Abstract
We previously identified a negative correlation between histamine release to histamine releasing factor/translationally controlled tumor protein (HRF/TCTP) and protein levels of the Src homology 2 domain–containing inositol 5′ phosphatase (SHIP) in basophils. We have also demonstrated that HRF/TCTP primes basophils to release mediators. The purpose of this study was to begin characterization of signal transduction events directly induced by HRF/TCTP and to investigate these events when HRF/TCTP is used as a priming agent for human basophil histamine release. Highly purified human basophils were examined for surface expression of bound HRF/TCTP, changes in calcium, and phosphorylation of Akt, mitogen-activated protein kinase kinase (MEK), extracellular signal–regulated kinase (ERK), Syk, and FcϵRIγ. Results showed that basophils from all donors bound HRF/TCTP. There was a biphasic calcium response to HRF/TCTP, which corresponded to the magnitude of histamine release. Furthermore, those donors who have direct histamine release when exposed to HRF/TCTP (HRF/TCTP responder [HRF/TCTP-R] donors) have phosphorylation of Syk, Akt, MEK, and ERK. Remarkably, basophils from HRF/TCTP-nonresponder (HRF/TCTP-NR) donors do not show phosphorylation of these molecules. This finding is different from IL-3, which also primes basophils for histamine release, but does show phosphorylation of these events. We conclude that priming induced by HRF/TCTP is distinct from that induced by IL-3.
Introduction
Translationally controlled tumor protein (TCTP) was originally identified in the 1980s by Brawerman's group as a tumor protein in a mouse acidic tumor and in mouse erythroleukemia. However, no function for the protein was identified.1,2 We identified a histamine releasing factor (HRF) found in late-phase fluids from nasal lavages, bronchoalveolar lavage fluids, and skin blister fluids that directly induced histamine release from basophils isolated from a subpopulation of allergic donors.3 We have previously published that approximately 50% of allergic donor basophils respond to HRF/TCTP.4 This HRF was distinct from histamine release induced by monocyte chemotactic protein-1 (MCP-1), which is a potent activation stimulus of basophils from all donors.5–7 After purification and cloning, HRF was found to be identical to TCTP, which is also known as p23.8 This recombinant molecule was found to have the same properties as the originally described HRF derived from nasal secretions: namely, an ability to induce histamine release from selected donors. This protein is ubiqui-tously expressed as an intracellular protein, and homologs of HRF/TCTP have been described in parasites, including Plasmodium falciparum, Wucheria bancrofti, Brugiia malea, and Schistosoma mansinai, all of which possess mast cell/basophil histamine–releasing activity.9–11
HRF/TCTP appears to have both intracellular and extracellular functions. One group identified the antiapoptotic activities of this molecule and renamed it fortilin.12 Our group, as well as another group, has identified the interaction between HRF/TCTP and elongation factor-1δ, also known as eElongation factor 1B-β.13,14 Thus, HRF/TCTP may have an intracellular role in interfering with the elongation step of protein synthesis.
We, as well as others, have focused on the extracellular functions of HRF/TCTP. HRF/TCTP was initially described as a complete secretagog for histamine and IL-4 secretion from basophils of certain allergic donors.15 Initially, it was thought that these donors had a certain type of IgE that interacted with HRF/TCTP to induce secretion.15 However, it was subsequently demonstrated that HRF/TCTP primed HRF/TCTP non-responder (HRF/TCTP-NR) basophils for histamine release as well as IL-4 and IL-13 secretion regardless of the type of IgE.16 Additional studies demonstrated that while HRF/TCTP functions as a histamine-releasing factor, it can also modulate secretion of cytokines from human basophils, eosinophils, and T cells.17–20 Thus, these studies demonstrated that HRF/TCTP does not appear to directly interact with IgE. In addition, Kang et al identified this molecule as a B-cell growth factor.21 This group demonstrated that HRF/TCTP bound to B cells and induced cytokine production from these cells. More recently, HRF/TCTP was shown to stimulate bronchial epithelial cells to produce IL-8 and GM-CSF.22
IL-3 is known to be a direct secretagog of IL-13 and mediator release, particularly in basophils from allergic patients.23–27 In addition, IL-3 is the most potent stimulus of augmentation of IgE-mediated histamine release.5,28 This enhanced response is referred to as priming. Furthermore, several studies have demonstrated that IL-3 induces phosphorylation of various signaling pathways, including JAK2, Shc, STAT5, Grb2, ras, and ERK.29 In addition, the priming of IgE-mediated induced histamine release was shown to exert its effects at localized points of the signaling cascade.30 Notably, there was significant phosphorylation of signal molecules induced by IL-3 alone in all of these studies.30
This present study was designed to elucidate specific actions of HRF/TCTP on human basophils and begin to characterize the nature of intracellular signaling that follows stimulation with HRF/TCTP directly or by priming with HRF/TCTP. Given the similarities in secretion kinetics following IgE-mediated stimulation, we hypothesized there would be some signaling characteristics similar to those previously found for IgE-mediated release. However, due to the differential sensitivity to treatment with rottlerin between HRF/TCTP and anti-IgE,17 we also sought differences in signaling. Because HRF/TCTP primes basophils for mediator release, we also expected to see phosphorylation of signal transduction molecules in HRF/TCTP-NR donors. In fact, that was not the case. IL-3, the most potent known priming agent for human basophils alone, is known to cause phosphorylation of many intracellular molecules.30 HRF/TCTP, on the other hand, does not cause phosphorylation of molecules in HRF/TCTP-NR donors despite HRF/TCTP's ability to prime or enhance histamine release. This demonstrates a distinguishing characteristic of this cytokine.
Methods
The following materials were purchased: PIPES, BSA, D-glucose, IGEPAL CA-630 (NP-40), NaF, Na4P2O7, Na3VO4, Tris-HCL, EDTA, Accuspin tubes, glutathione S–transferase (GST; Sigma-Aldrich, St Louis, MO); crystallized human serum albumin (HSA; Bayer, Emeryville, CA); Percoll (GE Healthcare, Chalfont St Giles, United Kingdom); DTT (Roche, Indianapolis, IN); protease inhibitor cocktail (BD PharMingen, San Diego, CA); anti–phospho-Akt, anti–phospho– mitogen-activated protein kinase kinase (MEK), and anti–phospho– extracellular signal–regulated kinase (ERK; Cell Signaling Technology, Danvers, MA); anti-Syk monoclonal antibody, anti–ERK-1 polyclonal antibody, and PY20 (antiphosphotyrosine; Santa Cruz Biotechnology, Santa Cruz, CA); antiphosphotyrosine monoclonal antibody (4G10; Upstate Biotechnology, Lake Placid, NY); anti-mouse IgG1 (Beckman Coulter, Fullerton, CA); rabbit anti-human HRF (Medical and Biological Laboratories, Woburn, MA); goat F(Ab′)2, anti-rabbit Ig's R-PE conjugate (Biosource, Camarillo, CA); Fura 2-AM (Molecular Probes, Eugene, OR); basophil isolation kit (StemCell Technologies, Vancouver, BC); Dulbecco phosphate-buffered saline (PBS), Tris-glycine gels (Invitrogen, Carlsbad, CA); rabbit anti-human PI3 kinase (p85; Upstate Biotechnology); gp 120 IgE and ovalalbumin gp 120 antigen (Tanox, Houston, TX). Goat anti-human IgE was prepared as previously described.31 Anti-FcϵRIγ antibody was obtained from Upstate Biotechnology. Glaxo Smith Kline (Stevenage, United Kingdom) Syk kinase inhibitor NVP-QAB205 was a gift.
