffoin, E. Zrenner, P. Amalric, C. Eliaou, S.Y. Liu, E. Harris, T.M. Redmond, B. Arnaud, et al. 1997. Mutations in RPE65 cause Leber's congenital amaurosis. Nat. Genet. 17:139–141. [DOI] [PubMed] [Google Scholar]
- Matsumoto, H., and T. Yoshizawa. 1978. Recognition of opsin to the longitudinal length of retinal isomers in the formation of rhodopsin. Vision Res. 18:607–609. [DOI] [PubMed] [Google Scholar]
- Maw, M.A., B. Kennedy, A. Knight, R. Bridges, K.E. Roth, E.J. Mani, J.K. Mukkadan, D. Nancarrow, J.W. Crabb, and M.J. Denton. 1997. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat. Genet. 17:198–200. [DOI] [PubMed] [Google Scholar]
- McBee, J.K., V. Kuksa, R. Alvarez, A.R. de Lera, O. Prezhdo, F. Haeseleer, I. Sokal, and K. Palczewski. 2000. Isomerization of all-trans-retinol to cis-retinols in bovine retinal pigment epithelial cells: dependence on the specificity of retinoid-binding proteins. Biochemistry. 39:11370–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell, J.H. 1993. Preparing rod outer segment membranes, regenerating rhodopsin, and determining rhodopsin concentration. Methods Neurosci. 15:123–130. [Google Scholar]
- Morimura, H., G.A. Fishman, S.A. Grover, A.B. Fulton, E.L. Berson, and T.P. Dryja. 1998. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc. Natl. Acad. Sci. USA. 95:3088–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura, M., Y. Hotta, A. Tanikawa, H. Terasaki, and Y. Miyake. 2000. A high association with cone dystrophy in fundus albipuncatatus caused by mutations of the RDH5 gene. Invest. Ophthalmol. Vis. Sci. 41:3925–3932. [PubMed] [Google Scholar]
- Nathans, J., C.J. Weitz, N. Agarwal, I. Nir, and D.S. Papermaster. 1989. Production of bovine rhodopsin by mammalian cell lines expressing cloned cDNA: spectrophotometry and subcellular localization. Vision Res. 29:907–914. [DOI] [PubMed] [Google Scholar]
- Noy, N., and Z.-J. Xu. 1990. Thermodynamic parameters of the binding of retinol to binding proteins and to membranes. Biochemistry. 29:3888–3892. [DOI] [PubMed] [Google Scholar]
- Okada, T., I. LeTrong, B.A. Fox, C.A. Behnke, R.E. Stenkamp, and K. Palczewski. 2000. X-Ray diffraction analysis of three-dimensional crystals of bovine rhodopsin obtained from mixed micelles. J. Struct. Biol. 130:73–80. [DOI] [PubMed] [Google Scholar]
- Okajima, T.I., D.R. Pepperberg, H. Ripps, B. Wiggert, and G.J. Chader. 1989. Interphotoreceptor retinoid-binding protein: role in delivery of retinal to the pigment epithelium. Exp. Eye Res. 49:629–644. [DOI] [PubMed] [Google Scholar]
- Ong, D.E., D.L. Page, and F. Chytil. 1975. Retinoic acid binding protein: occurrence in human tumors. Science. 190:60–61. [DOI] [PubMed] [Google Scholar]
- Ostapenko, I.A., and V.V. Furayev. 1973. 9-cis isomerization of all-trans retinal during in vitro regeneration of visual pigment. Nat. New Biol. 243:185–186. [DOI] [PubMed] [Google Scholar]
- Palczewski, K., T. Kumasaka, T. Hori, C.A. Behnke, H. Motoshima, B.A. Fox, I. LeTrong, D.C. Teller, T. Okada, R.E. Stenkamp, et al. 2000. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 289:739–745. [DOI] [PubMed] [Google Scholar]
- Parkes, J.H., and P.A. Liebman. 1984. Temperature and pH dependence of the Metarhodopsin I - Metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 23:5054–5061. [DOI] [PubMed] [Google Scholar]
- Rando, R.R. 1991. Membrane phospholipids as the energy source in the operation of the visual cycle. Biochemistry. 30:595–602. [DOI] [PubMed] [Google Scholar]
- Rando, R.R. 1992. Molecular mechanisms in visual pigment regeneration. Photochem. Photobiol. 56:1145–1156. [DOI] [PubMed] [Google Scholar]
- Rando, R.R., and A. Chang. 1983. Studies on the catalyzed interconversion of Vitamin A derivatives. J. Am. Chem. Soc. 105:2879–2882. [Google Scholar]
- Redmond, T.M., S. Yu, E. Lee, D. Bok, D. Hamasaki, N. Chen, P. Goletz, J.-X. Ma, R.K. Crouch, and K. Pfeifer. 1998. Rpe65 is necessary for the production of 11-cis-vitamin A in the retinal visual cycle. Nat. Genet. 20:344-351. [DOI] [PubMed] [Google Scholar]
- Romert, A., P. Tuvendal, A. Simon, L. Dencker, and U. Eriksson. 1998. The identification of a 9-cis-retinol dehydrogenase in the mouse embryo reveals a pathway for synthesis of 9-cis-retinoic acid. Proc. Natl. Acad. Sci. USA. 95:4404–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romert, A., P. Tuvendal, K. Tryggvason, L. Dencker, and U. Eriksson. 2000. Gene structure, expression analysis, and membrane topology of RDH4. Exp. Cell Res. 256:338–345. [DOI] [PubMed] [Google Scholar]
- Rotmans, J.P., F.J.M. Daemen, and S.L. Bonting. 1972. Biochemical aspects of the visual process. XIX. Formation of isorhodopsin from photolyzed rhodopsin by bacterial action. Biochim. Biophys. Acta. 267:583–587. [DOI] [PubMed] [Google Scholar]
