Abstract
Numerous studies have reported the presence of oxidatively modified high-density lipoprotein (OxHDL) within the intima of atheromatous plaques as well as in plasma; however, its role in the pathogenesis of thrombotic disease is not established. We now report that OxHDL, but not native HDL, is a potent inhibitor of platelet activation and aggregation induced by physiologic agonists. This antithrombotic effect was concentration and time dependent and positively correlated with the degree of lipoprotein oxidation. Oxidized lipoproteins are known ligands for scavenger receptors type B, CD36 and scavenger receptor B type I (SR-BI), both of which are expressed on platelets. Studies using murine CD36−/− or SR-BI−/− platelets demonstrated that the antithrombotic activity of OxHDL depends on platelet SR-BI but not CD36. Binding to SR-BI was required since preincubation of human and murine platelets with anti–SR-BI blocking antibody abrogated the inhibitory effect of OxHDL. Agonist-induced aggregation of platelets from endothelial nitric oxide synthase (eNOS)−/−, Akt-1−/−, and Akt-2−/− mice was inhibited by OxHDL to the same degree as platelets from wild-type (WT) mice, indicating that the OxHDL effect is mediated by a pathway different from the eNOS/Akt pathway. These novel findings suggest that contrary to the prothrombotic activity of oxidized low-density lipoprotein (OxLDL), HDL upon oxidation acquires antithrombotic activity that depends on platelet SR-BI.
Introduction
The protective role of high-density lipoprotein (HDL) in coronary heart disease is well established and attributed to the ability of HDL to promote reverse cholesterol transport as well as its antioxidant, anti-inflammatory, and antithrombotic activities.1–6 One potential mechanism for the antithrombotic activity of HDL is via a direct effect on platelets. However, conflicting results on the effect of HDL on platelet activation and aggregation have been reported. Most work to date has supported an inhibitory effect of HDL on platelet activation, while a few have reported increased activation or lack of effect.7–14
Hyperlipidemia is associated with oxidative stress, generation of oxidized lipoproteins, and increased risk for thrombosis.15 Activation of platelets by oxidized low-density lipoprotein (OxLDL) and specific oxidized phospholipids via platelet scavenger receptor CD36 contributes significantly to the prothrombotic phenotype associated with dyslipoproteinemia.16 HDL is at least as susceptible to oxidation as LDL both in vivo and in vitro due to the depletion of antioxidants and decreased paraoxonase-1 (PON1) activity in HDL during hyperlipidemia.17–22 Evidences for the presence of OxHDL in vivo are abundant. Lipids isolated from HDL and LDL found in atherosclerotic lesions have been reported to be oxidized to a comparable extent, and increases with increasing severity of disease.17,23–25 Apo A-I in HDL recovered from atherosclerotic lesions showed significant oxidative modifications.17 Studies of animals and humans have indicated that OxHDL is also detected in plasma.18,19,23
Scavenger receptor B type I (SR-BI), the principal HDL receptor, is expressed on the surface of various cell types, including hepatocytes and endothelial cells, and contributes to the antiatherogenic properties of HDL.4,26–33 SR-BI, a multiligand transmembrane protein of the scavenger receptor CD36 superfamily, binds to a variety of ligands, including native and oxidized lipoproteins.29,34 Binding of HDL to SR-BI on endothelial cells induces the production of nitric oxide (NO) by up-regulating endothelial NO synthase (eNOS) expression through a kinase cascade and has a protective effect on hypercholesterolemia-induced vascular disease.34–37
Recently, SR-BI has been shown to be expressed on human platelets, and reduced levels of SR-BI were associated with increased platelet aggregation, suggesting a role in platelet activation.38 We hypothesized that HDL can control platelet activation via platelet SR-BI. Using both human and mouse platelets, here we demonstrate that OxHDL, but not native HDL, is a strong inhibitor of agonist-induced platelet activation and aggregation. By using platelets from SR-BI−/− and CD36−/− mice and including anti–SR-BI blocking antibody in aggregation assays, we demonstrate that the effect of OxHDL on platelets is mediated mainly by platelet SR-BI. The HDL/SR-BI/Akt/eNOS pathway that functions in endothelial cells was ruled out as a mediator of the OxHDL effect in platelets. This surprising finding suggests that contrary to LDL, HDL upon oxidation acquires antithrombotic function mediated by SR-BI.
Methods
Materials
Human α-thrombin was purchased from Enzyme Research Laboratories (South Bend, IN); phorbol-12-myristate-13-acetate (PMA) was purchased from Calbiochem (San Diego, CA); apyrase grade 1, prostaglandin E1 (PGE1), sepharose 2B, BSA, NaBr, diethylenetriamine penta acetic acid (DTPA), and monoclonal antibody against β-actin were from Sigma-Aldrich (St Louis, MO); chrono-lume luciferin-luciferase reagent to measure ATP release, collagen type I (equine), and ADP were purchased from Chrono-log (Havertown, PA); FITC-conjugated anti–P-selectin antibody (FITC-CD62P) and FITC-conjugated anti-αIIbβ3 antibody (FITC-PAC1) were purchased from Becton Dickinson (San Jose, CA); antibodies against SR-BI were from Novus Biologicals (Littleton, CO); isotype matched nonimmune antibody from Jackson ImmunoResearch (West Grove, PA); Na[125I] was from ICN Pharmaceutical (Costa Mesa, CA); and 1-hexadecanoyl-2-eicosatetra-5′,8′,11′,14′-enoyl-sn-glycero-3-phosphocholine (PAPC) was from Avanti Polar Lipids (Alabaster, AL).
Animals
Wild-type (WT), SR-BI−/−, Akt-1−/−, Akt-2−/−, and eNOS−/− mice were purchased from Jackson Laboratories (Bar Harbor, ME). CD36−/− animals were generated as described.39 All strains were at least 99% C57Bl/6 background. Animals were housed in ventilated cages with ad libitum access to food and water, on a 14:10 light-dark cycle. All animal procedures were approved prior to study by the Institutional Animal Care and Use Committee of the Cleveland Clinic. WT and knockout mice were used at 10 to 14 weeks of age.
Preparation of platelets
All experiments done with human blood were approved by the Institutional Review Board of the Cleveland Clinic. Informed consent was obtained in accordance with the Declaration of Helsinki. Human venous blood was drawn from healthy donors into acid-citrate-dextrose (ACD; 85 mM tri-sodium citrate, 65 mM citric acid, and 111 mM D-glucose [pH 4.6]) solution containing PGE1 (1 μg/mL of ACD) and apyrase (0.02 U/mL). Platelet-rich plasma (PRP) was separated by centrifugation at 100g for 20 minutes at 22°C. Platelets were pelleted from PRP by centrifugation at 800g for 15 minutes at 22°C, resuspended in Tyrode buffer (137 mM NaCl, 12 mM NaHCO3, and 2.5 mM KCl [pH 7.2]) containing 0.35% BSA and 0.1% glucose, and further purified by gel filtration on a sepharose 2B column. The concentration of platelets was adjusted accordingly with Tyrode buffer containing 0.35% BSA, 0.1% glucose, 2 mM CaCl2, and 1 mM MgCl2. For the preparation of mouse platelets, 600 μL of blood was drawn from the abdominal vein of WT or knockout mice into a syringe containing ACD and PGE1. PRP was separated by centrifugation at 100g for 10 minutes at 22°C, followed by pelleting the platelets at 625g for 10 minutes. The gel-filtered platelets were prepared from a pool of 3 to 4 mice as described.