Buffers
PIPES buffer consisted of 25 mM PIPES, 110 mM NaCl, and 5 mM KCl. PIPES–albumin glucose (PAG) is made up of PIPES, 0.1% glucose, and 0.003% HSA. PAGCM is PAG supplemented with 1 mM CaCl2 and 1 mM MgCl2. For histamine release assays, PAG was supplemented with 5 mM CaCl2. For signal transduction experiments, PAGCM was used. Lysis buffer (1 ×) was 20 mM Tris (pH 7.5) containing 1% NP-40, 5 mM EDTA, 1 mM Na3VO4, 5 mM Na4P2O7, 5 mM DDT, 50 mM NaF, and protease inhibitor cocktail. Buffered 4% paraformaldehyde was made with 0.1 M Na2HPO4 ullet 7H2O and 0.1 M NaH2 PO4 ullet H2O (pH 7.4). Magnetic-activated cell sorter (MACS) column buffer consisted of PIPES, 0.5% BSA, and 0.002 M EDTA. Complete lysis buffer (CLB) consisted of 20 mM Tris-HCl (pH 7.5), 100 g/mL aprotinin, 10 mM benzamidine, 1 mM PMSF, 50 mM NaF, 1 mM Na3VO4, 1% NP-40, and 10% glycerol. Electrophoresis sample buffer (ESB) was Invitrogen buffer containing 5% 2-ME. Stripping buffer (1 ×) consisted of 2.3 M SDS and 2.5 M Trizma-Base (pH 6.7) with 0.1 M β-mercaptoethanol.
Basophil purification
For all studies, except histamine release, basophils were purified from 150 mL of peripheral blood drawn from volunteers after informed consent was obtained in accordance with the Declaration of Helsinki. All blood drawing was conducted under a protocol approved by the Johns Hopkins University Institutional Review Board. Blood was placed onto a discontinuous double Percoll gradient consisting of 53.2% and 62% Percoll, respectively, in Accuspin tubes and centrifuged at 300g for 20 minutes at room temperature. After centrifugation, 2 bands of white cells were visible above the filter and most red blood cells (RBCs) sedimented below the filter. The cell suspension from the lower white band, consisting primarily of basophils and lymphocytes, trace numbers of RBCs, and infrequently eosinophils and neutrophils, was then further purified by means of negative selection. The human basophil enrichment cocktail and pump feed column (StemCell Technologies) were used to further purify basophils isolated from the double Percoll gradient. The purity of basophils was determined by alcian blue staining and generally exceeded 99%.
Mediator secretion and donor classification
Histamine release was determined by automated fluorometry of stimulated basophil supernatants.32 Stimulation was done with 0.1 μg/mL polyclonal anti-IgE as a positive control or 2.1 to 17.4 μM glutathionine S–transferase HRF/TCTP fusion protein (GST-HRF/TCTP) for 45 minutes at 37°C on dextran-sedimented basophils. The method of obtaining basophils by dextran sedimentation for histamine release was previously published.33 Partially purified double percoll basophils (see “Basophil purification”) were used for the Syk inhibitor experiments. Total cellular histamine was determined from cells lysed with 1.6% perchloric acid. Spontaneous histamine release from basophils incubated with buffer alone was subtracted from the values from all stimulated samples.33 Donors were classified as HRF/TCTP-R if they released greater than or equal to 10% total histamine content to HRF/TCTP. With the exception of the donors allergic to cats used for LTC4 generation, all other HRF/TCTP-NR were nonallergic donors.
Recombinant HRF/TCTP production
HRF/TCTP preparations used in these studies include several GST-HRF/TCTP fusion protein productions. The average potency of these batches was 1.1 plus 0.17 μM of HRF/TCTP, with potency defined as the concentration that elicits 20% histamine release. GST-HRF/TCTP was produced in the baculovirus system and purified, as previously described.34 Purified HRF/TCTP was judged to be greater than 95% by Coomassie blue staining of an SDS-PAGE gel. The protein concentration was determined by BCA protein assay (Pierce, Rockford, IL) and calculated based on the molecular mass of the HRF/TCTP (23 kDa) as a portion of the total fusion protein.
LTC4 measurements
A radioimmunoassay was performed using 100 μL supernatant to determine LTC4 levels as previously described.35
Flow cytometry
Basophils were isolated according to our routine negative selection protocol described under “Basophil purification” using the human basophil enrichment cocktail and the pump feed column (StemCell Technologies). Cells were eluted with MACS column buffer, pelleted, and counted. Basophils (106) were transferred to a tube and pelleted at 400g for 5 minutes at 4°C. The supernatant was removed, and the cells were fixed in 300 μL buffered 4% paraformaldehyde at room temperature for 5 minutes. Cells were flooded with 1 mL ice-cold water, filtered in 0.1% BSA, and pelleted at 400g for 5 minutes at 4°C. The supernatant was removed, and the cells were resuspended in 250 μL of 10% DMSO in PBS. Cells were frozen at −80°C until further use. For the flow analysis, the cells were thawed and washed twice with MACS column buffer and divided so that each condition had 100 000 cells.
Cells were incubated with buffer or HRF/TCTP (0.16–1.3 μM) for 2 hours at 0°C on ice, then washed twice with 0.2% BSA PBS. Cells were labeled in the presence of 1 mg/mL human IgG to minimize nonspecific binding to FcγR as previously described.36 Antibodies for these studies included an irrelevant rabbit IgG control, rabbit anti–human HRF, and goat F(ab′)2 anti–rabbit Ig's R-PE conjugate. Samples were analyzed on a Coulter EPICS Profile Flow Cytometer (Beckman Coulter). At least 5000 events per experimental condition were counted. Data were expressed as percentage increase in mean fluorescence intensity (MFI): [HRF/TCTP-specific MFI − control MFI] / control MFI.
[Ca2+]i measurements
Basophils were labeled with 1 mM Fura 2-AM for 20 minutes at 37°C in RPMI 1640 containing 2% FCS (300 000–500 000 cells in 200 μL). After washing once with 200 μL PAG, the cells were resuspended in PAG for loading in the microscope observation chamber. Changes in [Ca2+]i were obtained by digital videomicroscopy using techniques previously described in detail.37 Briefly, 15 μL cells (20 000–30 000) were loaded onto a siliconized coverslip of the microscope chamber and, after settling, overlayed with 1 mL PAG with 5 mM CaCl2 buffer. After warming to 37°C, monitoring of the cells was begun, and after 4 frames (each frame is a single ratio measurement of a field of 30–100 cells) of prechallenge, [Ca2+]i levels were acquired. The cells were challenged with 1 mL stimulus in buffer with 5 mM CaCl2. Data were then obtained for 50 to 150 frames at intervals of 3 to 10 seconds to determine the subsequent [Ca2+]i response.