- Ruiz, A., A. Winston, Y.-H. Lim, B.A. Gilbert, R.R. Rando, and D. Bok. 1999. Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 274:3834–3841. [DOI] [PubMed] [Google Scholar]
- Saari, J.C., and D.L. Bredberg. 1982. Enzymatic reduction of 11-cis-retinal bound to cellular retinal-binding protein. Biochim. Biophys. Acta. 716:266–272. [DOI] [PubMed] [Google Scholar]
- Saari, J.C., L. Bredberg, and G.G. Garwin. 1982. Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J. Biol. Chem. 257:13329–13333. [PubMed] [Google Scholar]
- Saari, J.C., D.L. Bredberg, and N. Noy. 1994. Control of substrate flow at a branch in the visual cycle. Biochemistry. 33:3106–3112. [DOI] [PubMed] [Google Scholar]
- Saari, J.C., S. Futterman, G.W. Stubbs, J.T. Heffernan, L. Bredberg, K.Y. Chan, and D.M. Albert. 1978. Cellular retinol- and retinoic acid-binding proteins in transformed mammalian cells. Invest. Ophthalmol. Vis. Sci. 17:988–992. [PubMed] [Google Scholar]
- Saari, J.C., G.G. Garwin, J.P. van Hooser, and K. Palczewski. 1998. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 38:1325–1333. [DOI] [PubMed] [Google Scholar]
- Saari, J.C., M. Nawrot, B.N. Kennedy, G.G. Garwin, J.B. Hurley, J. Huang, D.E. Possin, and J.W. Crabb. 2001. Visual cycle impairment in cellular retinaldehyde binding protein (CRALBP) knockout mice results in delayed dark adaptation. Neuron. 29:739–748. [DOI] [PubMed] [Google Scholar]
- Shukla, P., and J.M. Sullivan. 1999. Normal and mutant rhodopsin activation measured with the early receptor current in a unicellular expression system. J. Gen. Physiol. 114:609–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon, A., U. Hellman, C. Wernstedt, and U. Eriksson. 1995. The retinal pigment epithelial-specific 11-cis-retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J. Biol. Chem. 270:1107–1112. [PubMed] [Google Scholar]
- Spalink, J.D., and H. Stieve. 1980. Direct correlation between the R2 component of the early receptor potential and the formation of metarhodopsin II in the excised bovine retina. Biophys. Struct. Mech. 6:171–174. [DOI] [PubMed] [Google Scholar]
- Stecher, H., M.H. Gelb, J.C. Saari, and K. Palczewski. 1999. Preferential release of 11-cis-retinol from retinal pigment epithelial cells in the presence of cellular retinaldehyde-binding protein. J. Biol. Chem. 274:8577–8585. [DOI] [PubMed] [Google Scholar]
- Stillman, B.W., and Y. Gluzman. 1985. Replication and supercoiling of Simian virus 40 DNA in cell extracts from human cells. Mol. Cell. Biol. 5:2051–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, J.M. 1998. Low-cost monochromatic microsecond flash microbeam apparatus for single-cell photolysis of rhodopsin or other photolabile pigments. Rev. Sci. Inst. 69:527–539. [Google Scholar]
- Sullivan, J.M., and P. Shukla. 1999. Time-resolved rhodopsin activation currents in a unicellular expression system. Biophys. J. 77:1333–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, J.M., and M.F. Satchwell. 2000. Development of stable cell lines expressing high levels of point mutants of human opsin for biochemical and biophysical studies. Methods Enzymol. 315:30–58. [DOI] [PubMed] [Google Scholar]
- Sullivan, J.M., L. Brueggemann, and P. Shukla. 2000. An electrical approach to study rhodopsin activation in single cells with the early receptor current assay. Methods Enzymol. 315:268–293. [DOI] [PubMed] [Google Scholar]
- Thompson, D.A., Y. Li, C.L. McHenry, T.J. Carlson, X. Ding, P.A. Sieving, E. Apfelstedt-Sylla, and A. Gal. 2001. Mutations in the gene encoding lecithin retinal acyltransferase are associated with early-onset severe retinal dystrophy. Nat. Genet. 28:123–124. [DOI] [PubMed] [Google Scholar]
- Wang, J., X. Chai, U. Eriksson, and J.L. Napoli. 1999. Activity of human 11-cis-retinol dehydrogenase (Rdh5) with steroids and retinoids and expression of its mRNA in extra-ocular human tissue. Biochem. J. 338:23–27. [PMC free article] [PubMed] [Google Scholar]
- Williams, T.P. 1964. Photoreversal of rhodopsin bleaching. J. Gen. Physiol. 47:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, T.P. 1965. Rhodopsin bleaching: relative effectiveness of high and low intensity flashes. Vision Res. 5:633–638. [DOI] [PubMed] [Google Scholar]
- Williams, T.P. 1974. Upper limits to the bleaching of rhodopsin by high intensity flashes. Vision Res. 14:603–607. [DOI] [PubMed] [Google Scholar]
- Winston, A., and R.R. Rando. 1998. Regulation of isomerohydrolase activity in the visual cycle. Biochemistry. 37:2044–2050. [DOI] [PubMed] [Google Scholar]
- Yau, K.-W., G. Matthews, and D.A. Baylor. 1979. Thermal activation of the visual transduction mechanism in retinal rods. Nature. 279:806–807. [DOI] [PubMed] [Google Scholar]
- Zhai, Y., D. Higgins, and J.L. Napoli. 1997. Coexpression of the mRNAs encoding retinol dehydrogenase isozymes and cellular retinol-binding protein. J. Cell. Physiol. 173:36–43. [DOI] [PubMed] [Google Scholar]