Platelet aggregation and ATP release
Platelet aggregation was monitored by using a Lumi-Aggregometer type 500 VS (Chrono-log). Thrombin (0.1 U/mL), collagen (5 μg/mL), PMA (100 nM), or adenosine diphosphate (ADP; 10 μM) were used as agonists. Adenosine triphosphate (ATP) release from platelets was measured after the addition of luciferin-luciferase reagent (Chrono-log) and 0.1 U/mL thrombin according to the manufacturer's protocol.
Preparation of lipoproteins
Lipoproteins were isolated from human or mouse plasma by ultracentrifugation of discontinuous NaBr gradients as described by Havel et al.40 Lipoprotein fractions were dialyzed extensively against 0.9% NaCl containing 0.02% EDTA at 4°C. Immediately before use in aggregation assays with platelets, lipoproteins were dialyzed against 0.9% NaCl. The protein content in each lipoprotein fraction was estimated by BCA protein assay kit from Pierce Biotechnology (Rockford, IL). The purity of lipoprotein preparation was assessed by SDS-PAGE analysis using 4% to 20% gradient gels (Bio-Rad Laboratories, Hercules, CA) and stained with Bio-Safe Coomassie (Bio-Rad Laboratories). Oxidative modification of human and mouse HDL was performed by dialysis at lipoprotein concentration of 0.8 mg protein/mL against PBS containing 5 μM CuSO4 for 24 hours at 37°C or by myeloperoxidase (MPO)–mediated oxidation as described previously.41,42
Flow cytometry
Platelets isolated by gel filtration (2.5 × 106) in Tyrode buffer containing 2 mM Ca2+ and 1 mM Mg2+ were incubated with lipoproteins for 5 minutes at room temperature followed by stimulation with thrombin (0.1 U/mL). The activated platelets were incubated with either FITC-conjugated anti–P-selectin antibody (1:1000) or FITC-conjugated anti-αIIbβ3 antibody (1:100) for 15 minutes. Data for 20 000 FITC-positive cells were acquired using a FACSCalibur instrument (Becton Dickinson, San Jose, CA) and analyzed by the FlowJo software program (Tree Star, Ashland, OR). For the surface expression analysis of SR-BI, human platelets isolated by gel filtration (3 × 107) in Tyrode buffer containing 2 mM Ca2+ and 1mM Mg2+ were incubated with 0.1 U/mL thrombin for 5 minutes at room temperature. Both quiescent and activated platelets were fixed in 2% formaldehyde for 10 minutes, washed with Tyrode buffer, and incubated with anti–SR-BI antibody (1:100) for 1 hour. Platelets were washed and incubated with FITC-conjugated anti-rabbit secondary antibody (1:100) for 30 minutes and washed twice with Tyrode buffer; the surface expression of SR-BI was analyzed by flow cytometry as described.
Western blotting
Platelets from WT and SR-BI−/− mice were lysed in cell lysis buffer and mixed with equal volume SDS-PAGE sample buffer. Equal amounts of protein (50 μg) from both WT and SR-BI−/− platelets were separated on a 10% gel and transferred to PVDF membranes. Blots were blocked and probed with anti–SR-BI antibody (1:1000) for 1 hour, followed by incubation with peroxidase-labeled secondary antibody and developed with enhanced chemiluminescence.
Binding of [125I]HDL to SR-BI–overexpressing HEK-293T cells
HDL isolated from fresh plasma by sequential ultracentrifugation was iodinated with Na[125I], and the resulting [125I]HDL was oxidized by either MPO/H2O2/NO2 system or Cu2+ to obtain [125I]NO2-HDL and [125I]Cu2+-HDL as described previously.40–42 Full-length human/mouse SR-BI was polymerase chain reaction (PCR)–amplified from liver Marathone-Ready cDNA (BD Biosciences, Rockville, MD) and cloned into pEF6V5-His vector (Invitrogen, Carlsbad, CA). HEK-293T cells were stably transfected using Lipofectamine 2000 (Invitrogen) and blasticidine as a resistance marker. Clones overexpressing SR-BI were selected based on Western blot for SR-BI. Cells transfected with vector alone served as a control. Confluent cell monolayers were washed with serum-free media and incubated with 5 μg/mL of [125I]HDL, [125I]Cu2+-HDL, and [125I]NO2-HDL in 250 μL of media for 3 hours at 37°C. Lipoprotein binding to SR-BI–overexpressing cells and control vector cells was determined by measuring the bound radioactivity to cells as reported previously.41,42
Platelet-lipoprotein binding assay
Human platelets isolated by gel filtration (108) were incubated with 5 μg/mL of [125I] lipoproteins for 30 minutes at room temperature in Tyrode buffer containing 2 mM Ca2+ in the presence or absence of competitors. Unbound [125I] lipoprotein was separated from platelets by centrifugation of the platelet suspension through a 20% sucrose cushion, and the bound radioactivity was quantified. Final concentrations of competitors were 200 μg/mL for lipoproteins, 20 μg/mL for antibodies, and 40 μg/mL for unilamellar phospholipid vesicles.
Statistical analysis
Values are expressed as means plus or minus SEM or SD as indicated in the figure legends. The statistical significance was evaluated using a 2-sample t test. Results were considered statistically significant with P values less than .05.
Results
OxHDL but not native HDL inhibits agonist-induced platelet aggregation
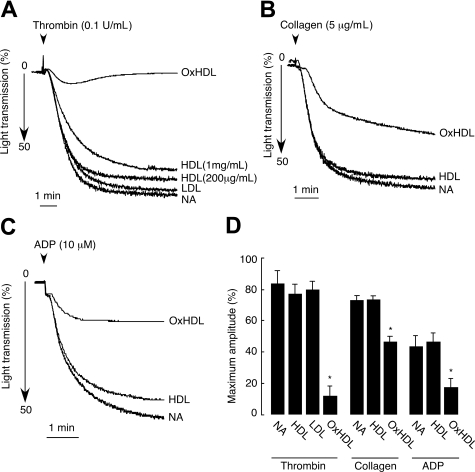
We first tested the effect of native HDL on platelet aggregation induced by thrombin by preincubating isolated human platelets with human native HDL. Extreme care was taken to prevent potential oxidation of HDL by including DTPA during HDL isolation, protecting against light, overlaying isolated HDL with argon, and finally, by using only fresh preparations of HDL in the aggregation assays. Native HDL did not have significant effects on thrombin-mediated platelet aggregation at concentrations from 200 μg/mL to 1 mg/mL (Figure 1A,D). In contrast, short 2-minute preincubation of platelets with 200 μg/mL of OxHDL resulted in approximately 90% inhibition of thrombin-induced platelet aggregation (Figure 1A,D). The inhibitory effect was not dependent on the method of HDL oxidation, since both HDL oxidized by Cu2+ and MPO/H2O2/NO2 systems showed similar inhibitory activity in platelet aggregation assays. Native LDL served as an additional control and at a concentration of 200 μg/mL had no significant effect on thrombin-induced platelet aggregation (Figure 1A,D). To demonstrate that the inhibition of platelet aggregation by OxHDL is a general phenomenon and not restricted only to thrombin as agonist, we tested the effect of OxHDL on platelet aggregation induced by other physiologically relevant agonists. The inhibition of platelet aggregation by OxHDL was found to be universal. OxHDL significantly inhibited platelet aggregation induced by both fibrillar collagen (approximatey 40% inhibition) and by ADP (approximately 55% inhibition; Figure 1B-D). At the same time, native HDL had no effect on ADP as well as on collagen-induced platelet aggregation. Thus, it appears that OxHDL inhibits platelet aggregation via a pathway that interferes with multiple physiologic agonists and multiple platelet activation pathways.