Syk and FcϵRIγ phosphorylation
Syk phosphorylation was detected with the antiphosphotyrosine antibody clone 4G10 after immunoprecipitation of Syk from basophil lysates. After stimulating basophils (2 × 106) in PAGCM buffer at 37°C, the reaction was stopped by adding ice-cold PAG buffer and centrifuged in a microfuge for 15 seconds. The pellets were immediately lysed in CLB by vortexing and incubating on ice for 10 minutes. The lysates were then centrifuged for 3 minutes at 16 000g and precleared with protein G–sepharose beads for 30 minutes at 4°C. Then, the clarified lysates were incubated with anti-Syk antibody prebound to protein G–sepharose beads (1 μg antibody per 20 μL beads) for 1 hour at 4°C. The beads were washed 3 times, and the immunoprecipitated proteins were eluted by boiling for 5 minutes in ESB. After electrophoresis and transfer, the membranes were blotted with 4G10 antibody. The membranes were then stripped with SDS buffer and reblotted with anti-Syk antibody to determine loading. Data from the antiphosphotyrosine blots were normalized for loading differences using the band intensities from the anti-Syk reblot. For the measurements of FcϵRIγ phosphorylation, the cell lysates were immunoprecipitated with antiphosphotyrosine (PY20), and the blots were developed with an anti-FcϵRIγ.
Akt, MEK, and ERK-1/2 phosphorylation
After isolation, basophils were washed twice in PAG buffer and aliquoted for stimulation with various doses of HRF/TCTP from 0 to 8.7 μM at 37° for 15 minutes in PAGCM. For ERK-1/2 activation, 2 × 105 basophils were stimulated. For Akt or MEK activation, 5 × 105 basophils were used. The reactions were quenched by addition of ice-cold PAG, pelleting the cells at 4°C at 18 000g for 10 seconds, aspirating the supernatants, and lysing the cell pellet in 25 μL lysis buffer. After solubilizing for 20 minutes on ice, the lysate was clarified and mixed with an equal volume of 2 × Tris-glycine SDS sample buffer with 5% β-mercaptoethanol. Samples were boiled for 5 minutes and electrophoresed on 10% Tris-glycine gels. Proteins from the gel were transferred to a nitrocellulose membrane and Western blotted with anti–phospho-Akt (Thr308), anti–phospho-MEK (Ser217/221), and anti–phospho-ERK (Thr202, Tyr204). The blots were then stripped and reprobed with anti–ERK-1 antibody, which cross-reacts with anti–ERK-2. A fixed amount of a Western blotting standard prepared from antigen-stimulated rat basophilic leukemia (RBL) cells was included on each gel to allow for comparison between blots. Linear ranges for the antibodies used were determined based on net intensities of increasing doses of stimulated RBL lysates on Western blots. All values depicted fell within this linear range of staining. For each individual experiment, the ratio of phospho-Akt to ERK-1 (phospho-Akt/ERK-1) was calculated by scanning the phospho-Akt blots, normalizing the values to the scanned value of the standard, and dividing by the scanned value from the ERK-1 reprobe. Data were represented as a percentage of maximum to account for donor variability. Similar calculations were performed to determine the ratio of phospho-MEK/ERK-1 and phospho-ERK/ERK-1. Kinetics of phosphorylation was performed at optimal doses of HRF/TCTP.
Data presentation
Chemiluminescence films (Amersham Hyperfilm™ ECL; GE Healthcare) were converted to digital images with a Bio-Rad Gel Doc EQ (Bio-Rad, Hercules, CA), and in most cases were analyzed with that instrument or, in the case of the Syk experiments, National Institutes of Health (NIH; Bethesda, MD) Image software was used.
Results
Cell-surface binding of HRF/TCTP to purified human basophils
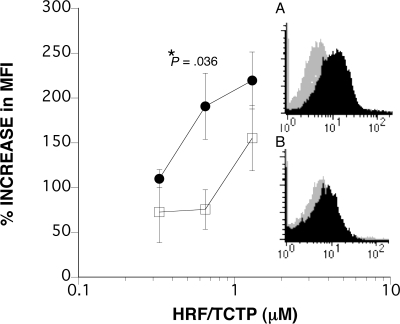
To examine the binding of HRF/TCTP to purified human basophils, we used flow cytometry with an anti-HRF antibody using 4% paraformaldehyde-fixed basophils from HRF/TCTP-R and HRF/TCTP-NR donors. We determined that 11 μg/mL anti-HRF antibody was an optimal concentration (data not shown; n = 9 using 5 different donors). The shift in MFI was measured by incu-bating varying doses of HRF/TCTP (0–1.3 μM) for 2 hours on ice, followed by extensive washes and incubation with 11 μg/mL anti-HRF. There was a consistent shift in MFI over control antibody in 8 donors (4 HRF/TCTP-R donors and 4 HRF/TCTP-NR donors) whose cells were preincubated with HRF/TCTP and visualized by flow cytometry. The inset panels in Figure 1 show representative data. Figure 1A shows a shift in mean fluorescence that is 175% over that of control in an HRF/TCTP-R donor, while Figure 1B shows a shift of 49% increase over that of control in an HRF/TCTP-NR donor. Both of these were performed using 0.65 μM HRF/TCTP. The increase over control was consistently seen at the 0.65-μM concentration of HRF/TCTP. As shown on the left side of the figure, there is a dose response of HRF/TCTP in the flow cytometry experiments (n = 4). There is a statistical difference between HRF/TCTP-R and HRF/TCTP-NR donors of HRF/TCTP binding to the cell surface only at the 0.65-μM concentration of HRF/TCTP (P = .036). Since HRF/TCTP acts as a direct secretagog and primes HRF/TCTP-NR basophils for anti-IgE–induced histamine release, the data showing binding by flow cytometry in the 2 populations of donors is not surprising.
Figure 1.
Cell-surface binding of HRF/TCTP to purified human basophils. Purified human basophils were incubated with HRF/TCTP and then with an anti-HRF antibody. Flow cytometry was performed as described in “Methods.” (A) Shift in MFI (dark histogram) is compared with an IgG control antibody (gray histogram) in an HRF/TCTP-R donor. (B) Shift in the MFI in an HRF/TCTP-NR donor. Panels A and B are representative of a total of 4 HRF/TCTP-R and 4 HRF/TCTP-NR donors, respectively. The left-hand side of the figure depicts the percentage increase in MFI in 4 HRF/TCTP-R donors (●) compared with 4 HRF/TCTP-NR donors (□) when stimulated with increasing doses of HRF/TCTP. There is a statistical difference between the 2 types of donors only at the 0.65-μM dose of HRF/TCTP with a P value of .036. The standard error is represented by the error bars.
Early ITAM and Syk phosphorylation
Progressing inward from the receptor, a receptor-associated kinase may mediate early signaling, possibly being recruited to an immunotyrosine activating motif (ITAM)–like structure. In human basophils, there are pertussis toxin–sensitive G protein–linked receptors, but previous studies found that stimulation with HRF/TCTP is not sensitive to pertussis toxin. In 2 experiments, we were unable to demonstrate phosphorylation of FcϵRIγ by HRF/TCTP (data not shown). While phosphorylation of FcϵRIγ has been observed following stimulation with optimal concentrations of anti-IgE antibody, the procedure demands approximately 5 million basophils per condition and does not easily detect weaker signals.38 The requirement for so many basophils limited our experiments to 2 conditions, stimulation with or without HRF/TCTP, without a positive control (with the caveat that the donors chosen for the study were known to release histamine well to stimulation with anti-IgE antibody).
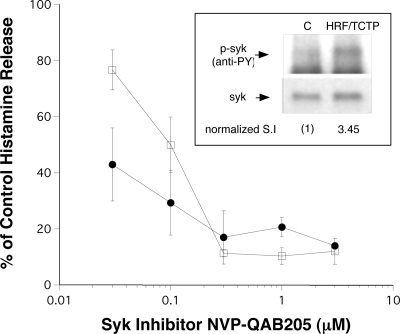
Since many molecules, with the exception of IL-3, are known to operate through recruitment of Syk kinase,30,38,39 we next investigated this signaling event. Approximately 2 million basophils per lane were stimulated with 2.8 μM HRF/TCTP or buffer for 15 minutes at 37°C. As shown in Figure 2 inset, HRF/TCTP induced a 3.45 stimulation index of Syk phosphorylation compared with buffer control. This is one of 2 experiments. There are several second- or third-generation selective inhibitors of Syk kinase, and we have previously characterized a Novartis compound for its influence of various functional endpoints in human basophils. NVP-QAB205 was found to inhibit HRF/TCTP-induced histamine release with an IC50 that is similar to its effect on IgE-mediated histamine release (Figure 2). It is expected that a weaker stimulus such as HRF/TCTP would have a lower IC50 for inhibition than IgE-mediated release, and Figure 2 shows that this is indeed the case with an IC50 approximately 3.3-fold lower.
Figure 2.
Phosphorylation of Syk by HRF/TCTP and inhibition of HRF/TCTP and anti-IgE–induced histamine release by a Syk inhibitor. (Inset) A total of 2 million basophils per lane were stimulated with buffer (“C”) or 2.8 μM HRF/TCTP. Pelleted cells were lysed, and a Western blot was probed with anti-4G10 to detect phosphorylated Syk (p-Syk/anti-PY). The blot was reprobed with anti-Syk to determine total cellular levels of Syk (syk). The levels of Syk phosphorylation were normalized to the stimulation index (SI) of 1 for control and calculated to be 3.45 after HRF/TCTP stimulation. Data are representative of 2 separate experiments. The graph shows partially purified double percoll basophils from HRF/TCTP-R donors preincubated with NVP-QAB205, the Syk inhibitor (.03–3μM), for 10 minutes at 37°C, and then stimulated with suboptimal concentrations of HRF/TCTP (●) or anti-IgE (□) (n = 3). The standard error is represented by the error bars. Inhibition is illustrated as percentage of control, which is stimulus without inhibitor.
Intracellular calcium response induced by HRF/TCTP
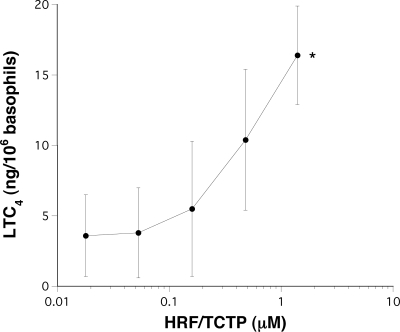
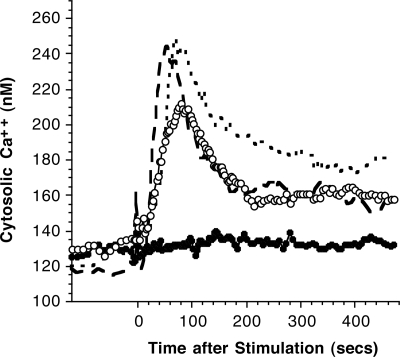
Previous studies have demonstrated that in HRF/TCTP-R donors, HRF/TCTP could induce secretion of both histamine and IL-4.15 In human basophils, histamine release does not need to be accompanied by changes in cytosolic calcium; phorbol esters induce degranulation without elevations in cytosolic calcium and induce histamine release even if extracellular calcium is removed and internal calcium buffered with cell-permeant calcium chelator.40,41 On the other hand, there is some indirect evidence that IL-4 secretion,42 which HRF/TCTP also induces,15 requires elevations in cytosolic calcium, so we expected that HRF/TCTP would induce a calcium response. This expectation is made nearly certain if one considers that HRF/TCTP induces LTC4 release because there is considerable evidence that generation of LTC4 requires a reasonable increase in cytosolic calcium.43 However, no previously published studies have examined generation of LTC4 following HRF/TCTP. Figure 3 demonstrates that HRF/TCTP does indeed result in LTC4 release at levels close to expectations for an IgE-like stimulus, 15 ng/106 basophils. These 5 donors were all allergic to cats, but only 2 of 5 showed direct histamine release to HRF/TCTP. Since all released LTC4 to HRF/TCTP, this suggests that LTC4 might be a more sensitive measure of HRF/TCTP's ability to be a secretagog. We also studied the calcium response in 4 different donors with varying responses to HRF/TCTP, as evidenced by histamine release. Among the 4 donors, the basophil response to HRF/TCTP varied from no release to histamine release of 49%. Figure 4 demonstrates that for the 2 releasing donors, there was a strong calcium response approaching that observed following stimulation with anti-IgE. In the 2 HRF/TCTP-NR donors, anti-IgE antibody induced a typical response, while the HRF/TCTP-stimulated cytosolic calcium response was near 0. It should be noted that IL-3 alone also does not cause an elevation of calcium at a dose where mediator release is not seen.30 The character of elevations, when observed with HRF/TCTP, was very similar to stimulation with anti-IgE antibody; the timing of the initial release from internal stores and the subsequent influx-dependent phases were similar. At the single-cell level (not shown), there was a characteristic long time lag (30–180 seconds), an abrupt rise in cytosolic calcium, and often many asynchronous spikes or oscillations.
Figure 3.
LTC4 levels after stimulation of basophils with doses of HRF/TCTP. Dextran-sedimented basophils from donors allergic to cats were incubated with increasing doses of HRF/TCTP. A total of 100 μL of reaction volume was assayed for LTC4 levels (n = 5). *P < .02 compared with buffer control; paired Student t test. The standard error is represented by the error bars.
Figure 4.
Intracellular calcium response induced by HRF/TCTP. Purified basophils were labeled with Fura 2, and calcium was measured by digital videomicroscopy as described in “Methods.” Basophils from 2 HRF/TCTP-R donors were stimulated with 24 μM HRF/TCTP (○) and from 2 HRF/TCTP-NR (●). Basophils from 2 HRF/TCTP-R were stimulated with 0.1 μg/mL AIgE (small dashed line) and from 2 HRF/TCTP-NR donors (large dark dashed line).
PIP3 and MAPK pathways
We have previously published that HRF/TCTP-R basophils express, on a per-cell basis, lower levels of SHIP.34 In addition, we have previously demonstrated that histamine release induced by HRF/TCTP in basophils from HRF/TCTP-R donors is inhibited by the P13-kinase inhibitor LY294002.34 This inhibitor has been used to prevent PIP3 formation in a variety of cell types.44 These observations suggest a role for PIP3 in the HRF/TCTP response. In addition, since we have shown that HRF/TCTP acts as a priming agent in HRF/TCTP-NR basophils,16 stimulation of this type of basophil might still result in increases in PIP3 levels and/or changes in the mitogen-activated protein kinase (MAPK) pathway that involves MEK and ERK. This prediction follows from the fact that IL-3, also a priming agent, induces these pathways.30
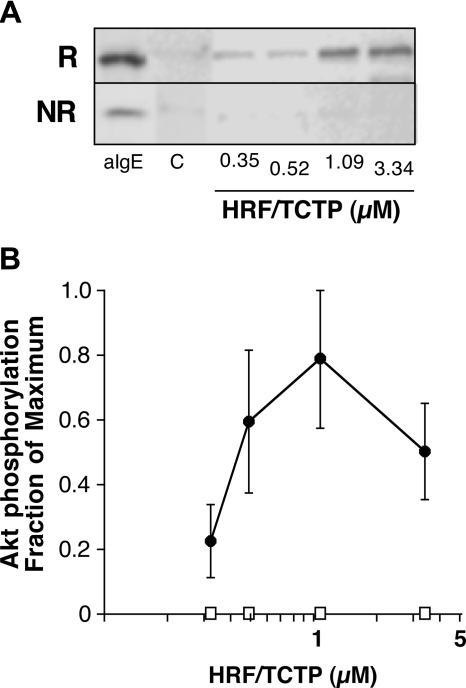
Since PIP3 measurements require many more basophils than can be obtained by simple venipuncture from selected donors, phosphorylation of Akt was used as a surrogate marker of the presence of PIP3. Akt phosphorylation was examined in the basophils obtained from the HRF/TCTP-R and HRF/TCTP-NR categories, 3 distinct donors for each group, and representative data are shown in Figure 5A. Figure 5B shows average phosphorylation data from these donors. At no concentration of HRF/TCTP did we observe a change in Akt phosphorylation in basophils from the HRF/TCTP-NR group. This is distinctly different from published results when IL-3 is used alone as a stimulus.