Figure 1.
Inhibition of platelet aggregation by OxHDL in response to thrombin, collagen, and ADP. (A) Human platelets isolated by gel filtration (2.5 × 108/mL) containing 2 mM Ca2+ and 1 mM Mg2+ were incubated with 200 μg/mL HDL, OxHDL, or LDL and 1 mg/mL of HDL for 2 minutes at 37°C, followed by stimulation with 0.1 U/mL thrombin. Platelet aggregation was monitored in a Lumi-Aggregometer. (B) Platelets were incubated with 200 μg/mL HDL or OxHDL for 5 minutes at 37°C and stimulated with 5 μg/mL collagen type I. (C) Platelets in the presence of 300 nM of human fibrinogen were incubated with 200 μg/mL HDL or OxHDL for 5 minutes at 37°C and stimulated with 10 μM of ADP. (D) Quantification of the data (means ± SD) panels A-C from 5 independent experiments. NA indicates no addition. *P < .001.
OxHDL inhibits agonist-induced platelet aggregation in a concentration- and time-dependent manner
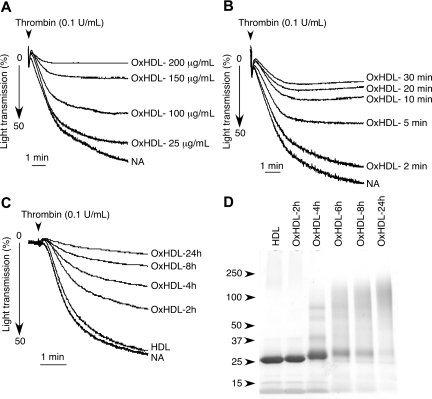
To further characterize the effect of OxHDL on platelets, platelet aggregation assays were carried out using various concentrations of OxHDL or by increasing preincubation times with OxHDL. Inhibition of thrombin-induced platelet aggregation by OxHDL was concentration dependent; complete inhibition was achieved at 200 μg/mL (Figure 2A). In experiments where washed platelets were incubated with low concentrations of OxHDL (25 μg/mL) for increasing periods of time, we found that the inhibitory effect increased with increasing time, and complete inhibition was achieved after 30 minutes of preincubation (Figure 2B). To determine whether the inhibitory activity of OxHDL depends on the degree of oxidation, we oxidized HDL for an increasing time by Cu2+ and tested in platelet aggregation assays (Figure 2C,D). Upon oxidation, HDL apoproteins formed a characteristic ladder consisting of apo A-II and apo A-I homo- and hetero-multimers that were apparent by SDS-PAGE, as described previously (Figure 2D).18 Interestingly, even HDL oxidized for a short period of time (HDL oxidized for 2 hours [OxHDL-2h]) that did not show significant signs of oxidative damage by SDS-PAGE (Figure 2D) showed very significant inhibition (approximately 40%) of thrombin-induced platelet aggregation (Figure 2C). The inhibitory capacity of OxHDL continued to increase with continued oxidation (up to 24 hours; Figure 2C). Taken together, these data demonstrate that even at a low degree of oxidation, OxHDL becomes a potent inhibitor of platelet aggregation.
Figure 2.
Concentration and time dependence of OxHDL-mediated inhibition of platelet aggregation. (A) Human platelets were incubated with the indicated concentrations of OxHDL for 2 minutes at 37°C, stimulated with 0.1 U/mL thrombin, and platelet aggregation was optically monitored. (B) Platelets were incubated with 25 μg/mL OxHDL for the indicated time at 37°C, and thrombin-stimulated platelet aggregation was monitored. (C) HDL was oxidized by Cu2+ for the indicated time and 200 μg/mL of Cu2+-OxHDL was incubated with washed platelets followed by stimulation with 0.1 U/mL thrombin. (D) SDS-PAGE analysis of HDL oxidized by Cu2+ at different time points. NA indicates no addition.
The effect of OxHDL on P-selectin expression, integrin αIIbβ3 activation, and ATP release
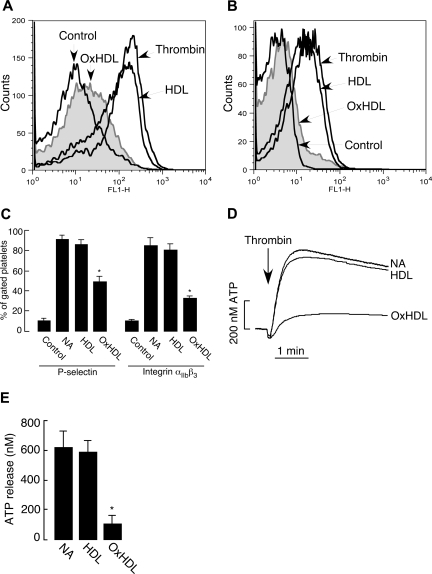
Platelet activation is characterized by a series of events that includes conformational change in the integrin αIIbβ3, enabling fibrinogen binding and ultimately platelet aggregation. A critical event in platelet activation is secretion of granular contents, a process that serves multiple purposes including amplification of the activation event via release of additional agonists and various adhesive proteins. Granular secretion also results in exposure of activation-dependent glycoproteins, such as P-selectin, on the platelet surface. We tested whether OxHDL suppresses platelet aggregation by inhibiting these critical platelet activation processes. Induction of P-selectin expression in response to thrombin was significantly inhibited by OxHDL (Figure 3A,C). αIIbβ3 conformational change in response to thrombin was also significantly inhibited by OxHDL (Figure 3B,C). As a measure of platelet-dense granule secretion, we assessed ATP release during platelet activation. There was significant inhibition in thrombin-induced release of ATP when the platelets were preincubated with OxHDL (Figure 3D,E). Native HDL had no significant effect on any of these parameters (Figure 3). Similar effects of OxHDL were observed on αIIbβ3 activation, and platelet granule release when other physiologic agonists were used (data not shown). Collectively, these data demonstrate that OxHDL inhibits agonist-induced platelet aggregation by a mechanism that prevents αIIbβ3 activation and granule release.
Figure 3.