Figure 5.
Phosphorylation of Akt by HRF/TCTP. (A) Purified basophils were stimulated with various doses of HRF/TCTP, and cell lysates were prepared and Western blotted for phospho-Akt as described in “Methods.” This panel shows phosphorylation of Akt in basophils from an HRF/TCTP-R donor (top row; R) or an HRF/TCTP-NR donor (bottom row; NR). Cells were stimulated with anti-IgE (aIgE) as a positive control, buffer as a negative control (“C”), or increasing doses of HRF/TCTP. (B) Percentage of maximum of Akt phosphorylation with increasing doses of HRF/TCTP (●) in 3 HRF/TCTP donors. There is no detectable phosphorylation induced by HRF/TCTP in 3 HRF/TCTP-NR donors (□). The standard error is represented by the error bars.
In contrast, basophils from the HRF/TCTP-R group showed elevations in Akt phosphorylation at concentrations of HRF/TCTP associated with strong histamine release. The kinetics varied among the responder donors, and the characteristics also depended on the concentration of HRF/TCTP (data not shown).
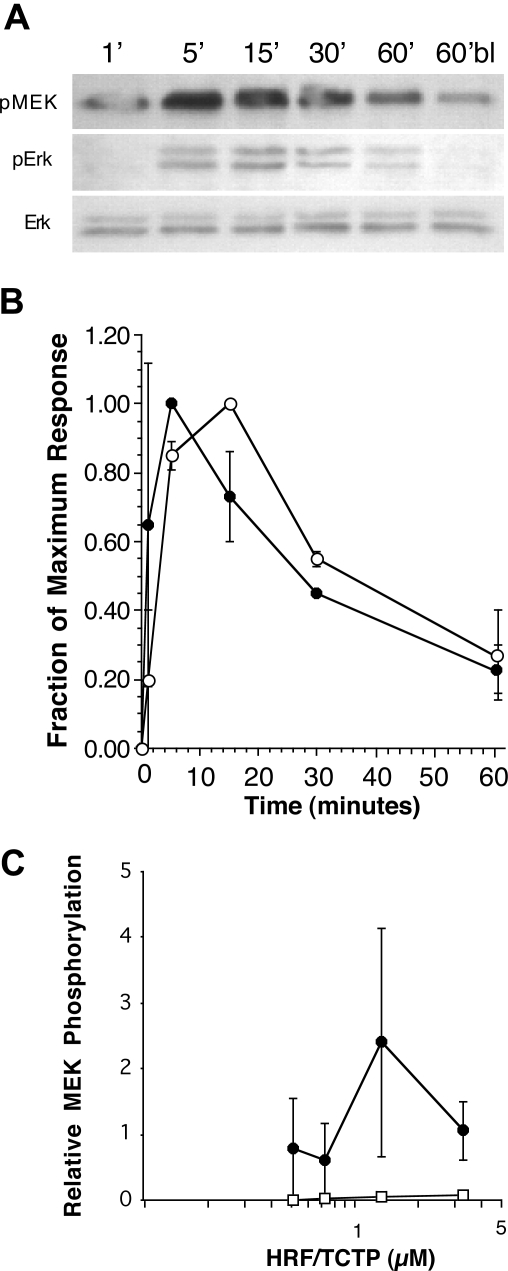
Since LTC4 release follows stimulation with HRF-TCTP, and since LTC4 release has been shown to require activation of MEK and ERK, it follows that MEK and ERK phosphorylation will occur in basophils stimulated with HRF/TCTP. It is less clear whether MEK or ERK phosphorylation will occur in basophils considered nonreleasers to HRF/TCTP, even though these cells can be primed by HRF/TCTP.16 As observed in basophils stimulated with anti-IgE antibody, HRF/TCTP induced MEK phosphorylation in HRF/TCTP-R basophils with a peak at 5 minutes (Figure 6A). Also shown in Figure 6A, ERK phosphorylation occurred in HRF/TCTP-R basophils peaking between 5 and 15 minutes. Total ERK protein is shown as a control. Figure 6B shows the kinetics of ERK (n = 2) and MEK (n = 3) phosphorylation by HRF/TCTP in HRF/TCTP-R basophils. Finally, Figure 6C demonstrates the concentration dependence of HRF/TCTP-induced MEK phosphorylation in HRF/TCTP-R donors. Once again, unlike IL-3, no phosphorylation of MEK was observed in basophils that did not secrete in response to HRF/TCTP.
Figure 6.
Phosphorylation of MEK and ERK by HRF/TCTP. Basophils were stimulated with HRF/TCTP, and then cell lysates were prepared and Western blotted for phospho-MEK and phospho-ERK as described in “Methods.” (A) Representative experiment showing phosphorylation of MEK in an HRF/TCTP-R donor (top row), phosphorylation of ERK in an HRF/TCTP-R donor (middle row), and equal loading of lanes as determined by anti-ERK (bottom row). (B) Phosphorylation of kinetics of MEK in 3 HRF/TCTP-R donors (●) compared with phosphorylation of kinetics of ERK in 2 HRF/TCTP-R donors (○). All stimulation was done with 1.09 μM HRF/TCTP. (C) Dose response of HRF/TCTP-induced MEK phosphorylation in 3 HRF/TCTP-R donors (●). There was no HRF/TCTP induced phosphorylation of MEK in HRF/TCTP-NR donors (□; n = 3). The standard error is represented by the error bars.
Parenthetically, the GST-HRF/TCTP fusion protein was used for these phosphorylation studies. GST alone at matched concentrations did not phosphorylate ERK (data not shown). This means that the phosphorylation of ERK in HRF/TCTP-R basophils is due to the HRF/TCTP portion of the molecule.