Inhibition of P-selectin expression, integrin αIIbβ3 activation, and ATP release by OxHDL. (A,B) Human platelets isolated by gel filtration (2.5 × 106) in buffer containing 2 mM Ca2+ and 1 mM Mg2+ were incubated with or without 200 μg/mL HDL or OxHDL for 5 minutes at room temperature, followed by stimulation with 0.1 U/mL thrombin. Samples were incubated either with 1:1000 FITC-conjugated anti–P-selectin antibody (A) or with 1:100 FITC-conjugated anti-αIIbβ3 antibody (B) for 15 minutes and analyzed by flow cytometry. (C) Quantification of the data (means ± SEM) as in panels A and B from 5 independent experiments. (D) ATP release was measured in human platelets containing luciferin-luciferase reagent incubated with or without 200 μg/mL HDL or OxHDL for 5 minutes, followed by stimulation with 0.1 U/mL thrombin. (E) Quantification of the data (means ± SD) in panel D from 3 independent experiments. NA indicates no addition. *P < .001.
The inhibitory effect of OxHDL is mediated by platelet SR-BI
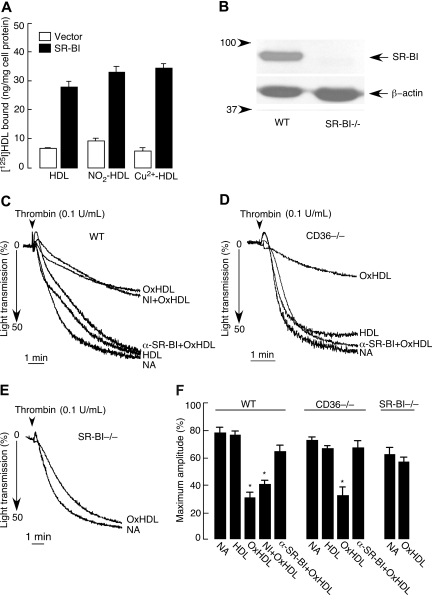
Oxidized lipoproteins are known to serve as ligands for scavenger receptors type B, CD36 and SR-BI. CD36 is a major platelet glycoprotein and was reported to recognize OxHDL.43 SR-BI has recently also been reported to be expressed on the surface of human platelets38; however, its presence on murine platelets and its ability to recognize OxHDL were unknown. Accordingly, we tested whether these scavenger receptors were involved in the inhibitory effect of OxHDL. We first assessed whether OxHDL interacts with SR-BI by using [125I]-labeled OxHDL and SR-BI–overexpressing 293T cells. [125I]-HDL showed significantly increased binding to SR-BI–overexpressing 293T cells compared with control, vector-transfected cells, as anticipated (Figure 4A). As shown in Figure 4A, [125I]-OxHDL, oxidized by various methods, bound to SR-BI–overexpressing cells at least as well as native HDL. Western blot analysis of platelets isolated from WT mice showed abundant expression of SR-BI, and this was absent in platelets isolated from SR-BI−/− mice (Figure 4B). Gel-filtered platelets were prepared from WT, CD36−/−, and SR-BI−/− mice, and the effect of oxidized murine HDL on thrombin-induced platelet aggregation was assessed. OxHDL inhibited thrombin-induced aggregation of WT platelets, whereas native HDL had no effect (Figure 4C,F). Likewise, preincubation of CD36−/− platelets with OxHDL resulted in almost complete inhibition of platelet aggregation similar to WT platelets, ruling out a significant contribution of this receptor to the observed inhibitory effect (Figure 4D,F). In contrast, OxHDL failed to significantly inhibit thrombin-induced aggregation in platelets from SR-BI−/− mice (Figure 4E,F). Similar effects were observed using collagen and ADP as agonists (data not shown). Moreover, preincubation of WT or CD36−/− platelets with anti–SR-BI blocking antibody (but not with isotype-matched nonimmune antibody) prior to the addition of OxHDL, abrogated the inhibitory effect of OxHDL (Figure 4C,D,F). These experiments clearly demonstrate that the inhibitory effect of OxHDL on murine platelet aggregation is mediated mainly by SR-BI.
Figure 4.
Inhibition of platelet aggregation by OxHDL depends on platelet SR-BI. (A) Confluent monolayers of SR-BI–overexpressing or control, vector-transfected cells were incubated with 5 μg/mL [125I]-HDL, [125I]-NO2HDL, or [125I]-Cu2+-HDL for 3 hours at 37°C, and bound radioactivity was quantified. Data shown are means plus or minus SEM from 3 independent experiments. (B) Immunoblot analysis of SR-BI protein in platelets isolated from WT and SR-BI−/− mice. β-actin was used as a loading control, and the molecular weight markers are shown on the left side of the panel. (C) Murine platelets isolated by gel filtration were preincubated with or without anti–SR-BI blocking antibody (20 μg/mL) or nonimmune isotype control antibody for 5 minutes, followed by incubation with 200 μg/mL murine OxHDL or native HDL for 5 minutes at 37°C and stimulation with 0.1 U/mL thrombin. (D) Platelets prepared from CD36−/− mice were preincubated with or without anti–SR-BI blocking antibody for 5 minutes, followed by incubation with 200 μg/mL murine OxHDL or native HDL for 5 minutes at 37°C and stimulation with 0.1 U/mL thrombin. (E) Platelets isolated from SR-BI−/− mice were incubated with or without 200 μg/mL murine OxHDL for 5 minutes at 37°C, followed by stimulation with 0.1 U/mL thrombin; platelet aggregation was optically monitored. (F) Data from panels C-E are presented as means plus or minus SEM of 3 to 5 independent experiments. NA indicates no addition; α-SR-BI, anti–SR-BI blocking antibody; and NI, nonimmune antibody. *P < .001.
SR-BI mediates inhibition of platelet aggregation by OxHDL in human platelets
We next tested the role of SR-BI in inhibition of human platelet aggregation by OxHDL. Preincubation of human platelets with anti–SR-BI blocking antibody or isotype-matched nonimmune antibody did not show any significant effect on thrombin-induced platelet aggregation (Figure 5A,B). However, preincubation of human platelets with anti–SR-BI blocking antibody (but not isotype control) significantly reduced the inhibitory effect of OxHDL on thrombin-induced platelet aggregation (Figure 5A,B), as well as the inhibitory effect of OxHDL on platelet aggregation induced by other agonists (Figure 5C,D). Taken together, these experiments demonstrate that the inhibitory effect of OxHDL on platelets is mediated primarily by OxHDL binding to SR-BI.
Figure 5.
OxHDL inhibits human platelet aggregation via SR-BI. (A) Human platelets isolated by gel filtration were preincubated with or without anti–SR-BI blocking antibody or nonimmune isotype control antibody for 5 minutes, followed by incubation with 200 μg/mL OxHDL for 5 minutes at 37°C and stimulation with 0.1 U/mL thrombin. (B) Data from panel A are presented as means plus or minus SD of 5 independent experiments. (C) Platelet aggregation was performed as in panel A, but platelets were stimulated with 5 μg/mL collagen type I. (D) Platelet aggregation was performed as in panel A, but platelets were stimulated with 10 μM ADP in the presence of 300 nM human fibrinogen. (E) Human platelets isolated by gel filtration were incubated with 5 μg/mL [125I] lipoproteins for 30 minutes at room temperature in the presence or absence of indicated competitors, and platelet-bound radioactivity was quantified. Final concentrations of competitors were 200 μg/mL for lipoproteins, 40 μg/mL for phospholipids, and 20 μg/mL for antibodies. Data shown are means plus or minus SD from 3 independent experiments. (F) Human platelets isolated by gel filtration were activated by thrombin as described in “Materials and methods.” Activated and resting platelets were fixed with 2% formaldehyde, and SR-BI expression was analyzed by flow cytometry (left panel). Quantification of the data (means ± SD) from 3 independent experiments is shown in right panel. NA indicates no addition; α-SR-BI, anti–SR-BI blocking antibody; NI, nonimmune antibody; PAPC, unilamellar vesicles containing unoxidized phospholipids; and OxPAPC, unilamellar vesicles containing oxidized phospholipids. *P < .005; **P < .001.