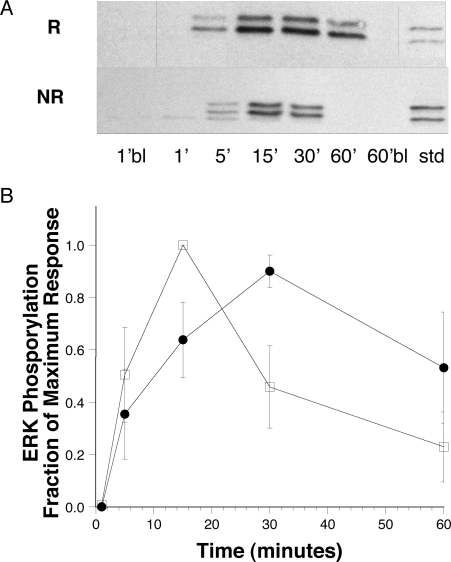
Since we did not observe phosphorylation of signaling molecules in HRF/TCTP-NR donors, despite documented priming, we investigated anti-IgE–induced signaling in these 2 groups of donors. Figure 7 demonstrates sustained anti-IgE–induced ERK phosphorylation only in HRF/TCTP-R basophils. Figure 7A (top row) is a representative experiment of the mean data shown in Figure 7B showing ERK phosphorylation in HRF/TCTP-R donors. The kinetics of this phosphorylation is sustained up to 60 minutes. As expected, anti-IgE induced transient ERK phosphorylation in HRF/TCTP-NR basophils with a peak of phosphorylation at 15 minutes (Figure 7A bottom row). Figure 7B further demonstrates the anti-IgE–induced sustained ERK phosphorylation in 4 HRF/TCTP-R donors as compared with transient ERK phosphorylation in 3 HRF/TCTP-NR basophils. In HRF/TCTP-R donors, the peak of ERK phosphorylation occurs at a later time point (30 minutes) and is sustained, compared with that of HRF/TCTP-NR donors.
Figure 7.
Sustained phosphorylation of ERK in HRF/TCTP-R basophils. (A) Representative anti–phospho-ERK blots from an HRF/TCTP-R (top row) and HRF/TCTP-NR (bottom row) donor. Basophils were stimulated with 0.3 μg/mL anti-IgE for the times noted. There was no phosphorylation in the 1-minute blank (1′bl) nor in the 60-minute blank (60′bl). The standard (Std) is 5 μL of an RBL standard sensitized with DNP-specific IgE and stimulated with DNP35 BSA antigen. Vertical lines have been inserted to indicate a repositioned gel lane. (B) Summary of kinetics of ERK phosphorylation stimulated with anti-IgE in HRF/TCTP-R donors (●; n = 4) and HRF/TCTP-NR donors (□; n = 3). The blots were scanned and values normalized as described in “Methods.” The standard error is represented by the error bars.
Discussion
In the current study, we demonstrate some of the signal transduction events induced by HRF/TCTP on human basophils from 2 donor populations, HRF/TCTP-R and HRF/TCTP-NR. Consistent with the ability of HRF/TCTP to either induce secretion directly in HRF/TCTP-R or prime HRF/TCTP-NR basophils, Figure 1 shows binding of HRF/TCTP to both donor populations.
Most of the figures in this study demonstrate that HRF/TCTP induces activation of intracellular signal transduction events in basophils only from those donors who directly release histamine to HRF/TCTP; namely, HRF/TCTP-R. The characteristics of the responses are very similar to those observed for stimulation with anti-IgE antibody or antigens with a couple of exceptions. Notably, there is no phosphorylation of FcϵRIγ. Additionally, stimulation of HRF/TCTP-R donor basophils have prolonged ERK phosphorylation up to 60 minutes when stimulated with anti-IgE as compared with the kinetics of anti-IgE–induced ERK phosphorylation in HRF/TCTP-NR basophils, which peaks at 15 minutes. More specifically, responder donors show a peak of ERK phosphorylation at 30 minutes and have significant phosphorylation that persists until at least 60 minutes. On the other hand, the nonresponder donors peak at 15 minutes and show marked decreased phosphorylation of ERK at both the 30-minute and 60-minute time points. It should be noted that phosphorylation of Syk is observed. This would occur whether HRF/TCTP is acting through the FcϵRI or another adapter molecule, which uses an ITAM to mediate early signal transduction events.39 What is novel about these data are that despite the fact that HRF/TCTP primes basophils in HRF/TCTP-NR for anti-IgE–induced histamine release, there is no phosphorylation of any signal transduction events in these donors. This is very different from the most studied basophil priming cytokine, IL-3.30
It would be expected that if Syk is phosphorylated by HRF/TCTP, that the events that follow would mimic, at least to some degree, the kind of signaling observed for aggregation of FcϵRI. In this respect, the elevation in cytosolic calcium has characteristics like those observed for IgE-mediated signaling. Specifically, the magnitude of the calcium response, relative to the amount of histamine release, is similar to the IgE-mediated response. Furthermore, there is a timelag between the addition of stimulus and the earliest rapid elevation in calcium, despite the micromolar concentrations of stimulus, which is again a feature of the IgE-mediated calcium response.
We have previously published a negative association with histamine release to HRF/TCTP and levels of SHIP in human basophils.34 Furthermore, we have shown a requirement for PIP3 for HRF/TCTP-mediated secretion in HRF/TCTP-R basophils as demonstrated by our experiments with the PI3-kinase inhibitor Ly294002.34 Inactivation of PI3-kinase with the specific inhibitor LY294002 prevented HRF/TCTP-induced degranulation in HRF/TCTP-R basophils, which is consistent with the requirement for PIP334. It has been reported that Akt phosphorylation is an accurate indicator of the presence of PIP345. Furthermore, Akt phosphorylation remains sensitive to the presence of PIP3 throughout the reaction. To further support this concept, we have demonstrated in Figure 5 that Akt is phosphorylated in a dose-dependent manner in HRF/TCTP-R donor basophils, peaking at 1 μM of HRF/TCTP. Again, notably, there was no phosphorylation of Akt induced by HRF/TCTP in HRF/TCTP-NR donor basophils. It has been previously published that MEK kinase signaling in human basophils is important in LTC4 generation.46 In addition, activation of ERK appears to be a key step necessary for the phosphorylation and activation of cPLA2, which is important for liberating free aracadonic acid for LTC4 generation in human basophils.46 Furthermore, eliminating the second phase of the intracellular calcium response, which appears to result from an influx of extracellular calcium, ablates LTC4 release in human basophils.47 Therefore, it was logical to address phosphorylation of both MEK and ERK in HRF/TCTP-R basophils.
To this point, we have presented data that demonstrates that HRF/TCTP phosphorylates signal transduction molecules only in HRF/TCTP-R donor basophils and not in HRF/TCTP-NR basophils. However, as demonstrated in Figure 7, both HRF/TCTP-R and HRF/TCTP-NR respond to anti-IgE and demonstrate ERK phosphorylation. This further supports that the HRF/TCTP-R donors are a different donor population in that the phosphorylation of ERK is sustained. This is not the case in HRF/TCTP-NR. Since we did not see phosphorylation of FcϵRIγ, it appears that HRF/TCTP might be acting through another ITAM-associated receptor other than IgE. Furthermore, as previously mentioned, HRF/TCTP activates cells that do not have the FcϵRI. Therefore, the previous concept of IgE heterogeneity is not solely responsible for the function of HRF/TCTP.
Given that HRF/TCTP causes priming in HRF/TCTP-NR basophils, it is notable that no form of signaling was observed in these donors. Currently, our only context for understanding the priming phenomenon in basophils is the nature of signaling induced by IL-3. There are several phases of IL-3: an acute phase requiring only 2 to 5 minutes of exposure to IL-3, an intermediate phase, requiring 18 to 24 hours, and a long phase requiring several days.30 The acute phase might have provided a model for the priming effect of HRF/TCTP. A second priming pathway of IL-3 induces a sustained signaling in the ERK; however, HRF/TCTP, using HRF/TCTP-NR basophils, did not induce activity of the ERK pathway. Neither did it induce any Akt phosphorylation, another pathway induced by IL-3. Therefore, the mechanism underlying HRF/TCTP-induced priming remains unclear. A previous study has noted that transfection of RBL cells with DAP10 and SIRPβ can, following activation of the SIRPβ, augment IgE-dependent signaling without directly inducing secretion. Replacing DAP10 with DAP12 allows crosslinking of SIRPβ to directly induce secretion.48 The underlying mechanism is unclear but it may be related to costimulatory effects of the DAP10 YxxM motif. This work suggests the hypothesis that differences in responsiveness to HRF/TCTP may relate to the particular adaptor protein associated with or used by the HRF/TCTP receptor.