SR-BI is the major receptor on platelets for OxHDL
We next characterized interaction of platelets with OxHDL. Platelets isolated from human blood bound significant amounts of both [125I]-HDL and [125I]-OxHDL, the latter showing slightly better binding than the former (Figure 5E). Binding of [125I]-OxHDL to platelets was completely inhibited by excess of unlabeled OxHDL, while excess of unlabeled HDL was less effective inhibitor. Preincubation of platelets with anti–SR-BI blocking antibody abrogated the binding, whereas nonimmune antibody did not show any effect (Figure 5E), indicating that SR-BI is the major receptor on platelets for OxHDL. Oxidized phospholipids in oxidized lipoproteins represent a potent ligand for scavenger receptors type B. We next tested the effect of oxidized phospholipids on the binding. Platelets were preincubated with PAPC phospholipid vesicles or PAPC vesicles that were oxidized by reactive nitrogen species as described before.16 Preincubation of platelets with OxPAPC but not with PAPC significantly inhibited binding of OxHDL to platelets (Figure 5E). These results demonstrate that oxidized lipids present in OxHDL may represent at least one ligand responsible for the binding to platelet SR-BI. These results suggest that interaction of OxHDL and platelet SR-BI may involve oxidized phospholipid moiety. Thus, both HDL and OxHDL bind to SR-BI (Figure 4A) and to platelets (Figure 5E), but only OxHDL inhibits platelet activation, suggesting that inhibitory activity may be due to the presence of oxidized lipids such as oxidized phospholipids in OxHDL.
Platelet surface expression of SR-BI is not activation dependent
To test whether SR-BI is constitutively expressed on the surface of platelets, we performed flow cytometric analysis of platelet SR-BI expression in both quiescent and activated platelets using anti–SR-BI antibody, which recognizes the extracellular domain of SR-BI. Surface expression of SR-BI was similar in resting and activated platelets (Figure 5F). Similar results were obtained when analysis of SR-BI expression was performed using confocal fluorescent microscopy (data not shown).
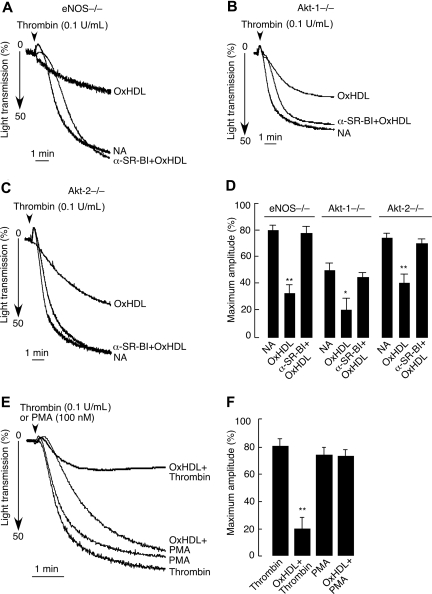
The eNOS/Akt pathway is not involved in SR-BI–dependent inhibition of platelet aggregation by OxHDL
Several studies implicated eNOS and its upstream activator Akt kinase in SR-BI mediated effects of HDL.34–37,44,45 Accordingly, we assessed possible roles for eNOS, Akt-1, and Akt-2 in SR-BI–mediated inhibition of platelet aggregation by OxHDL. To this end, we used platelets from corresponding knockout mice (ie, eNOS−/−, Akt-1−/−, and Akt-2−/−) in aggregation assays. In these 3 knockout models tested, OxHDL was able to inhibit thrombin-induced platelet aggregation to an extent similar to what we observed in WT platelets (Figure 6A-D; compare with Figure 4C,F). The inhibitory capacity of OxHDL was abrogated by preincubation of eNOS−/−, Akt-1−/−, and Akt-2−/− platelets with anti–SR-BI blocking antibody, confirming the specificity of the effect (Figure 6A-D). These results demonstrate that although the eNOS/Akt signaling pathway is known to be downstream of SR-BI, it is not involved in SR-BI–mediated inhibition of platelet aggregation by OxHDL. As shown in Figures 6E and 6F, OxHDL was not able to inhibit platelet aggregation induced by the model agonist PMA, which is known to activate protein kinase C (PKC). Moreover, treatment with PMA was able to reverse the inhibitory effect of OxHDL preincubation on platelet aggregation induced by thrombin (data not shown). These results demonstrate that OxHDL interferes with agonist-induced signaling pathways at the level of PKC (or upstream of PKC) and is independent of Akt/eNOS.
Figure 6.
Inhibition of platelet aggregation by OxHDL in eNOS−/−, Akt-1−/−, and Akt-2−/− platelets. Platelets prepared from eNOS−/− (A), Akt-1−/− (B), or Akt-2−/− (C) mice by gel filtration were preincubated with or without SR-BI blocking antibody, followed by incubation with 200 μg/mL murine OxHDL for 5 minutes at 37°C and stimulation with 0.1 U/mL thrombin. Platelet aggregation was monitored. (D) Data from panels A-C are presented as means plus or minus SD of 3 independent experiments. (E) Human platelets isolated by gel filtration were incubated with 200 μg/mL human OxHDL for 5 minutes at 37°C and stimulated with either 0.1 U/mL thrombin or 100 nM PMA. Platelet aggregation was monitored. (F) Data from panel E are presented as means plus or minus SD of 3 independent experiments. NA indicates no addition; α-SR-BI, anti–SR-BI blocking antibody. *P < .005; **P < .001.
Discussion
A variety of antithrombotic mechanisms protective against arterial and venous thrombosis have been proposed in the literature for HDL. These include stimulation of NO production in endothelial cells, activation of prostacyclin synthesis, attenuation of the expression of tissue factor and selectins, down-regulation of thrombin generation via the PKC pathway, and direct inhibition of platelet activation.4,46,47 Cardioprotective functions of HDL are presumed to be lost upon oxidative damage.17,18,21,22,48 Here, we report the surprising finding that HDL, upon oxidative modification, acquires a potent antithrombotic activity. Using several approaches, we clearly demonstrate that in both human and murine platelets, this effect requires platelet scavenger receptor SR-BI. Using several knockout models, we further demonstrate that inhibition of agonist-induced platelet aggregation mediated by OxHDL is not through the eNOS/Akt pathway, which mediates cardioprotective effects of HDL in endothelial cells.
The modulation of platelet functions by lipoproteins has been investigated intensively. Numerous studies have demonstrated interaction of platelets with LDL and the platelet-activating property of OxLDL.14,49–55 We recently found that a novel family of specific oxidized phospholipids in OxLDL (oxPCCD36) serve as high-affinity ligands for CD36 and mediate the effects of OxLDL on platelets.16 oxPCCD36 accumulates in the plasma of hyperlipidemic mice and in humans with low HDL levels and promote a prothrombotic state via platelet scavenger receptor CD36.16 However, the effect of HDL on platelet aggregation is less clear. Different studies reported either no effect, activation, or inhibition for HDL on agonist-induced platelet aggregation.7–14 Some of the discrepancies could be attributed to HDL subclass specificity.9,11,12 In this study, we used native HDL, HDL2, and HDL3, and found that none of them had any substantial effect on agonist-induced platelet aggregation (Figure 1; data shown only for HDL), even at supraphysiologic concentrations (> 1 mg/mL). An explanation for the inconsistent results may be inadvertent oxidation of HDL during isolation. Interestingly, the inhibitory effect of auto-oxidized HDL3 on the ADP-induced aggregation of platelets has been described earlier,56 but the detailed mechanism of the effect and the platelet receptors mediating these effects were not elucidated. In our study, we took extra care to prevent oxidation of HDL during isolation and used only fresh preparations in aggregation assays. Contrary to native HDL, we observed a potent inhibition of platelet aggregation by OxHDL, even at concentrations as low as 25 μg/mL. This inhibitory effect appears to be a general phenomenon for all physiologically relevant agonists tested, including thrombin, collagen, and ADP. The inhibitory effect was concentration and time dependent and correlated with the extent of HDL oxidation, indicating its specificity. Importantly, even minimally oxidized HDL exerted a potent inhibitory effect (Figure 2C,D) supporting a potential physiologic significance for the observed phenomenon. Even though both native and OxHDL bind to SR-BI and to platelets (Figures 4A, 5E), only the binding of OxHDL to SR-BI resulted in inhibition of agonist-induced platelet activation. Oxidation of lipoproteins results in accumulation of biologically active lipids in lipoproteins. One explanation of our results is that inhibitory activity of OxHDL may be due to the presence of biologically active oxidized lipids such as oxidized phospholipids in OxHDL.
SR-BI is a member of the CD36 superfamily of proteins. It shares 30% sequence homology with CD36, and both proteins are structurally comprised of 2 transmembrane and 2 cytoplasmic domains, as well as a large extracellular domain containing conserved sites for N-linked glycosylation.26–28 Although both these receptors bind multiple common ligands, including HDL, LDL, OxLDL, and anionic phospholipids, they have different lipid-related functions. CD36 facilitates the intracellular uptake of oxidized lipoproteins and long-chain fatty acids, while the major function of SR-BI is to mediate the selective transport of cholesteryl esters from HDL particles.28,34,39,57–59 CD36 on macrophages plays a proatherosclerotic role, while SR-BI is atheroprotective, probably due to its capacity to mediate cholesterol efflux in cholesterol-loaded cells.42,60,61 On platelets, CD36 was described as a major glycoprotein decades ago, while platelet SR-BI expression was reported only recently.38,62 The role of these 2 scavenger receptors in platelet physiology is not well defined. CD36 was reported to contribute to initial stages of platelet adhesion to collagen.63 Reduced levels of SR-BI were associated with increased platelet aggregation.38 Our studies demonstrate that platelet SR-BI plays a role opposite to that of CD36. In the context of dyslipidemia and enhanced oxidative stress, platelet CD36 interaction with specific oxidized phospholipids present in OxLDL results in enhanced platelet reactivity and a prothrombotic phenotype.16 In contrast, SR-BI interaction with OxHDL, a particle that is present in circulation in chronic inflammatory conditions, apparently blunts platelet activation. It is interesting to note that while OxHDL can bind CD36,43 this does not affect agonist-induced platelet activation (Figure 4D,F). Thus, it is tempting to speculate that under conditions of dyslipidemia and oxidative stress, 2 platelet scavenger receptors function to counterbalance each other. In general, lipid oxidation and oxidative stress appear to trigger not only prothrombotic effects but also to produce a previously unrecognized defense mechanism against thrombosis. In patients, the predisposition to thrombotic events may be determined not merely by the accumulation of oxidized lipids, but by its subtype (ie, the ratio of OxLDL to OxHDL).
On a mechanistic level, OxHDL appears to inhibit all key events associated with agonist-induced platelet activation, including the conformational change/activation of integrin αIIbβ3, α-granule secretion (measured by P-selectin surface exposure), and dense granule release (measured by ATP release; Figure 3). Altogether, these data show that OxHDL interferes with agonist-induced signaling pathways, ultimately leading to platelet aggregation. Binding of HDL to SR-BI on endothelial cells was reported to activate eNOS through the PI3K/Akt kinase cascade, resulting in the production of NO.34–37 Our experiments using eNOS−/−, Akt-1−/−, and Akt-2−/− platelets clearly ruled out the PI3K/Akt/eNOS pathway as playing any significant role in the observed inhibitory effects of OxHDL.
OxHDL was able to interfere with platelet activation triggered by several physiologically relevant agonists, suggesting that it affects a signaling pathway common to all agonists. PKC activation is a central event in platelet aggregation induced by thrombin, collagen, and ADP.64–67 OxHDL had no effect on platelet activation and aggregation induced by the PKC activator PMA (Figure 6E,F), indicating that OxHDL inhibits platelet aggregation either at PKC or upstream of PKC. In sum, our studies establish a novel mechanism for regulation of platelet activity by oxidized lipoproteins. Oxidative stress leads to generation of at least 2 types of products circulating in blood and affecting platelet function, OxLDL and OxHDL. While the OxLDL/CD36 axis triggers platelet activation and promotes aggregation, OxHDL and its receptor SR-BI appear to serve as a natural shield mechanism restricting excessive platelet stimulation and subsequent thrombotic events.
Acknowledgments
We thank V. Verbovetskaya for technical assistance.
This work was supported in part by National Institutes of Health grants HL077213 and HL053315 (E.A.P.), HL071625 (T.V.B.), and HL70083 (M.F.).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.V. designed the research, performed the experiments, analyzed the data, and wrote the paper; N.K. and M.Z.A. designed and performed the experiments and analyzed the data; T.V.B. and M.F. designed the research, discussed the results, and wrote the paper; and E.A.P. designed the research, wrote the paper, and provided overall direction.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eugene A. Podrez, Cleveland Clinic, Lerner Research Institute, Department of Molecular Cardiology, 9500 Euclid Ave, ND-50, Cleveland, OH 44195; e-mail: podreze@ccf.org.