In summary, these data demonstrate for the first time some of the characteristics of HRF/TCTP-induced signaling and furthermore illustrate that HRF/TCTP priming is distinct from IL-3 priming. Taken together, data to date suggest that although HRF/TCTP is a unique cytokine. It initiates a signal transduction process that mimics many of events associated with IgE-mediated secretion due to the initiation of Syk kinase activity. However, it does not phosphorylate FcϵRIγ, suggesting it may act through an adaptor protein to associate with the HRF/TCTP receptor.
Acknowledgments
We thank Ms Nancy Van Keuren for secretarial assistance in preparing the manuscript.
These studies were supported by NIH grants R01 AI32651 (S.M.M.), R01 AI20253 (D.W.M. Jr), and K22 AI49288 (B.M.V.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.M.V., N.V., J.M.L., and R.S.S. performed experiments and analyzed data. B.M.V., J.M.L., and D.W.M. Jr contributed to the writing of the manuscript. S.M.M. defined the experimental strategy, supervised and interpreted the experiments, and contributed to the writing of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan M. MacDonald, The Johns Hopkins Asthma and Allergy Center, 5501 Hopkins Bayview Circle, Rm 2A.38, Baltimore, MD 21224; e-mail: smacdona@jhmi.edu.
References
- 1.Yenofsky R, Cereghini S, Krowczynska A, Brawerman G. Regulation of mRNA utilization in mouse erythroleukemia cells induced to differentiate by exposure to dimethyl sulfoxide. Mol Cell Biol. 1983;3:1197–1203. doi: 10.1128/mcb.3.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitpatima ST, Makrides S, Bandyopadhyay R, Brawerman G. Nucleotide sequence of a major messenger RNA for a 21 kilodalton polypeptide that is under translational control in mouse tumor cells. Nucleic Acids Res. 1988;16:2350. doi: 10.1093/nar/16.5.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald SM, Lichtenstein LM, Proud D, Plaut M, Naclerio RM, Kagey-Sobotka A. Studies of IgE-dependent histamine releasing factors: heterogeneity of IgE. J Immunol. 1987;139:506–512. [PubMed] [Google Scholar]
- 4.MacDonald SM, Lichtenstein LM. Histamine releasing factors and heterogeneity of IgE. Springer Semin Immunopathol. 1990;12:415–428. doi: 10.1007/BF00225327. [DOI] [PubMed] [Google Scholar]
- 5.Kuna P, Reddigari SR, Rucinski D, Oppenheim JJ, Kaplan AP. Monocyte chemotactic and activating factor is a potent histamine-releasing factor for human basophils. J Exp Med. 1992;175:489–493. doi: 10.1084/jem.175.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischoff SC, Krieger M, Brunner T, Dahinden CA. Monocyte chemotactic protein 1 is a potent activator of human basophils. J Exp Med. 1992;175:1271–1275. doi: 10.1084/jem.175.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitschke M, Sohn K, Dieckmann D, Gibbs BF, Wolff HH, Amon U. Effects of basophil-priming and stimulating cytokines on histamine release from isolated human skin mast cells. Arch Dermatol Res. 1996;288:463–468. doi: 10.1007/BF02505236. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald SM, Rafnar T, Langdon J, Lichtenstein LM. Molecular identification of an IgE-dependent histamine releasing factor. Science. 1995;269:688–690. doi: 10.1126/science.7542803. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald SM, Bhisutthibhan J, Shapiro TA, et al. Immune mimicry in malaria: Plasmodium falciparum secretes a functional histamine-releasing factor homolog in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:10829–10832. doi: 10.1073/pnas.201191498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao KV, Chen L, Gnanasekar M, Ramaswamy K. Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J Biol Chem. 2002;277:31207–31213. doi: 10.1074/jbc.M204114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gnanasekar M, Rao KV, Chen L, et al. Molecular characterization of a calcium binding translationally controlled tumor protein homologue from the filarial parasites Brugia malayi and Wuchereria bancrofti. Mol Biochem Parasitol. 2002;121:107–118. doi: 10.1016/s0166-6851(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Zhang D, Fujise K. Characterization of fortilin, a novel antiapoptotic protein. J Biol Chem. 2001;276:47542–47549. doi: 10.1074/jbc.M108954200. [DOI] [PubMed] [Google Scholar]
- 13.Langdon JM, Vonakis BM, MacDonald SM. Identification of the interaction between the human recombinant histamine releasing factor/translationally controlled tumor protein and elongation factor-1 delta (also known as eElongation Factor-1B beta). Biochem Biophys Acta. 2004;1688:232–236. doi: 10.1016/j.bbadis.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Cans C, Passer BJ, Shalak V, et al. Translationally controlled tumor protein acts as a guanine nucleotide dissociation inhibitor on the translation elongation factor eEF1A. Proc Natl Acad Sci U S A. 2003;100:13892–13897. doi: 10.1073/pnas.2335950100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder JT, Lichtenstein LM, MacDonald SM. An immunoglobulin E-dependent recombinant histamine releasing factor induces IL-4 secretion from human basophils. J Exp Med. 1996;183:1265–1270. doi: 10.1084/jem.183.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroeder JT, Lichtenstein LM, MacDonald SM. Recombinant histamine-releasing factor enhances IgE-dependent IL-4 and IL-13 secretion by human basophils. J Immunol. 1997;159:447–452. [PubMed] [Google Scholar]
- 17.Bheekha-Escura R, Chance SR, Langdon JM, MacGlashan DW, Jr, MacDonald SM. Pharmacologic regulation of histamine release by the human recombinant histamine-releasing factor. J Allergy Clin Immunol. 1999;103:937–943. doi: 10.1016/s0091-6749(99)70442-2. [DOI] [PubMed] [Google Scholar]
- 18.Wantke F, MacGlashan DW, Jr, Langdon JM, MacDonald SM. The human recombinant histamine releasing factor rHRF: functional evidence that rHRF does not bind to the IgE molecule. J Allergy Clin Immunol. 1999;103:642–648. doi: 10.1016/s0091-6749(99)70237-x. [DOI] [PubMed] [Google Scholar]
- 19.Bheekha-Escura R, MacGlashan DW, Jr, Langdon JM, MacDonald SM. The human recombinant histamine releasing factor (HrHRF) activates human eosinophils and the eosinophilic-like cell line, AML 14-3D10. Blood. 2000;96:2191–2198. [PubMed] [Google Scholar]
- 20.Vonakis BM, Sora R, Langdon JM, Casolaro V, MacDonald SM. Inhibition of cytokine gene transcription by human recombinant histamine-releasing factor in human T lymphocytes. J Immunol. 2003;171:3742–3750. doi: 10.4049/jimmunol.171.7.3742. [DOI] [PubMed] [Google Scholar]
- 21.Kang HS, Lee MJ, Song H, et al. Molecular identification of IgE-dependent histamine-releasing factor as a B cell growth factor. J Immunol. 2001;166:6545–6554. doi: 10.4049/jimmunol.166.11.6545. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda K, Rokutan K, Nakamura Y, Yanagawa H, Kondo-Teshima S, Sone S. Stimulation of human bronchial epithelial cells by IgE-dependent histamine-releasing factor. Am J Physiol Lung Cell Mol Physiol. 2004;286:L174–L181. doi: 10.1152/ajplung.00118.2003. [DOI] [PubMed] [Google Scholar]
- 23.Haak-Frendscho M, Arai N, Arai K, Baeza ML, Finn A, Kaplan AP. Human recombinant granulocyte-macrophage colony-stimulating factor and interleukin 3 cause basophil histamine release. J Clin Invest. 1988;82:17–20. doi: 10.1172/JCI113567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald SM, Schleimer RP, Kagey-Sobotka A, Gillis S, Lichtenstein LM. Recombinant IL-3 induces histamine release from human basophils. J Immunol. 1989;142:3527–3532. [PubMed] [Google Scholar]
- 25.Redrup AC, Howard BP, MacGlashan DW, Jr, Kagey-Sobotka A, Lichtenstein LM, Schroeder JT. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol. 1998;160:1957–1964. [PubMed] [Google Scholar]
- 26.Sin AZ, Roche EM, Togias A, Lichtenstein LM, Schroeder JT. Nerve growth factor or IL-3 induces more IL-13 production from basophils of allergic subjects than from basophils of nonallergic subjects. J Allergy Clin Immunol. 2001;108:387–393. doi: 10.1067/mai.2001.117459. [DOI] [PubMed] [Google Scholar]
- 27.Ochensberger B, Daepp GC, Rihs S, Dahinden CA. Human blood basophils produce interleukin-13 in response to IgE-receptor-dependent and -independent activation. Blood. 1996;88:3028–3037. [PubMed] [Google Scholar]
- 28.Chen YH, Bieneman AP, Creticos PS, Chichester KL, Schroeder JT. IFN-alpha inhibits IL-3 priming of human basophil cytokine secretion but not leukotriene C4 and histamine release. J Allergy Clin Immunol. 2003;112:944–950. doi: 10.1016/j.jaci.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 29.Miura K, Saini SS, Gauvreau G, MacGlashan DW., Jr Differences in functional consequences and signal transduction induced by IL-3, IL-5, and nerve growth factor in human basophils. J Immunol. 2001;167:2282–2291. doi: 10.4049/jimmunol.167.4.2282. [DOI] [PubMed] [Google Scholar]
- 30.Vilariño N, Miura K, MacGlashan DW., Jr Acute IL-3 priming up-regulates the stimulus-induced Raf-1-Mek-Erk cascade independently of IL-3-induced activation of Erk. J Immunol. 2005;175:3006–3014. doi: 10.4049/jimmunol.175.5.3006. [DOI] [PubMed] [Google Scholar]
- 31.MacGlashan DW, Jr, Peters SP, Warner J, Lichtenstein LM. Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol. 1986;136:2231–2239. [PubMed] [Google Scholar]
- 32.Siraganian RP. Refinements in the automated fluorometric histamine analysis system. J Immunol Methods. 1975;7:283–290. doi: 10.1016/0022-1759(75)90025-3. [DOI] [PubMed] [Google Scholar]
- 33.Schleimer RP, MacGlashan DW, Jr, Gillespie E, Lichtenstein LM. Inhibition of basophil histamine release by anti-inflammatory steroids, II: studies on the mechanism of action. J Immunol. 1982;129:1632–1636. [PubMed] [Google Scholar]
- 34.Vonakis BM, Gibbons S, Sora R, Langdon JM, MacDonald SM. Src homology 2 domain-containing inositol 5′ phosphatase is negatively associated with histamine release to human recombinant histamine-releasing factor in human basophils. J Allergy Clin Immunol. 2001;108:822–831. doi: 10.1067/mai.2001.119159. [DOI] [PubMed] [Google Scholar]
- 35.Hayes EC, Lombardo DL, Girard Y, et al. Measuring leukotrienes of slow reacting substance of anaphylaxis: development of a specific radioimmunoassay. J Immunol. 1983;131:429–433. [PubMed] [Google Scholar]
- 36.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, et al. Down-regulation of human basophil IgE and FC epsilon RI alpha surface densities and mediator release by anti-IgE-infusions is reversible in vitro and in vivo. J Immunol. 1999;162:5624–5630. [PubMed] [Google Scholar]
- 37.MacGlashan DW, Jr, Gleich GJ, Thomas LL. Increases in cytosolic Ca2+ accompany basophil activation by eosinophil granule major basic protein. Immunol Lett. 1997;58:37–42. doi: 10.1016/s0165-2478(97)02710-7. [DOI] [PubMed] [Google Scholar]
- 38.MacGlashan DW, Jr, Vilariño N. Nonspecific desensitization, functional memory and the characteristics of SHIP phosphorylation following IgE-mediated stimulation of human basophils. J Immunol. 2006;177:1040–1051. doi: 10.4049/jimmunol.177.2.1040. [DOI] [PubMed] [Google Scholar]
- 39.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 40.Warner JA, MacGlashan DW., Jr Signal transduction events in human basophils. A comparative study of the role of protein kinase C in basophils activated by anti-IgE antibody and formyl-methionyl-leucyl-phenylalanine. J Immunol. 1990;145:1897–1905. [PubMed] [Google Scholar]
- 41.MacGlashan DW, Jr, Botana LM. Biphasic Ca2+ responses in human basophils. Evidence that the initial transient elevation associated with the mobilization of intracellular calcium is an insufficient signal for degranulation. J Immunol. 1993;150:980–991. [PubMed] [Google Scholar]
- 42.MacGlashan DW., Jr Desensitization of IgE-mediated IL-4 release from human basophils. J Leuk Biol. 1998;63:59–67. doi: 10.1002/jlb.63.1.59. [DOI] [PubMed] [Google Scholar]
- 43.MacGlashan DW, Jr, Hubbard WC. Interleukin-3 alters free arachidonic acid generation in C5a-stimulated human basophils. J Immunol. 1993;151:6358–6369. [PubMed] [Google Scholar]
- 44.Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 45.Miura K, Lavens-Phillips S, MacGlashan DW., Jr Localizing a control region in the pathway to leukotriene C(4) secretion following stimulation of human basophils with anti-IgE antibody. J Immunol. 2001;167:7027–7037. doi: 10.4049/jimmunol.167.12.7027. [DOI] [PubMed] [Google Scholar]
- 46.Miura K, Schroeder JT, Hubbard WC, MacGlashan DW., Jr Extracellular signal-regulated kinases regulate leukotriene C4 generation, but not histamine release or IL-4 production from human basophils. J Immunol. 1999;162:4198–4206. [PubMed] [Google Scholar]
- 47.Miura K, Hubbard WC, MacGlashan DW., Jr Phosphorylation of cytosolic phospholipase A2 by IL-3 is associated with increased free arachidonic acid generation and leukotriene C4 release in human basophils. J Allergy Clin Immunol. 1998;102:512–520. doi: 10.1016/s0091-6749(98)70142-3. [DOI] [PubMed] [Google Scholar]
- 48.Tomasello E, Cant C, Bühring HJ, et al. Association of signal-regulatory proteins beta with KARAP/DAP-12. Eur J Immunol. 2000;30:2147–2156. doi: 10.1002/1521-4141(2000)30:8<2147::AID-IMMU2147>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]