References
- 1.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease: the Framingham Study. Am J Med. 1977;62:707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 2.Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. 2005;96:1221–1232. doi: 10.1161/01.RES.0000170946.56981.5c. [DOI] [PubMed] [Google Scholar]
- 3.Gordon DJ, Rifkind BM. High-density lipoprotein: the clinical implications of recent studies. N Engl J Med. 1989;321:1311–1316. doi: 10.1056/NEJM198911093211907. [DOI] [PubMed] [Google Scholar]
- 4.Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res. 2006;98:1352–1364. doi: 10.1161/01.RES.0000225982.01988.93. [DOI] [PubMed] [Google Scholar]
- 5.Assmann G, Nofer JR. Atheroprotective effects of high-density lipoproteins. Annu Rev Med. 2003;54:321–341. doi: 10.1146/annurev.med.54.101601.152409. [DOI] [PubMed] [Google Scholar]
- 6.Barter PJ, Nicholls S, Rye KA, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res. 2004;95:764–772. doi: 10.1161/01.RES.0000146094.59640.13. [DOI] [PubMed] [Google Scholar]
- 7.Hassall DG, Owen JS, Bruckdorfer KR. The aggregation of isolated human platelets in the presence of lipoproteins and prostacyclin. Biochem J. 1983;216:43–49. doi: 10.1042/bj2160043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naqvi TZ, Shah PK, Ivey PA, et al. Evidence that high-density lipoprotein cholesterol is an independent predictor of acute platelet-dependent thrombus formation. Am J Cardiol. 1999;84:1011–1017. doi: 10.1016/s0002-9149(99)00489-0. [DOI] [PubMed] [Google Scholar]
- 9.Nofer JR, Walter M, Kehrel B, et al. HDL3-mediated inhibition of thrombin-induced platelet aggregation and fibrinogen binding occurs via decreased production of phosphoinositide-derived second messengers 1,2-diacylglycerol and inositol 1,4,5-tris-phosphate. Arterioscler Thromb Vasc Biol. 1998;18:861–869. doi: 10.1161/01.atv.18.6.861. [DOI] [PubMed] [Google Scholar]
- 10.Chen LY, Mehta JL. Inhibitory effect of high-density lipoprotein on platelet function is mediated by increase in nitric oxide synthase activity in platelets. Life Sci. 1994;55:1815–1821. doi: 10.1016/0024-3205(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 11.Desai K, Bruckdorfer KR, Hutton RA, Owen JS. Binding of apoE-rich high density lipoprotein particles by saturable sites on human blood platelets inhibits agonist-induced platelet aggregation. J Lipid Res. 1989;30:831–840. [PubMed] [Google Scholar]
- 12.Desai K, Mistry P, Bagget C, Burroughs AK, Bellamy MF, Owen JS. Inhibition of platelet aggregation by abnormal high density lipoprotein particles in plasma from patients with hepatic cirrhosis. Lancet. 1989;1:693–695. doi: 10.1016/s0140-6736(89)92207-1. [DOI] [PubMed] [Google Scholar]
- 13.Takahashi Y, Chiba H, Matsuno K, et al. Native lipoproteins inhibit platelet activation induced by oxidized lipoproteins. Biochem Biophys Res Commun. 1996;222:453–459. doi: 10.1006/bbrc.1996.0765. [DOI] [PubMed] [Google Scholar]
- 14.Korporaal SJ, Akkerman JW. Platelet activation by low density lipoprotein and high density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:270–280. doi: 10.1159/000093220. [DOI] [PubMed] [Google Scholar]
- 15.Lacoste L, Lam JY, Hung J, Letchacovski G, Solymoss CB, Waters D. Hyperlipidemia and coronary disease: correction of the increased thrombogenic potential with cholesterol reduction. Circulation. 1995;92:3172–3177. doi: 10.1161/01.cir.92.11.3172. [DOI] [PubMed] [Google Scholar]
- 16.Podrez EA, Byzova TV, Febbraio M, et al. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greilberger J, Jurgens G. Oxidation of high-density lipoprotein HDL3 leads to exposure of apo-AI and apo-AII epitopes and to formation of aldehyde protein adducts, and influences binding of oxidized low-density lipoprotein to type I and type III collagen in vitro1. Biochem J. 1998;331:185–191. doi: 10.1042/bj3310185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowry VW, Stanley KK, Stocker R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc Natl Acad Sci U S A. 1992;89:10316–10320. doi: 10.1073/pnas.89.21.10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deakin S, Moren X, James RW. HDL oxidation compromises its influence on paraoxonase-1 secretion and its capacity to modulate enzyme activity. Arterioscler Thromb Vasc Biol. 2007;27:1146–1152. doi: 10.1161/ATVBAHA.107.141747. [DOI] [PubMed] [Google Scholar]
- 21.Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL–an evolving field. Nat Clin Pract Endocrinol Metab. 2006;2:504–511. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- 22.Shao B, Oda MN, Oram JF, Heinecke JW. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr Opin Cardiol. 2006;21:322–328. doi: 10.1097/01.hco.0000231402.87232.aa. [DOI] [PubMed] [Google Scholar]
- 23.Pankhurst G, Wang XL, Wilcken DE, et al. Characterization of specifically oxidized apolipoproteins in mildly oxidized high density lipoprotein. J Lipid Res. 2003;44:349–355. doi: 10.1194/jlr.M200256-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Upston JM, Niu X, Brown AJ, et al. Disease stage-dependent accumulation of lipid and protein oxidation products in human atherosclerosis. Am J Pathol. 2002;160:701–710. doi: 10.1016/S0002-9440(10)64890-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suarna C, Dean RT, May J, Stocker R. Human atherosclerotic plaque contains both oxidized lipids and relatively large amounts of alpha-tocopherol and ascorbate. Arterioscler Thromb Vasc Biol. 1995;15:1616–1624. doi: 10.1161/01.atv.15.10.1616. [DOI] [PubMed] [Google Scholar]
- 26.Acton SL, Scherer PE, Lodish HF, Krieger M. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J Biol Chem. 1994;269:21003–21009. [PubMed] [Google Scholar]
- 27.Acton S, Rigotti A, Landschulz KT, Xu S, Hobbs HH, Krieger M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science. 1996;271:518–520. doi: 10.1126/science.271.5248.518. [DOI] [PubMed] [Google Scholar]
- 28.Krieger M, Kozarsky K. Influence of the HDL receptor SR-BI on atherosclerosis. Curr Opin Lipidol. 1999;10:491–497. doi: 10.1097/00041433-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Connelly MA, Williams DL. Scavenger receptor BI: a scavenger receptor with a mission to transport high density lipoprotein lipids. Curr Opin Lipidol. 2004;15:287–295. doi: 10.1097/00041433-200406000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Kozarsky KF, Donahee MH, Rigotti A, Iqbal SN, Edelman ER, Krieger M. Overexpression of the HDL receptor SR-BI alters plasma HDL and bile cholesterol levels. Nature. 1997;387:414–417. doi: 10.1038/387414a0. [DOI] [PubMed] [Google Scholar]
- 31.Krieger M. Charting the fate of the “good cholesterol”: identification and characterization of the high-density lipoprotein receptor SR-BI. Annu Rev Biochem. 1999;68:523–558. doi: 10.1146/annurev.biochem.68.1.523. [DOI] [PubMed] [Google Scholar]
- 32.Varban ML, Rinninger F, Wang N, et al. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci U S A. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huby T, Doucet C, Dachet C, et al. Knockdown expression and hepatic deficiency reveal an atheroprotective role for SR-BI in liver and peripheral tissues. J Clin Invest. 2006;116:2767–2776. doi: 10.1172/JCI26893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger M. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiologic systems. J Clin Invest. 2001;108:793–797. doi: 10.1172/JCI14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mineo C, Shaul PW. HDL stimulation of endothelial nitric oxide synthase: a novel mechanism of HDL action. Trends Cardiovasc Med. 2003;13:226–231. doi: 10.1016/s1050-1738(03)00098-7. [DOI] [PubMed] [Google Scholar]
- 36.Yuhanna IS, Zhu Y, Cox BE, et al. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 37.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 38.Imachi H, Murao K, Cao W, et al. Expression of human scavenger receptor B1 on and in human platelets. Arterioscler Thromb Vasc Biol. 2003;23:898–904. doi: 10.1161/01.ATV.0000067429.46333.7B. [DOI] [PubMed] [Google Scholar]
- 39.Febbraio M, Abumrad NA, Hajjar DP, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–19062. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- 40.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Podrez EA, Schmitt D, Hoff HF, Hazen SL. Myeloperoxidase-generated reactive nitrogen species convert LDL into an atherogenic form in vitro. J Clin Invest. 1999;103:1547–1560. doi: 10.1172/JCI5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Podrez EA, Febbraio M, Sheibani N, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest. 2000;105:1095–1108. doi: 10.1172/JCI8574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thorne RF, Mhaidat NM, Ralston KJ, Burns GF. CD36 is a receptor for oxidized high density lipoprotein: implications for the development of atherosclerosis. FEBS Lett. 2007;581:1227–1232. doi: 10.1016/j.febslet.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 44.Assanasen C, Mineo C, Seetharam D, et al. Cholesterol binding, efflux, and a PDZ-interacting domain of scavenger receptor-BI mediate HDL-initiated signaling. J Clin Invest. 2005;115:969–977. doi: 10.1172/JCI200523858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojanovic A, Marjanovic JA, Brovkovych VM, et al. A phosphoinositide 3-kinase-AKT-nitric oxide-cGMP signaling pathway in stimulating platelet secretion and aggregation. J Biol Chem. 2006;281:16333–16339. doi: 10.1074/jbc.M512378200. [DOI] [PubMed] [Google Scholar]
- 46.Andrews RK, Gardiner EE, Shen Y, Berndt MC. Platelet interactions in thrombosis. IUBMB Life. 2004;56:13–18. doi: 10.1080/15216540310001649831. [DOI] [PubMed] [Google Scholar]
- 47.Stouffer GA, Smyth SS. Effects of thrombin on interactions between beta3-integrins and extracellular matrix in platelets and vascular cells. Arterioscler Thromb Vasc Biol. 2003;23:1971–1978. doi: 10.1161/01.ATV.0000093470.51580.0F. [DOI] [PubMed] [Google Scholar]
- 48.Wu Z, Wagner MA, Zheng L, et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14:861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- 49.Matsumoto N, Nomura S, Kamihata H, Kimura Y, Iwasaka T. Increased level of oxidized LDL-dependent monocyte-derived microparticles in acute coronary syndrome. Thromb Haemost. 2004;91:146–154. doi: 10.1160/TH03-04-0247. [DOI] [PubMed] [Google Scholar]
- 50.Siess W, Zangl KJ, Essler M, et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weidtmann A, Scheithe R, Hrboticky N, Pietsch A, Lorenz R, Siess W. Mildly oxidized LDL induces platelet aggregation through activation of phospholipase A2. Arterioscler Thromb Vasc Biol. 1995;15:1131–1138. doi: 10.1161/01.atv.15.8.1131. [DOI] [PubMed] [Google Scholar]
- 52.Ardlie NG, Selley ML, Simons LA. Platelet activation by oxidatively modified low density lipoproteins. Atherosclerosis. 1989;76:117–124. doi: 10.1016/0021-9150(89)90094-4. [DOI] [PubMed] [Google Scholar]
- 53.Korporaal SJ, Gorter G, van Rijn HJ, Akkerman JW. Effect of oxidation on the platelet-activating properties of low-density lipoprotein. Arterioscler Thromb Vasc Biol. 2005;25:867–872. doi: 10.1161/01.ATV.0000158381.02640.4b. [DOI] [PubMed] [Google Scholar]
- 54.Nofer JR, Noll C, Feuerborn R, Assmann G, Tepel M. Low density lipoproteins inhibit the Na+/H+ antiport in human platelets via activation of p38MAP kinase. Biochem Biophys Res Commun. 2006;340:751–757. doi: 10.1016/j.bbrc.2005.12.070. [DOI] [PubMed] [Google Scholar]
- 55.Leitinger N. Oxidized phospholipids as modulators of inflammation in atherosclerosis. Curr Opin Lipidol. 2003;14:421–430. doi: 10.1097/00041433-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Vlasova II, Azizova OA. [Effect of high density lipoproteins on the ADP-induced aggregation of thrombocytes in plasma]. Biull Eksp Biol Med. 1998;126:160–163. [PubMed] [Google Scholar]
- 57.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 58.Rigotti A, Acton SL, Krieger M. The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J Biol Chem. 1995;270:16221–16224. doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- 59.Tait JF, Smith C. Phosphatidylserine receptors: role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. J Biol Chem. 1999;274:3048–3054. doi: 10.1074/jbc.274.5.3048. [DOI] [PubMed] [Google Scholar]
- 60.Febbraio M, Podrez EA, Smith JD, et al. Targeted disruption of the class B scavenger receptor CD36 protects against atherosclerotic lesion development in mice. J Clin Invest. 2000;105:1049–1056. doi: 10.1172/JCI9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang W, Yancey PG, Su YR, et al. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2003;108:2258–2263. doi: 10.1161/01.CIR.0000093189.97429.9D. [DOI] [PubMed] [Google Scholar]
- 62.Tandon NN, Lipsky RH, Burgess WH, Jamieson GA. Isolation and characterization of platelet glycoprotein IV (CD36). J Biol Chem. 1989;264:7570–7575. [PubMed] [Google Scholar]
- 63.Tandon NN, Kralisz U, Jamieson GA. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989;264:7576–7583. [PubMed] [Google Scholar]
- 64.Yacoub D, Theoret JF, Villeneuve L, et al. Essential role of protein kinase C delta in platelet signaling, alpha IIb beta 3 activation, and thromboxane A2 release. J Biol Chem. 2006;281:30024–30035. doi: 10.1074/jbc.M604504200. [DOI] [PubMed] [Google Scholar]
- 65.Murugappan S, Shankar H, Bhamidipati S, Dorsam RT, Jin J, Kunapuli SP. Molecular mechanism and functional implications of thrombin-mediated tyrosine phosphorylation of PKCdelta in platelets. Blood. 2005;106:550–557. doi: 10.1182/blood-2004-12-4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia A, Shankar H, Murugappan S, Kim S, Kunapuli SP. Regulation and functional consequences of ADP receptor-mediated ERK2 activation in platelets. Biochem J. 2007;404:299–308. doi: 10.1042/BJ20061584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murugappa S, Kunapuli SP. The role of ADP receptors in platelet function. Front Biosci. 2006;11:1977–1986. doi: 10.2741/1939. [DOI] [PubMed] [Google Scholar]